Summary
Bloom’s helicase (BLM) is thought to prevent crossing-over during DNA double-strand-break repair (DSBR) by disassembling double Holliday Junctions (dHJs) or by preventing their formation. We show that Saccharomyces cerevisiae BLM ortholog, Sgs1, prevents aberrant crossing-over during meiosis by suppressing formation of joint molecules (JMs) comprising three and four interconnected duplexes. Sgs1 and pro-crossover factors, Msh5 and Mlh3, are antagonistic, since Sgs1 prevents dHJ formation in msh5 cells and sgs1 mutation alleviates crossover defects of both msh5 and mlh3 mutants. We propose that differential activity of Sgs1 and pro-crossover factors at the two DSB-ends effects productive formation of dHJs and crossovers, and prevents multi-chromatid JMs and counterproductive crossing-over. Strand-invasion of different templates by both DSB-ends may be a common feature of DSBR that increases repair efficiency but also the likelihood of associated crossing-over. Thus, by disrupting aberrant JMs, BLM-related helicases maximize repair efficiency while minimizing the risk of deleterious crossing-over.
Introduction
Homologous recombination (HR) occurs when a broken or damaged chromosome uses a homologous chromosome as template for its repair (Paques and Haber, 1999). HR can occur with one of two outcomes: a crossover, with exchange of chromosome arms, or a non-crossover involving only a local alteration of DNA. Unregulated crossing-over can cause chromosome rearrangements, missegregation and homozygosis of deleterious mutations (Richardson et al., 2004). To minimize these risks, mitotically dividing cells actively suppress crossovers and preferentially utilize the sister-chromatid as repair-template (Kadyk and Hartwell, 1992; Johnson and Jasin, 2001).
The RecQ family DNA helicase, Sgs1, acts to suppress mitotic crossing-over in budding yeast (Gangloff et al., 1994; Ira et al., 2003). Sgs1 is a homolog of human BLM, which is mutated in the cancer-prone Bloom’s Syndrome (Ellis et al., 1995; Watt et al., 1996). The signature of cells from Bloom’s patients is unregulated crossing-over (Chaganti et al., 1974). In vitro studies show that RecQ proteins are bona fide DNA helicases with a preference for branched structures including joint molecule (JM) HR intermediates (Opresko et al., 2004). BLM disrupts D-loops, in which one DSB-end has undergone strand-exchange with a homologous duplex, and both BLM and Sgs1 promote branch-migration of Holliday Junctions (HJs; Bennett et al., 1999; Karow et al., 2000; van Brabant et al., 2000; Bachrati and Hickson, 2006). Notably, combined action of BLM, its cognate type-I topoisomerase TOPIIIα, and the specificity factor BLAP75/RMI1 can catalyze the “dissolution” of double-Holliday Junctions (dHJs; Figure 2C) into two non-crossover duplexes (Wu et al., 2006; Wu and Hickson, 2003; Mullen et al., 2005; Plank et al., 2006). Hypercrossover phenotypes of both sgs1 and top3 mutants are consistent with the dissolvase model (Gangloff et al., 1994; Ira et al., 2003), but direct in vivo evidence for disruptase and/or dissolvase activities has been lacking.
Figure 2. Physical Assay System for Monitoring Recombination.
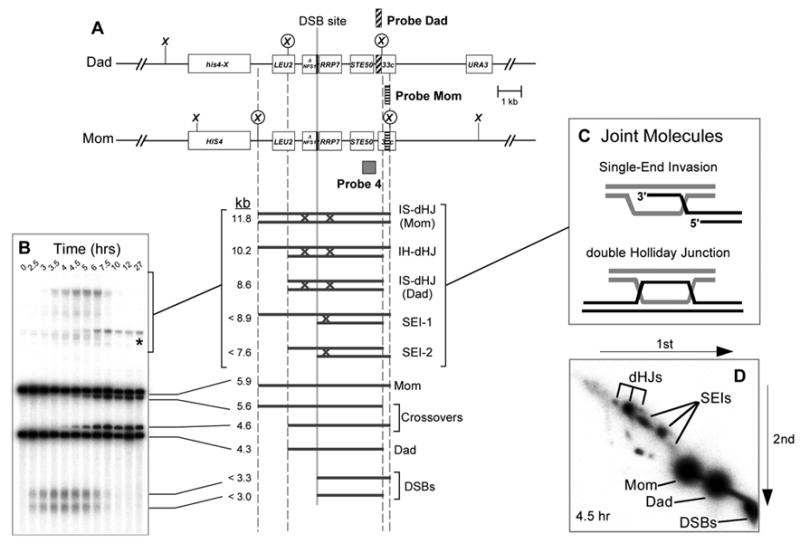
(A) Map of the HIS4LEU2 locus showing diagnostic restriction sites and the positions of probes. DNA species detected with Probe 4 are shown below. SEI-1 and SEI-2 are the two major SEI species detected with Probe 4 (see Hunter and Kleckner, 2001).
(B) Image of one-dimensional (1D) gel hybridized with Probe 4 showing DNA species detailed in (A). Asterisk indicates a meiosis-specific recombinant band resulting from “gene conversion” of the most DSB-proximal XhoI site.
(C) Presumed structures of SEI and dHJ joint molecules.
(D) Image of native/native two-dimensional (2D) gel hybridized with Probe 4. Species detailed in (A) are highlighted. The three dHJ species are highlighted by a trident; SEIs are indicated by a fork.
During meiosis, HR plays essential roles in homolog pairing and segregation (Hunter, 2006; Petronczki et al., 2003). Most critically, crossing-over between homologs facilitates their stable bipolar connection to the meiosis I spindle and thereby promotes regular homolog disjunction. HR is an integral part of the meiotic program, being initiated by DNA double-strand-breaks (DSBs) catalyzed by the transesterase, Spo11 (Keeney, 2001). DSB-ends are resected to form 3′-single-stranded tails which assemble into nucleoprotein filaments together with homologous-pairing and strand-exchange proteins, Rad51 and Dmc1 (Shinohara and Shinohara, 2004). The crossover or non-crossover fate is thought to be determined at the next stage, as DSB-ends pair with a homologous duplex and begin to exchange DNA strands (Allers and Lichten, 2001a; Bishop and Zickler, 2004; Borner et al., 2004; Hunter and Kleckner, 2001). Along the crossover pathway, two joint molecule (JM) intermediates have been identified in vivo: Single End Invasions (SEIs), which are thought to resemble D-loops (Hunter and Kleckner, 2001), and dHJs (see Figure 2C; Bell and Byers, 1983b; Schwacha and Kleckner, 1995; Allers and Lichten, 2001b). dHJs are resolved to give crossover products (Allers and Lichten, 2001a; Hunter and Kleckner, 2001). Molecular events leading to non-crossovers are less clear but likely involve a synthesis-dependent strand-annealing (SDSA) mechanism. In its simplest form, SDSA proposes that one DSB-end invades a homolog and primes DNA synthesis; the nascent strand is then displaced and anneals to complementary sequences on the second DSB-end (Nassif et al., 1994; Paques and Haber, 1999).
At least eleven genes appear to specifically promote the crossover outcome of meiotic HR. These genes encode proteins of diverse molecular function, i.e. DNA helicase, Mer3; DNA exonuclease, Exo1; homologs of the MutS and MutL DNA mismatch-repair proteins, the Msh4-5 and Mlh1-3 heterocomplexes; a major component of synaptonemal complexes (SCs), Zip1; a SUMO E3 ligase, Zip3; large WD-like and TPR-like repeat proteins, Zip2 and Zip4; and a protein with no clear functional motifs, Spo16 (for review see Hunter, 2006; Akira Shinohara, personal communication). All but three of these proteins are termed ZMMs (Zip, Mer, Msh) or SICs (Synapsis Initiation Complex). ZMMs show meiosis-specific expression, and their mutation leads to coordinate defects in recombination and formation of SCs (e.g. Borner et al., 2004). The remaining three – Exo1, Mlh1 and Mlh3 – function in mitotic and meiotic DNA mismatch correction, as well as meiotic crossing-over (Hoffmann and Borts, 2004; Kolas and Cohen, 2004). In contrast to zmm mutants, exo1, mlh1 and mlh3 mutants form SCs normally. Moreover, mlh1 and mlh3 mutants appear to be defective at a later stage of HR than do zmm mutants (Lipkin et al., 2002; Woods et al., 1999) (N. Hunter, A. Jambhekar, J.P. Lao, S.D. Oh, N. Kleckner and V.B. Borner, submitted).
In this study, we examine the function of Sgs1 in meiotic HR and its relationship to pro-crossover activities, represented by Msh5 and Mlh3. Our data argue that the major function of Sgs1 is not as a general regulator of the crossover/non-crossover decision; rather, Sgs1 acts at designated crossover sites, in conjunction with pro-crossover activities, to promote the orderly formation of interhomolog dHJs and productive crossing-over. In the absence of Sgs1, we detect high levels of a novel class of JMs that comprise three and four interconnected duplexes. Formation of these structures correlates with a specific increase in closely spaced double crossovers. We also provide direct in vivo evidence that Sgs1 suppresses dHJ formation between sister-chromatids. These findings have broad implications for understanding meiosis, DSB-repair and the functions of BLM-related helicases.
Results
Closely spaced double crossovers are specifically elevated in sgs1-ΔC795 mutants
To determine whether mutation of SGS1 causes a general increase in crossing-over, we utilized a diploid strain in which crossing-over within nine different intervals, located on three different chromosomes can be scored in a single cross (Figure 1A; Experimental Procedures). We also utilized the sgs1-ΔC795 truncation mutation (Mullen et al., 2000) because, as shown by Rockmill et al. (2003), cells carrying this mutation sporulate more efficiently than sgs1Δ null mutants and their vegetative growth rate is essentially normal. Sgs1-ΔC795 protein lacks the conserved helicase, RQC and HRDC domains but retains the N-terminal region implicated in several protein-protein interactions (Bachrati and Hickson, 2003). Wild-type and sgs1-ΔC795 cells were sporulated and segregation patterns analyzed by tetrad analysis (Figure 1; supplemental materials, Figure S1 and Tables S1 to S4). At least one interval along each of the three chromosomes analyzed shows a significant increase in map distance (Figure 1C and Tables S1 and S2). The largest increase, almost 1.7-fold, is observed along chromosome 3 in the interval LEU2-CEN3. Overall, however, the combined map distance for all intervals is increased by a modest 1.17-fold (from 165 cM in wild type to 193 cM in sgs1-ΔC795).
Figure 1. Tetrad Analysis of Wild-Type and sgs1-ΔC795 Cells.
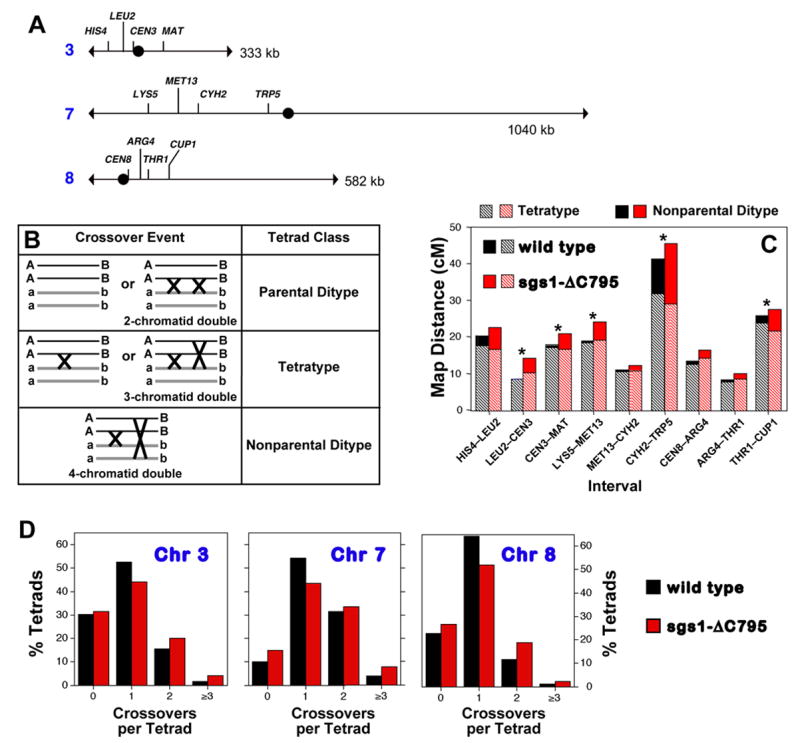
(A) Intervals analyzed. CEN3 is marked with the ADE2 gene; CEN8 is marked with URA3.
(B) Crossover classes within a single interval and their genetic outcomes.
(C) Contributions of tetratype and non-parental ditype tetrads to map distances in wild-type and sgs1-ΔC795 strains. Asterisks indicate significant differences between map distances in wild-type and sgs1-ΔC795 tetrads (see Tables S1 and S2; spore viability data are shown in Figure S1).
(D) Distribution of crossover classes for the combined intervals along the three chromosomes analyzed. Wild type and sgs1-ΔC795 distributions differ significantly for each chromosome: chromosome 3, P = 0.0008; chromosome 7, P = 3 × l0−5; chromosome 8, P = 1 × l0−5.
In a single genetic interval, various crossover classes are detected by tetrad analysis (Figure 1B): double crossovers involving all four chromatids result in a nonparental ditype tetrad (NPD); single crossovers or double crossovers involving three chromatids produce tetratype tetrads (TT); and zero crossovers or double crossovers involving the same two chromatids produce parental ditype tetrads (PD). Close inspection of sgs1-ΔC795 tetrad data reveals that expanded map distances are attributable to a disproportionate increase in closely spaced double crossovers, as represented by NPD tetrads (Figure 1C and Supplemental Table S1). This pattern is not expected from a general increase in crossing-over, i.e. all DSBs having an increased probability of a crossover outcome, which predicts a decrease in the zero (PD) tetrad class and proportional increases in single (TT) and double (NPD) crossover tetrads. The unique alteration of crossing-over in sgs1-ΔC795 cells is further illustrated by comparing distributions of crossover classes for the combined intervals along each of the three analyzed chromosomes (Figure 1D). For each chromosome, the fraction of sgs1-ΔC795 tetrads with zero detectable crossovers remains unchanged or is increased; the fraction with one detectable crossover is decreased; and the fractions with two and three-or-more crossovers are increased.
Adjacent crossovers between the same pair of homologs tend to be widely and evenly spaced, a phenomenon known as positive crossover interference (Muller, 1916). The disproportionate increase in double crossovers in sgs1-ΔC795 cells suggests that positive crossover interference may be diminished. This inference is confirmed by additional analysis presented in Supplemental Figure S2 and Tables S3 and S4.
Taken together, tetrad analysis indicates that while the sgs1-ΔC795 mutation moderately increases map distances, its major effect is not a general increase in the probability that any initiated recombination event will become a crossover. Rather, it appears that a fraction of the events that would normally form single crossovers in wild-type cells gives rise to closely-spaced double crossovers in sgs1-ΔC795 cells.
Intersister-dHJs are elevated in sgs1-ΔC795 cells
To understand the molecular defects underlying the aberrant crossover patterns in sgs1-ΔC795 cells, we analyzed the DNA events of recombination using the HIS4LEU2 physical assay system (Figure 2; Schwacha and Kleckner, 1995; Hunter and Kleckner, 2001). DNA events are monitored over time in synchronized cultures induced to undergo meiosis. Cell samples are treated with psoralen to produce DNA interstrand crosslinks, which stabilize SEI and dHJ intermediates. Species of interest are detected by gel electrophoresis and Southern hybridization with Probe 4 (Figure 2). XhoI polymorphisms between parental “Mom” and “Dad” homologs produce diagnostic restriction fragments for parental and recombinant chromosomes, DSBs and JMs (SEIs and dHJs). In addition, Mom and Dad chromosomes can be distinguished by probing for short heterologous insertions of ØX174 DNA (“Probe Mom” and “Probe Dad” in Figure 2A; Schwacha and Kleckner, 1994). Each hybridizing signal is quantified using a Phosphorimager. DSBs and crossovers are quantified from one-dimensional gels (Figure 2B). Native/native two-dimensional gels reveal the branched structure of JMs and are used to quantitate SEIs and dHJs (Figure 2C and D; Bell and Byers, 1983a; Hunter and Kleckner, 2001). To monitor the timing and efficiency of meiotic divisions, fixed cells are stained with DAPI and scored as having one, two or four nuclei.
Wild-type and sgs1-ΔC795 cultures were sporulated and analyzed in parallel. Analysis of one pair of time-courses is described below and in Figure 3. Data for two additional pairs of time-courses are presented in Supplemental Figure S3. Although the absolute levels of recombination intermediates vary between time-courses, all paired experiments are internally consistent and identical conclusions can be drawn.
Figure 3. Physical Analysis of Recombination in Wild-Type and sgs1-ΔC795 cells.
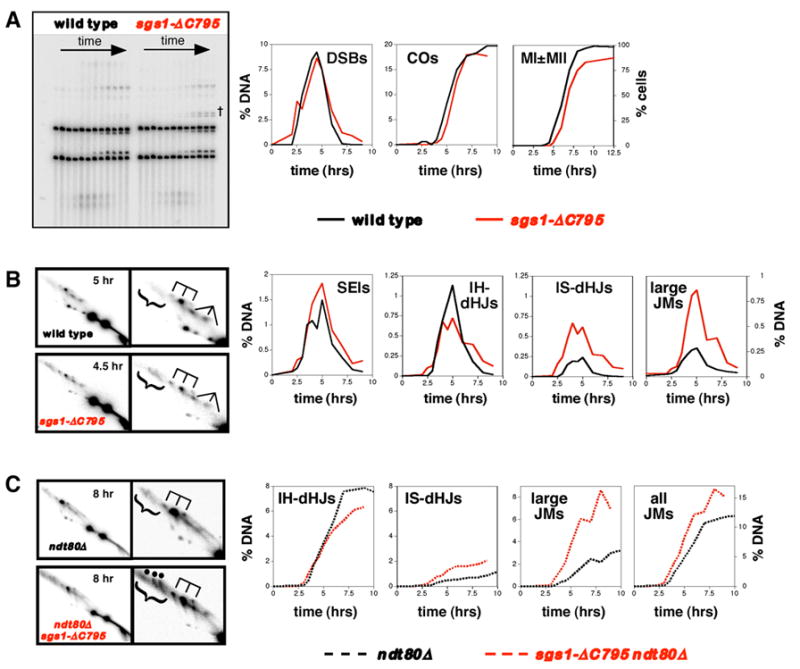
Row (A): 1D analysis of DSBs and crossovers (COs), and analysis of meiotic divisions (MI±MII). % DNA is percent of total hybridizing DNA. MI±MII is cells that have completed either the first or second meiotic divisions. †, bands resulting from ectopic recombination between HIS4LEU2 and the leu2::hisG allele at the native LEU2 locus (see Grushcow et al., 1999). The role of Sgs1 in preventing these events will be described elsewhere (S.D. Oh and N. Hunter, in preparation).
Row (B): 2D analysis of joint molecules in NDT80 cells. In each case a representative 2D panel is shown together with a blowup of the JM region. dHJ species are highlighted by a trident; SEIs are indicated by a fork; large JMs are bracketed.
Row (C): 2D analysis of joint molecules in ndt80Δ cells. “all JMs” = IH-dHJs + IS-dHJs + large JMs.
See also Supplemental Figure S2.
DSBs
In wild-type cells, DSBs are detected 2.5 hrs after induction of meiosis, peak at 4.5 hrs and are gone by 7 hrs (Figure 3A). The timing and level of DSBs in sgs1-ΔC795 cells are very similar to wild type except that a small number of DSBs may turn over more slowly.
Crossovers and meiotic divisions
Crossover bands first appear at 4 hrs for wild-type meiosis, and plateau after 8 hrs at 19.7% of hybridizing DNA. In sgs1-ΔC795 cells, crossovers show a very slight delay (≤30 minutes) and plateau at 18.1% of hybridizing DNA. This slight reduction in crossover frequency contradicts tetrad data, which show that crossing-over at HIS4LEU2 is, in fact, slightly increased in sgs1-ΔC795 cells (29.9 cM versus 26.9 cM in wild type; see Supplemental Tables S5 and S6). A possible explanation is that a small fraction of sgs1-ΔC795 cells fail to enter meiosis or to complete the first meiotic division, as reflected by the reduced efficiency of meiotic divisions in this strain (88% versus 97% in wild type; Figure 3A). Correcting for this difference gives a maximum crossover level of 19.9% for the sgs1-ΔC795 time-course shown in Figure 3. By averaging measurements from three independent time-courses for both wild type and sgs1-ΔC795 strains, a more accurate comparison of crossing-over was made. An average of 19.0 ± 0.6% (S.E.) crossovers was recorded for the three wild-type experiments compared to 19.9 ± 1.5% for sgs1-ΔC795 time-courses. In each case, meiotic divisions in sgs1-ΔC795 time-courses were less efficient (88, 85 and 88% versus 94, 95 and 97%). Assuming this difference reflects cells that failed to undergo meiosis, crossing-over could be as high as 21.7 ± 1.5% in sgs1-ΔC795 cells. This ~1.14-fold increase is consonant with the ~1.11-fold increase measured by tetrad analysis (above). In summary, crossing-over at HIS4LEU2 is either unaffected or slightly increased by the sgs1-ΔC795 mutation.
SEIs
In both wild-type and sgs1-ΔC795 cells, SEIs form with similar kinetics and peak at ~5 hrs (Figure 3B). SEIs reach slightly higher levels over several time points in sgs1-ΔC795 cells but this difference does not appear to be reproducible (Supplemental Figure S2).
dHJs
Restriction site polymorphisms between Mom and Dad homologs allow interhomolog dHJs (IH-dHJs) to be distinguished from intersister dHJs (IS-dHJs) (Figure 2A and 2D; (Schwacha and Kleckner, 1997). In wild-type cells, IH-dHJs form with a ~4.7-fold bias over IS-dHJs (peak steady-state levels of 1.13% for IH-dHJs versus 0.24% IS-dHJs; Figure 3B). This strong interhomolog bias is diminished in sgs1-ΔC795 mutant cells. Peak steady-state levels of IH-dHJs are slightly reduced relative to wild type (0.72% versus 1.13%), whereas IS-dHJs are increased ~2.5-fold (0.61% versus 0.24%).
ndt80Δ analysis
The apparent reduction of IH-dHJs in sgs1-ΔC795 cells is surprising, especially given that crossovers reach at least wild-type levels. To rule out the possibility that a subset of IH-dHJs turn over faster in sgs1-ΔC795 cells, we measured dHJ levels in an ndt80Δ background, which causes cells to arrest in pachytene and accumulate dHJs (Figure 3C; Allers and Lichten, 2001a). This analysis confirms the inferences made in NDT80 cells. Specifically, IH-dHJs accumulate to ~25% lower levels in sgs1-ΔC795 cells (7.9% in wild type versus 6.3% in sgs1-ΔC795), and IS-dHJs reach ~2-fold higher levels (0.87% in wild-type versus 1.75% in sgs1-ΔC795). Joint molecule analysis from ndt80Δ cells differs from that in NDT80 cells in that interhomolog bias appears to be more extreme in the ndt80Δ data set (4.7- versus 9-fold for SGS1 cells, and 1.2- versus 3.6-fold in sgs1-ΔC795 mutants). The reason for this difference is unclear. IH-dHJs and IS-dHJs may have different life-spans in NDT80 cells, or ndt80Δ may differentially affect the resolution of IH-dHJs relative to IS-dHJs. Regardless, this difference does not alter the basic inference that the sgs1-ΔC795 mutation decreases IH-dHJs and increases IS-dHJs.
Novel high molecular-weight JMs form at high levels in sgs1-ΔC795 cells
sgs1-ΔC795 ndt80Δ analysis reveals three, prominent, high molecular-weight, JM species on 2D gels (bracketed signals in Figure 3C; individual species highlighted by dots; also see Figure 4B and C). Two of these appear as discrete signals with sizes in the ~14–17kb range and their relative levels are essentially equal; a third signal is less discrete and larger, at ~20kb. These species can also be detected in wild type, sgs1-ΔC795 and ndt80Δ strains (Figure 3B and C) but the sgs1-ΔC795 mutation significantly increases their levels, by ~3-fold in both NDT80 and ndt80Δ backgrounds. Thus, large JMs are a major recombination intermediate during meiosis in sgs1-ΔC795 cells. Overall, when IH-dHJ, IS-dHJ and large JM signals are added together, sgs1-ΔC795 cells form ~35% more JMs than wild-type cells, due to conspicuous increases in IS-dHJs and large JMs.
Figure 4. Large JMs Contain Three and Four Chromatids.
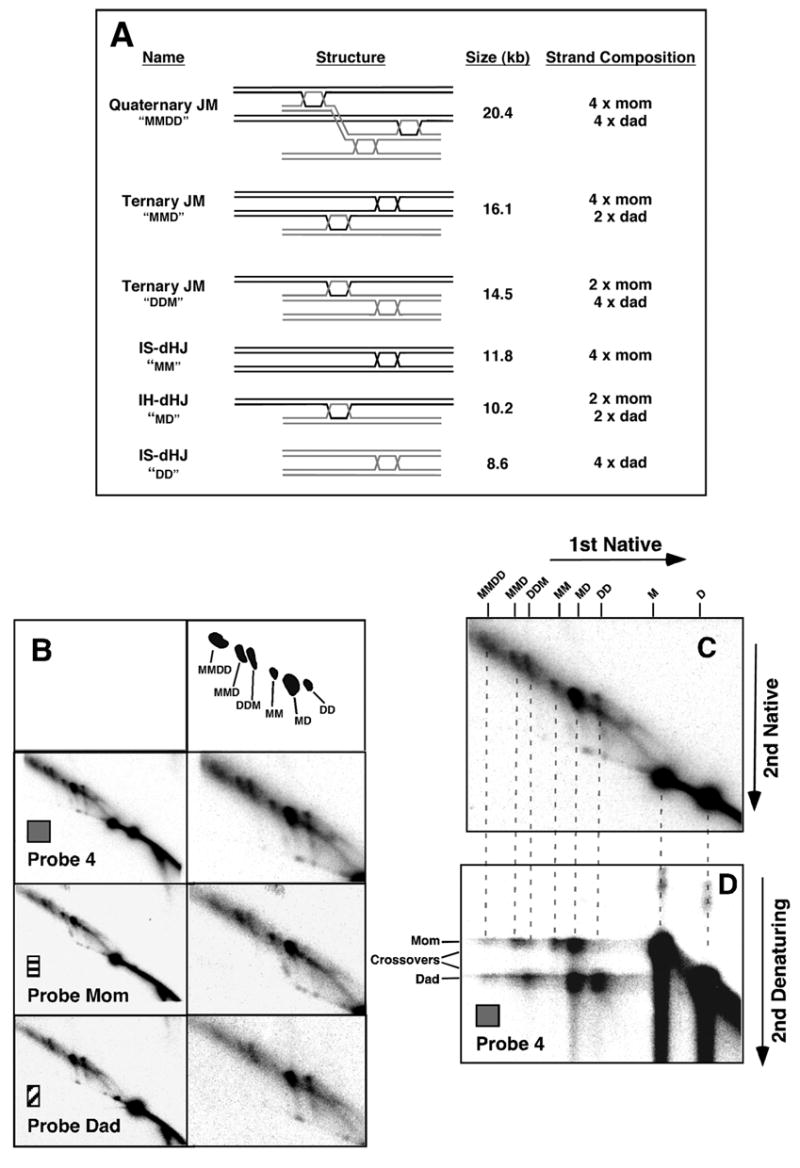
(A) Predicted structures, sizes and DNA strand composition of joint molecule species.
(B) Sequential probing of an 8 hr sample from an sgs1-ΔC795 ndt80Δ time-course with common probe, Probe 4, and homolog-specific probes, Probe Dad and Probe Mom (see Figure 2). Full panels from native/native 2D gels are shown on the left and blowups are shown on the right. Interpretative cartoon shows the positions of the JM species detailed in (A).
(C) and (D) “Pull-apart” analysis of component strands of the large JMs. (C) Native/native 2D gel highlighting the species of interest. (D) Native/denaturing 2D gel showing that large JMs comprise parental-length component single strands.
Large JMs comprise three or four interconnected homologous chromatids
The prominence of large JMs in sgs1-ΔC795 meiosis makes it critical to ascertain their identity. An attractive possibility is that large JMs comprise more than two duplexes, interconnected by Holliday Junctions (Figure 4A). Predicted sizes of these ternary and quaternary JMs are consistent with sizes of the large JMs detected on 2D gels: two Dad chromatids plus one Mom chromatid will produce a JM of 14.5 kb (ternary JM “DDM”); two Moms plus one Dad will give a JM of 16.1 kb (ternary JM “MMD”); and two Moms plus two Dads will form a JM of 20.4 kb (quaternary JM “DDMM”). Notably, the latter species can be resolved to give two closely-spaced interhomolog crossovers (see Figure 7C).
Figure 7. Model of Sgs1/BLM Function During Meiosis.
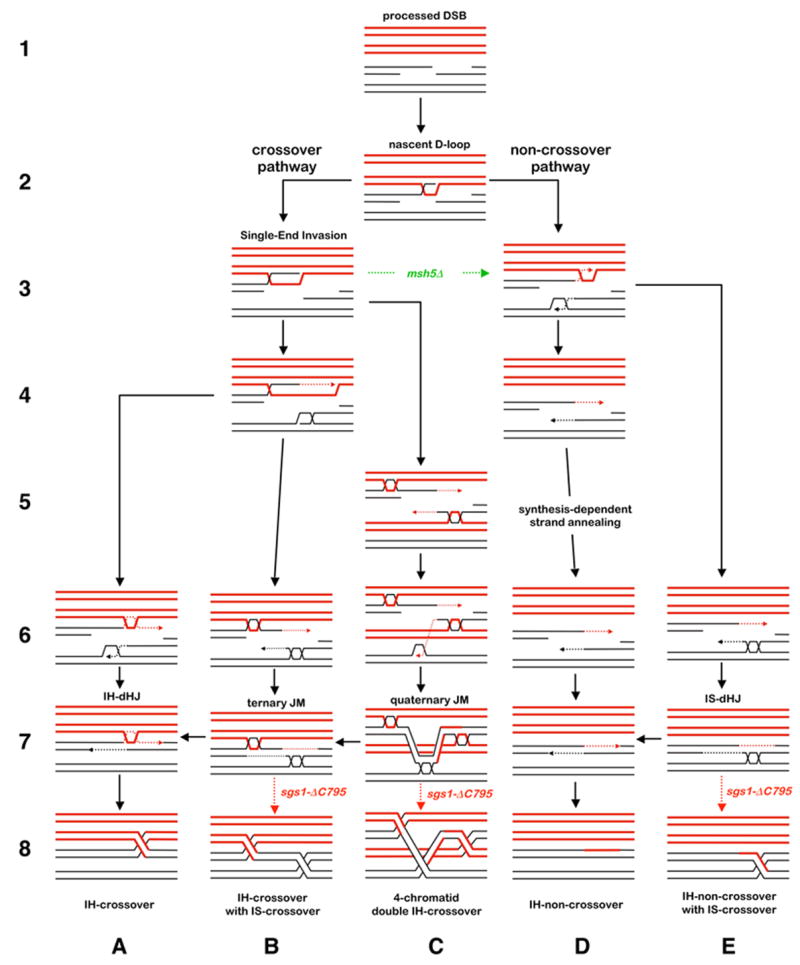
Homologs are shown in red and black, respectively; dashed lines indicate nascent DNA. Solid black arrows indicate pathways in wild-type cells. Dashed arrows indicate pathways in mutants. The crossover or non-crossover decision follows pairing and strand-invasion by one DSB-end to form a nascent D-loop (steps 1 and 2; Borner et al., 2004; Hunter and Kleckner, 2001). Along the crossover pathway, ZMM proteins convert the nascent JM into a SEI, which is then stabilized by Msh4-Msh5 and Mlh1-Mlh3 antagonizing Sgs1 (steps 3B, 4B and 6–8A; also see Jessop et al., 2006). Along the non-crossover pathway, the initial D-loop is not stabilized by ZMMs and ultimately disassembles even in the absence of Sgs1 (steps 3–8D). When homologs have successfully paired and synapsed, the sister-chromatid (or any second homologous template) may be invaded by the second DSB-end, e.g. steps 3D and 4B. Following extension by DNA synthesis, this end undergoes one of two annealing reactions with the first DSB-end. At a crossover-designated site, the second DSB-end anneals with the SEI to form a canonical dHJ, which is then resolved into a crossover (steps 6–8A). At a non-crossover site, the two DSB-ends anneal to seal the break (steps 6–8D). Along both pathways, the helicase activity of Sgs1 (± Top3 strand-passage activity) ensures that the second DSB-end completely dissociates from the template duplex. This could occur early, by disrupting the D-loop intermediate, or late by dissolving dHJs formed by the second DSB-end. In sgs1Δ-C795 cells, the second DSB-end does not efficiently disengage from its template and forms a stable dHJ independently of the first DSB-end, e.g. steps 5C, 6B, 6E. Along the crossover pathway, this will lead to formation of a ternary JM, which may be resolved into adjacent interhomolog (IH) and intersister (IS) crossovers (steps 6–8B). Successive invasion of two templates by a DSB-end will give a quaternary JM, whose resolution can produce a four-chromatid double crossover (steps 5–8C). Along the non-crossover pathway, stable dHJ formation by the second DSB-end will produce an IH-non-crossover associated with an intersister exchange (steps 6–8E). D-loop migration and “end-first” strand-displacement is proposed to be a common step that precedes strand-annealing to form mature JMs. This mechanism readily accommodates the formation of multi-chromatid JMs. Strand-displacement was previously proposed to explain the occurrence of DSB-distal JMs that lack intervening heteroduplex DNA (Allers and Lichten, 2001b).
To confirm the identity of large JMs, their composition was analyzed in two ways. First, 2D gels were hybridized with probes specific to either Mom or Dad homologs (Figure 2A; Figure 4B). With “Probe Mom”, the predicted pattern is observed; the larger of the two parental linear bands (1 x Mom), the IH-dHJ spot (Mom + Dad) and the larger of the two IS-dHJ spots (2 x Mom) are detected. In addition, Probe Mom detects the three large JM species; notably, the middle size species produces a signal with twice the intensity of the smaller species (signal ratio of 1.9:1.0), consistent with the prediction that the two ternary JMs should contain different numbers of Mom and Dad chromatids. With “Probe Dad” the reciprocal pattern is detected: the smaller parental linear band (1 x Dad), the IH-dHJ (Mom + Dad), the smaller IS-dHJ (2 x Dad) plus the three large JMs. Moreover, the relative intensity of the middle and smaller species is the reverse of that seen with Probe Mom (ratio of 1.0:1.9). Relative signal intensities for the largest JM (1.2:1.0) indicate approximately equal numbers of Mom and Dad chromatids, as expected for a quaternary JM.
We also analyzed component strands of large JMs using native/denaturing 2D gels in which psoralen crosslinks are removed prior to running the second dimension under denaturing conditions (Figure 4D). If large JMs contain three or four homologous duplexes interconnected by either dHJs or hemicatenanes, the component strands should all be parental in length, i.e. Mom- and Dad-length strands (Figure 4A). In the second dimension, as shown previously, IH-dHJs are denatured into Mom- and Dad-length strands, and the two IS-dHJs comprise either Mom or Dad strands (Schwacha and Kleckner, 1995). As predicted, large JMs are denatured into primarily parental-length Mom and Dad strands, consistent with proposed structures of three and four duplexes interconnected by two or three dHJs. Recombinant-length strands are not prominent, indicating that large JMs do not generally include single-HJs (or odd numbers of HJs). Thus, large JMs most likely comprise three and four chromatids interconnected by dHJs. We cannot rule out the possibility that a fraction of multi-chromatid JMs are connected by one or more single HJs, however.
Comparing relative levels of parental-length single-strands in ternary and quaternary JMs confirms the inferences made using homolog-specific probes (above). The quaternary JMs comprise approximately equal levels of Mom and Dad strands (~1.0:1.0), while the two ternary JMs, MMD and DDM, contain respectively ~1.7:1.0 and ~1.0:1.9 ratios of Mom:Dad strands (see Materials and Methods).
Direct visualization of multi-chromatid JMs by electron microscopy
The molecular analysis above is consistent with the idea that large JMs are intermediates containing three and four homologous duplexes. To visualize these structures directly, branched molecules from genomic DNA of ndt80Δ SGS1 and ndt80Δ sgs1ΔC795 cells were purified from 2D gels and examined by electron microscopy (EM) (Figure 5; Bell and Byers, 1983b; Cromie et al., 2006). Note that, unlike analysis at HIS4LEU2, this method visualizes JMs formed at loci throughout the genome.
Figure 5. Direct Visualization of Ternary JMs by Electron Microscopy.
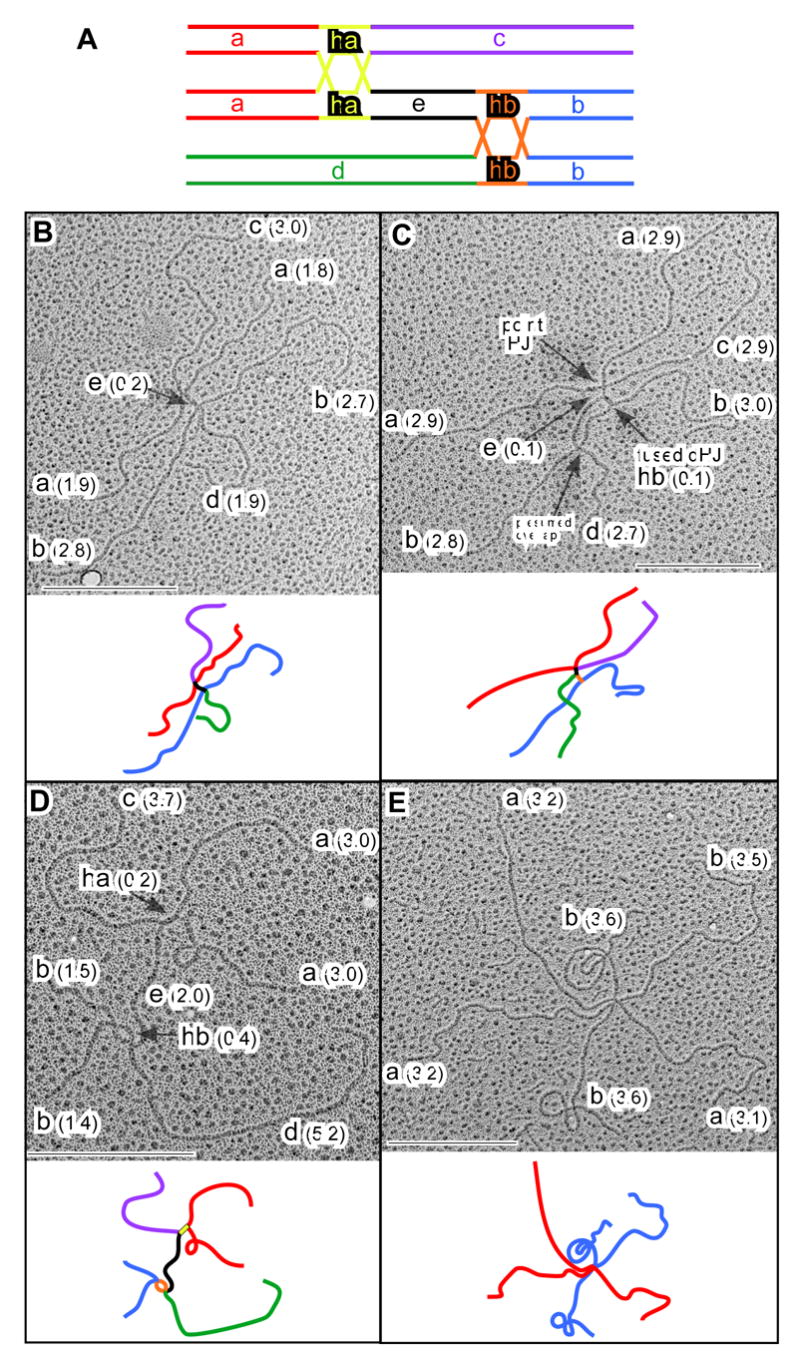
(A) Predicted relationships between segment lengths for fully homologous ternary JMs. (B-E) EM images and interpretative cartoons of ternary JMs from an sgs1-ΔC795 ndt80Δ DNA sample taken at 8 hrs: (B) ternary JM comprising three ~4.8 kb molecules interconnected by two closely spaced point junctions; (C) three ~4.8 kb molecules connected by a fused dHJ structure and a point junction; (D) three ~6.9 kb molecules connected by an open dHJ and a fused dHJ; (E) three 6.7 kb molecules connected at a single point, presumably by two very closely spaced point junctions. Segments correspond to those shown in A. Lengths are in kb (see Experimental Procedures). Scale bars = 0.5 μm. See also Figures S3 and S4.
Random sampling of EM grids reveals significantly different distributions of molecule types in ndt80Δ SGS1 and ndt80Δ sgs1ΔC795 samples. Six classes of molecules can be discerned by EM (Figures 5, S4, S5 and data not shown): linear molecules; Y-structures; binary JMs with four free ends (canonical Holliday Junctions; e.g. Figure S5F); ternary JMs with six ends; quaternary JMs with eight ends; and complex JMs with more than eight ends. JMs are interconnected by combinations of open dHJs, fused dHJs and point junctions, which could be single HJs or two very closely spaced HJs. In the ndt80Δ SGS1 sample, we counted 42 linears, 8 Ys, 55 binary JMs, 2 ternary JMs, 1 quaternary JM and no JMs with >8 ends. Consistent with the analysis at HIS4LEU2, multi-chromatid JMs are significantly enriched in the ndt80Δ sgs1ΔC795 sample, in which we counted 42 linears, 5 Ys, 58 binary JMs, 13 ternary JMs, 4 quaternary JMs and 3 JMs with >8 ends, representing a 5.8-fold increase in ternary and quaternary JMs (P=0.012 by G-test).
Ternary JMs are predicted to have six arms with the length relationships shown in Figure 5A. 10 of 21 six-armed structures analyzed showed exactly this relationship and can confidently be assigned as ternary JMs (Figures 5 and S4). In the other 11 six-armed JMs, one or more predicted arm lengths differed from expectations by >15%. Such structures have a variety of possible explanations, e.g. illegitimate strand-exchange (homeologous or non-homologous), strand-exchange within repetitive sequences (tandem or dispersed), hemicatenane formation at one or more junction points, and partial digestion or damage to the DNA during sample preparation. We can conclude, however, that ternary JMs of the predicted structure are a regular feature of sgs1ΔC795 meiosis. While the largest JMs formed at HIS4LEU2 clearly have the size, mobility and strand composition expected for quaternary JMs (Figure 4), we have been unable to unambiguously assign this structure to eight-armed molecules observed by EM (Supplemental Figure S5). Nonetheless, EM analysis clearly confirms the inferences made by analysis of DNA events at HIS4LEU2, i.e. that Sgs1 inhibits the formation of aberrant multi-chromatid JMs or promotes their disassembly.
sgs1-ΔC795 relieves the crossover defects of mlh3Δ and msh5Δ mutants
2-hybrid and immunoprecipitation assays have demonstrated an interaction between Sgs1 or BLM and the Mlh1-Mlh3 complex (Langland et al., 2001; Pedrazzi et al., 2001; Wang and Kung, 2002), but the biological relevance of this interaction is unclear. Also, Sgs1 immunostaining foci show extensive colocalization with pro-crossover factors along pachytene SCs (Rockmill et al., 2003). Similarly, in mouse, BLM colocalizes with MutS homolog, MSH4 (Moens et al., 2002). To understand the relationship between the specialized anti-crossover function of Sgs1, identified here, and meiotic pro-crossover functions, we examined the interaction between the sgs1-ΔC795 allele and deletion mutations of the MLH3 and MSH5 genes (Msh5 acts in a complex with Msh4; (Pochart et al., 1997). An isogenic set of single and double mutant strains was constructed and crossing-over was analyzed by tetrad analysis.
Crossing-over in the two intervals flanking HIS4LEU2 is reduced by ~1.4 -fold in mlh3Δ mutants and ~1.7-fold in msh5Δ mutants, consistent with published data (Figures 6A and 6B and Tables S5 and S6; Argueso et al., 2004; Hollingsworth et al., 1995; Wang et al., 1999). Strikingly, introduction of the sgs1-ΔC795 allele into mlh3Δ and msh5Δ mutant backgrounds completely restores crossing-over to at least wild-type levels. In fact, in msh5Δ sgs1-ΔC795 tetrads, map distances are significantly larger than in wild type, more closely resembling the sgs1ΔC795 single mutant (Tables S5 and S6). Importantly, however, the small increases in map distance observed in sgs1ΔC795 tetrads (<1.2-fold) cannot account for the large increases seen in mlh3Δ sgs1-ΔC795 (1.6 to 1.8-fold) and msh5Δ sgs1-ΔC795 (2.0 to 2.5-fold) double mutants. We infer that Sgs1 is responsible for the crossover defects of mlh3Δ and msh5Δ mutants.
Figure 6. sgs1-ΔC795 Relieves the Crossover Defects of msh5Δ and mlh3Δ Mutants.

(A) Tetrad analysis of crossing-over in two intervals flanking the HIS4LEU2 locus. Error bars indicate standard errors. See Tables S5 and S6.
(B) Spore viability. At least 200 tetrads were dissected for each strain.
Row (C): 1D analysis of DSBs and crossovers (COs), and analysis of meiotic divisions (MI±MII) from msh5Δ and msh5Δ sgs1Δ-C795 time-course experiments. Data for wild-type and sgs1Δ-C795 cells are from Figure 3A.
Row (D): 2D analysis of joint molecules in msh5Δ and msh5Δ sgs1Δ-C795.
Row (E): 2D analysis of joint molecules in msh5Δ ndt80Δ and msh5Δ sgs1Δ-C795 ndt80Δ cells.
Reduced crossing-over in msh5Δ and mlh3Δ mutants causes homologs to missegregate, which in turn results in some dead spores (Figure 5B; Argueso et al., 2004; Hollingsworth et al., 1995). Suppression of mlh3Δ and msh5Δ crossover defects by sgs1-ΔC795 is therefore expected to improve spore viability. This is clearly the case for msh5Δ sgs1-ΔC795 cells, which produce 82% viable spores compared to 44% for the msh5Δ single mutant (Figure 6B). While this is still lower than the 96% viable spores observed for wild type, msh5Δ sgs1-ΔC795 spore viability is not significantly different from that of the sgs1-ΔC795 single mutant (78%). The situation is less obvious for the mlh3Δ and mlh3Δ sgs1ΔC795 comparison, which produce 81% and 78% viable spores, respectively. However, the effects of the two mutations on spore viability are clearly not additive (expected viability, 63%). In fact spore viability of the mlh3Δ sgs1ΔC795 strain is also indistinguishable from that of the sgs1ΔC795 single mutant. We conclude that sgs1-ΔC795 relieves both crossover and homolog segregation defects of msh5Δ and mlh3Δ mutants.
This analysis indicates that Sgs1 can function as a general anti-crossover factor when pro-crossover activities, such as Msh5 or Mlh3, are absent. sgs1 mutation has recently been shown to variably suppress the crossover defects of zmm mutants, msh4Δ, mer3Δ, zip1Δ and zip2Δ (Jessop et al., 2006). Together, these data imply that pro-crossover activities of ZMMs generally antagonize Sgs1 during meiosis (but see Discussion).
The dHJ formation defect of msh5Δ cells is relieved by the sgs1-ΔC795 mutation
Sgs1 could prevent crossing-over by disrupting primary strand-exchange products, such as SEIs, and/or by dissolving dHJs in a concerted reaction together with the type I topoisomerase Top3 (see Introduction). To test these ideas, we analyzed intermediate steps of HR in msh5Δ and msh5Δ sgs1-ΔC795 cells by physical analysis at HIS4LEU2 (Figure 6C, D and E).
DSBs
In msh5Δ cells, DSBs form normally but their turnover is significantly delayed, consistent with previous analysis (Figure 6C; Borner et al., 2004). By 10 hrs, however, DSBs have disappeared, indicating efficient repair. In msh5Δ sgs1-ΔC795 cells, turnover is still delayed relative to wild-type and sgs1-ΔC795 cells, but DSBs disappear faster than in the msh5Δ single mutant.
Crossovers and meiotic divisions
Suppression of msh5Δ and mlh3Δ crossover defects by sgs1-ΔC795 is confirmed by physical assays (Figure 6C; for analysis of mlh3Δ, see Supplemental Figure S6). Crossing-over in msh5Δ cells is reduced 2.2-fold, relative to wild type. In the msh5Δ sgs1-ΔC795 double mutant, crossing-over is restored to near wild-type levels, reaching 17.1% of hybridizing DNA compared to 19.7% in wild type cells. Correcting for the fact that msh5Δ sgs1-ΔC795 strains undergo meiosis slightly less efficiently than wild type (Figure 6C) gives a crossover level of 20.2%, which implies complete restoration of crossing-over, consistent with tetrad analysis. Notably, however, msh5Δ sgs1-ΔC795 strains retain characteristics of the msh5Δ single mutant, specifically slight delays in DSB turnover, crossover formation and meiotic divisions (Figure 6C).
SEIs
msh5Δ strains form SEIs more slowly than wild type (Figure 6D; Borner et al., 2004). High steady-state levels of SEIs do eventually form but then persist until very late times, suggesting an additional defect at the SEI-to-dHJ transition. In msh5Δ sgs1-ΔC795 cells, these phenotypes are at least partially suppressed, with faster formation and turnover of SEIs. Clearly, however, the kinetics of SEI formation are still somewhat aberrant in msh5Δ sgs1-ΔC795 relative to wild type and sgs1-ΔC795 strains.
dHJs
In msh5Δ cells, IH-dHJ levels peak ~3 hrs later than in wild type and sgs1-ΔC795 cells, and reach lower levels (0.47%, 1.17% and 0.72%, respectively). Again, sgs1-ΔC795 partially suppresses the msh5Δ phenotype: IH-dHJs form with a delay of only ~1 hr and peak at the same level as in the sgs1-ΔC795 single mutant. Formation of IS-dHJs in msh5Δ and msh5Δ sgs1-ΔC795 cells follows similar patterns as those described for IH-dHJs. In this case, however, the msh5Δ sgs1-ΔC795 double mutant more clearly resembles the sgs1-ΔC795 single mutant, forming higher than normal levels of IS-dHJs.
Large JMs
Large JMs can be detected in msh5Δ cells. They form with relatively normal kinetics and reach near-wild-type levels but then persist at late times (Figure 6D). In the msh5Δ sgs1-ΔC795 double mutant, this pattern changes dramatically. Similar to the situation seen in the sgs1-ΔC795 single mutant, high levels of large JMs are seen in msh5Δ sgs1-ΔC795 cells, although their appearance is delayed by ~1 hr.
ndt80Δ analysis
Analysis of accumulated JMs in ndt80Δ cells reiterates the patterns observed in NDT80 cells (Figure 6E). Notably, accumulated JM levels in msh5Δ ndt80Δ cells are very low, no higher than the steady-state levels detected in msh5Δ NDT80 cells. This observation suggests that the moderate steady-state JM levels detected in msh5Δ NDT80 cells represent a small but very persistent population of molecules. Alternatively, the absence of Msh5 may permit JMs to be resolved via an Ndt80-independent mechanism, thereby preventing their accumulation in the ndt80Δ background.
Taken together, these data indicate that sgs1-ΔC795 suppresses the crossover defect of msh5Δ cells by removing an impediment to the formation of crossover-specific precursors, dHJs. The most obvious interpretation is that Msh5 and Sgs1 are antagonistic with respect to dHJ formation.
Discussion
The extraordinary meiotic recombination phenotype of sgs1Δ-C795 mutants
The observed patterns of crossing-over imply that DSBs that would normally form single crossovers in wild-type cells are more likely to result in closely spaced double crossovers in sgs1Δ-C795 cells. The simplest interpretation of our data is that Sgs1 prevents closely spaced crossovers by preventing formation of JMs involving more than two chromatids.
Sgs1 prevents formation of ternary and quaternary JMs
The novel three and four duplex JMs identified in this study form at ~3-fold higher levels in sgs1Δ-C795 cells. Ternary JMs are readily explained by a mechanism in which both DSB-ends stably engage and prime DNA synthesis from different templates (Figure 7). This mechanism also dictates that at least one of the resulting D-loops migrates away from the DSB site to displace the extended 3′-end. The resulting end(s) can then anneal to connect the two D-loops and ultimately form a three duplex JM connected by two dHJs. Quaternary JMs require that one of the DSB-ends sequentially invade two different templates before annealing occurs. Importantly, the DSB-end must retain a plectonemic association with both template chromosomes. The final structure contains all four chromatids, interconnected by three dHJs (Figures 4A). The fact that ternary and quaternary JMs are detected in wild-type cells indicates that strand-exchange at both DSB-ends and interaction with multiple templates is not peculiar to the sgs1Δ-C795 situation and reflects the normal mechanism of meiotic recombination (see below).
Sgs1 negatively regulates formation of intersister dHJs
The >2-fold increase in IS-dHJs we detect by molecular assays correlates with increased recombination between sister-chromatids in sgs1 mutants (A.B.H. Chaix and R.H. Borts, personal communication). This phenotype cannot be due to a loss of interhomolog bias because interhomolog events remain high in sgs1Δ-C795 tetrads. Instead, we suggest that while one DSB-end interacts with the homolog, the other end frequently engages the sister-chromatid, and that sgs1Δ-C795 does not alter the overall frequency of intersister interactions but does alter their outcome. Specifically, we propose that Sgs1 normally disassembles intersister strand-exchange intermediates so that stable IS-dHJs and intersister crossovers form only rarely. We further propose that events which would normally give rise to simple interhomolog non-crossovers in wild-type cells may result in aberrant interhomolog non-crossovers with an associated sister-chromatid exchange in sgs1Δ-C795 cells (Figure 7E).
A multi-template mechanism will improve the efficiency of homologous recombination
In canonical models of DSB-repair, only one DSB-end undergoes strand-invasion and extension by polymerase, and the other end subsequently anneals to the product of this reaction (Paques and Haber, 1999). We propose a significant revision of these models, specifically that either or both DSB-ends may undergo multiple rounds of invasion and extension from multiple templates. What could be the biological significance of allowing both DSB-ends to interact with different template chromosomes? Most obvious is that the ability to extend either or both DSB-ends will improve the efficiency of late steps of recombination. For example, during SDSA, extension of both DSB-ends will produce longer and thus more efficiently annealed homologous single-strands following end dissociation. Similarly, the transition from SEIs to dHJs will be more efficient if both DSB-ends can be extended prior to annealing. In addition, the flexibility afforded by being able to extend either DSB-end can overcome topological or steric hindrances in the template chromosome(s) that may limit extension from one or both ends. Finally, reiterative rounds of invasion coupled to weakly processive DNA synthesis may improve the fidelity of DSB-repair by limiting nonproductive interactions, e.g. with dispersed repeats and templates with limited homology.
Meiotic pro-crossover factors antagonize the anti-crossover activity of Sgs1
Suppression of the meiotic defects of msh5Δ and mlh3Δ mutants by sgs1Δ-C795 reveals a robust anti-crossover activity for Sgs1 in the absence of pro-crossover factors. Jessop et al. 2006 recently described a similar suppressive effect of sgs1 mutation on the crossover defects of msh4,Δ mer3,Δ zip1Δ and zip2Δ mutants. In that study, although the degree of suppression varied from mutant to mutant, msh4Δ was efficiently suppressed, as we have observed for msh5Δ. Together, these observations suggest that a key function of meiotic pro-crossover factors, particularly the Msh4-Msh5 and Mlh1-Mlh3 complexes, is to antagonize the anti-crossover activity of Sgs1. It should be noted, however, that msh5Δ sgs1Δ-C795 cells still progress through meiosis more slowly than MSH5 cells, indicating that Msh4-Msh5 has meiotic functions beyond simply antagonizing Sgs1. Moreover, the observations that: (i) the sgs1Δ-C795 single mutant does not show a simple hyper-crossover phenotype, and (ii) the crossover defects of mer3Δ and zip2Δ are relatively poorly suppressed by sgs1Δ-C795 (Jessop et al., 2006), indicate that, in addition to antagonizing Sgs1, some or all ZMMs are necessary for normal implementation of meiotic crossovers even in the absence of Sgs1.
Sgs1 prevents dHJ formation in the absence of Msh5
In the case of msh5Δ cells, our in vivo data directly confirm the proposal that Sgs1 (and, by extension, BLM) can prevent dHJ formation. Sgs1 could decrease detected dHJs by disrupting SEIs and/or by dissolving dHJs as soon as they form. Our data do not clearly discriminate between these two possibilities.
Differential activity of pro-crossover factors and Sgs1 at the two DSB-ends promotes the orderly formation of a single dHJ at designated crossover sites
The closely spaced crossovers, multi-chromatid JMs and increased IS-dHJs in sgs1Δ-C795 cells are reconciled by our proposal that both DSB-ends can engage different template chromosomes to more efficiently effect late steps of recombination (see above). How then can we also reconcile the interaction between pro-crossover activities, such as Msh4-Msh5 and Mlh1-Mlh3, and Sgs1? We propose that pro-crossover factors act at designated crossover sites to stabilize interhomolog strand-invasion by one DSB-end, in part by antagonizing Sgs1. The anti-recombination activity of Sgs1 then acts locally to disassemble JMs involving the second DSB-end and, perhaps, to disrupt crossover precursors formed at other nearby DSB sites. Our model also explains why immunostaining foci of Sgs1 and BLM colocalize with pro-crossover factors along yeast and mouse meiotic chromosomes (Moens et al., 2002; Rockmill et al., 2003). How Sgs1 is recruited to recombination sites is unclear, but Sgs1 and BLM interact with a number of repair factors including Mlh1, Rad51 and the single-strand binding protein, RPA (Hickson, 2003). Ultimately, this local coordination of pro- and anti-crossover activities effects a type of local positive interference by ensuring that only one dHJ forms at sites that have been designated a crossover fate. Consequently, the risk of forming closely spaced crossovers is minimized.
Closely spaced crossovers are predicted to be nonproductive for meiosis because there will be little if any intervening cohesion, i.e. the homologs will no longer be connected (see Maguire, 1980; Nilsson and Sall, 1995; van Veen and Hawley, 2003). In addition, Rockmill et al. (2006) demonstrated that aneuploidy due to precocious separation of sister-chromatids is associated with centromere-proximal crossing-over and proposed that such exchanges disrupt centromere cohesion. Undetected crossovers (between sisters or homologs) derived from multi-chromatid JMs could contribute to this effect. More generally, we conclude that the primary function of BLM and Sgs1 during homologous recombination, in both meiotic and somatic cells, is to help minimize the risk of potentially deleterious crossovers whilst also maximizing repair efficiency and fidelity by allowing strand-invasion and extension at both DSB-ends.
Experimental Procedures
Yeast strains and genetic techniques
Strains are derived from isolate SK1 (Table S7). Strains used to analyze crossing-over in Figure 1 are as described in de Los Santos et al. (2003), except that the can1 mutation was omitted and the arg4-bgl allele was introduced using two-step gene replacement. The HIS4LEU2 locus has been described (Hunter and Kleckner, 2001; Martini et al., 2006). The sgs1-ΔC795 allele was constructed via oligonucleotide-mediated truncation using the hphMX4 cassette (Goldstein and McCusker, 1999). msh5Δ and mlh3Δ mutations were made by replacing gene coding sequences with the kanMX4 cassette (Wach et al., 1994). The ndt80Δ mutation was kindly provided by Thorsten Allers and Michael Lichten, (Allers and Lichten, 2001a).
Tetrad Analysis
Haploid strains were mated briefly (≥3 hrs) on YPD plates and sporulated on plates containing 1% potassium acetate and 0.02% raffinose at 30°C for 48–72 hr. Asci were digested with zymolyase and dissected onto YPD plates. Only tetrads producing four viable spores were used in map distance calculations using the formula of Perkins (1949). Although the fraction of tetrads with four viable spores is reduced from 89% in wild type to 51% in sgs1-ΔC795, calculated map distances do not appear to be biased since crossover frequencies in spores from tetrads both with full viability and with less than four viable spores are not different (data not shown). Standard errors were calculated using Stahl Lab Online Tools (http://groik.com/stahl/). Heterogeneity in segregation patterns was tested using log-likelihood G-tests as described (Hoffmann et al., 2003).
Meiotic time courses and DNA physical assays
Meiotic time courses were essentially as described by Goyon and Lichten (1993). DNA physical assays were performed as described previously (Schwacha and Kleckner, 1995; Hunter and Kleckner, 2001; Martini et al., 2006). Ratios of Mom and Dad strands for the “pull-apart” experiment were estimated using ImageQuant Version 5.0 (Molecular Dynamics). Integrated pixel intensities of areas corresponding to the signals of interest were compared after subtracting the background baseline. A correction was made for the fact that non-specific nicking is incurred during crosslink reversal, which leads to biased reduction of signals for the longer Mom-length strands.
Electron microscopy
DNA was isolated from gels and prepared for EM by the formamide spreading technique (Cromie et al., 2006; Davis et al., 1971). JMs were measured as detailed by Cromie et al (2006). Contour lengths were converted to base-pairs using a conversion factor of 0.34 nm per bp.
Supplementary Material
Acknowledgments
We thank Rhona Borts for communicating unpublished data, Michael Lichten for stimulating discussions and suggestions, and Wolf Heyer, Amitabh Nimonkar and members of the Hunter lab for critical reading of the manuscript. N.H. is a Damon Runyon Scholar supported by the Damon Runyon Cancer Research Foundation (DRS-#40-04). This work was also supported by NIH NIGMS grants GM074223 to N.H. and GM031693 to G.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allers T, Lichten M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell. 2001a;106:47–57. doi: 10.1016/s0092-8674(01)00416-0. [DOI] [PubMed] [Google Scholar]
- Allers T, Lichten M. Intermediates of yeast meiotic recombination contain heteroduplex DNA. Mol Cell. 2001b;8:225–231. doi: 10.1016/s1097-2765(01)00280-5. [DOI] [PubMed] [Google Scholar]
- Argueso JL, Wanat J, Gemici Z, Alani E. Competing crossover pathways act during meiosis in Saccharomyces cerevisiae. Genetics. 2004;168:1805–1816. doi: 10.1534/genetics.104.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrati CZ, Hickson ID. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem J. 2003;374:577–606. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrati CZ, Hickson ID. Analysis of the DNA unwinding activity of RecQ family helicases. Methods Enzymol. 2006;409:86–100. doi: 10.1016/S0076-6879(05)09005-1. [DOI] [PubMed] [Google Scholar]
- Bell L, Byers B. Separation of branched from linear DNA by two-dimensional gel electrophoresis. Analyt Biochem. 1983a;130:527–535. doi: 10.1016/0003-2697(83)90628-0. [DOI] [PubMed] [Google Scholar]
- Bell LR, Byers B. Homologous association of chromosomal DNA during yeast meiosis. Cold Spring Harb Symp Quant Biol. 1983b;47(Pt 2):829–840. doi: 10.1101/sqb.1983.047.01.095. [DOI] [PubMed] [Google Scholar]
- Bennett RJ, Keck JL, Wang JC. Binding specificity determines polarity of DNA unwinding by the Sgs1 protein of S. cerevisiae. J Mol Biol. 1999;289:235–248. doi: 10.1006/jmbi.1999.2739. [DOI] [PubMed] [Google Scholar]
- Bishop DK, Zickler D. Early decision; meiotic crossover interference prior to stable strand exchange and synapsis. Cell. 2004;117:9–15. doi: 10.1016/s0092-8674(04)00297-1. [DOI] [PubMed] [Google Scholar]
- Borner GV, Kleckner N, Hunter N. Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell. 2004;117:29–45. doi: 10.1016/s0092-8674(04)00292-2. [DOI] [PubMed] [Google Scholar]
- Chaganti RS, Schonberg S, German J. A manyfold increase in sister chromatid exchanges in Bloom’s syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974;71:4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie GA, Hyppa RW, Taylor AF, Zakharyevich K, Hunter N, Smith GR. Single Holliday Junctions are intermediates of meiotic recombination. Cell. 2006;127:1167–1178. doi: 10.1016/j.cell.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RW, Simon RW, Davidson N. Electron microscope heteroduplex methods for mapping regions of base sequence homology in nucleic acids. Methods Enzymol. 1971;21:413–428. [Google Scholar]
- Gangloff S, McDonald JP, Bendixen C, Arthur L, Rothstein R. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol Cell Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J. The Bloom’s syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–1553. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Goyon C, Lichten M. Timing of molecular events in meiosis in Saccharomyces cerevisiae: stable heteroduplex DNA is formed late in meiotic prophase. Mol Cell Biol. 1993;13:373–382. doi: 10.1128/mcb.13.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grushcow JM, Holzen TM, Park KJ, Weinert T, Lichten M, Bishop DK. Saccharomyces cerevisiae checkpoint genes MEC1, RAD17 and RAD24 are required for normal meiotic recombination partner choice. Genetics. 1999;153:607–620. doi: 10.1093/genetics/153.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson ID. RecQ helicases: caretakers of the genome. Nature reviews. 2003;3:169–178. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- Hoffmann ER, Borts RH. Meiotic recombination intermediates and mismatch repair proteins. Cytogenet Genome Res. 2004;107:232–248. doi: 10.1159/000080601. [DOI] [PubMed] [Google Scholar]
- Hoffmann ER, Shcherbakova PV, Kunkel TA, Borts RH. MLH1 mutations differentially affect meiotic functions in Saccharomyces cerevisiae. Genetics. 2003;163:515–526. doi: 10.1093/genetics/163.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N. Meiotic Recombination. In: Aguilera A, Rothstein R, editors. Molecular Genetics of Homologous Recombination. Heidelberg: Springer-Verlag; 2006. [Google Scholar]
- Hunter N, Kleckner N. The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell. 2001;106:59–70. doi: 10.1016/s0092-8674(01)00430-5. [DOI] [PubMed] [Google Scholar]
- Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop L, Rockmill B, Roeder GS, Lichten M. Meiotic chromosome synapsis-promoting proteins antagonize the anti-Crossover activity of Sgs1. PLoS Genet. 2006;2:e155. doi: 10.1371/journal.pgen.0020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RD, Jasin M. Double-strand-break-induced homologous recombination in mammalian cells. Biochem Soc Trans. 2001;29:196–201. doi: 10.1042/0300-5127:0290196. [DOI] [PubMed] [Google Scholar]
- Kadyk LC, Hartwell LH. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics. 1992;132:387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow JK, Constantinou A, Li JL, West SC, Hickson ID. The Bloom’s syndrome gene product promotes branch migration of Holliday junctions. Proc Natl Acad Sci U S A. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney S. Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol. 2001;52:1–53. doi: 10.1016/s0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- Kolas NK, Cohen PE. Novel and diverse functions of the DNA mismatch repair family in mammalian meiosis and recombination. Cytogenet Genome Res. 2004;107:216–231. doi: 10.1159/000080600. [DOI] [PubMed] [Google Scholar]
- Langland G, Kordich J, Creaney J, Goss KH, Lillard-Wetherell K, Bebenek K, Kunkel TA, Groden J. The Bloom’s syndrome protein (BLM) interacts with MLH1 but is not required for DNA mismatch repair. J Biol Chem. 2001;276:30031–30035. doi: 10.1074/jbc.M009664200. [DOI] [PubMed] [Google Scholar]
- Lipkin SM, Moens PB, Wang V, Lenzi M, Shanmugarajah D, Gilgeous A, Thomas J, Cheng J, Touchman JW, Green ED, et al. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat Genet. 2002;31:385–390. doi: 10.1038/ng931. [DOI] [PubMed] [Google Scholar]
- Maguire MP. Adaptive advantage for chiasma interference: a novel suggestion. Heredity. 1980;45:127–131. doi: 10.1038/hdy.1980.56. [DOI] [PubMed] [Google Scholar]
- Martini E, Diaz RL, Hunter N, Keeney S. Crossover homeostasis in yeast meiosis. Cell. 2006;126:285–295. doi: 10.1016/j.cell.2006.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Kolas NK, Tarsounas M, Marcon E, Cohen PE, Spyropoulos B. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J Cell Sci. 2002;115:1611–1622. doi: 10.1242/jcs.115.8.1611. [DOI] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Brill SJ. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2000;154:1101–1114. doi: 10.1093/genetics/154.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Nallaseth FS, Lan YQ, Slagle CE, Brill SJ. Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1-Top3 complex. Mol Cell Biol. 2005;25:4476–4487. doi: 10.1128/MCB.25.11.4476-4487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. The mechanism of crossing over. Am Nat. 1916;50:193–221. [Google Scholar]
- Nassif N, Penney J, Pal S, Engels WR, Gloor GB. Efficient copying of nonhomologous sequences from ectopic sites via P-element-induced gap repair. Mol Cell Biol. 1994;14:1613–1625. doi: 10.1128/mcb.14.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson NO, Sall T. A model of chiasma reduction of closely formed crossovers. J Theor Biol. 1995;173:93–98. doi: 10.1006/jtbi.1995.0046. [DOI] [PubMed] [Google Scholar]
- Opresko PL, Cheng WH, Bohr VA. Junction of RecQ helicase biochemistry and human disease. J Biol Chem. 2004;279:18099–18102. doi: 10.1074/jbc.R300034200. [DOI] [PubMed] [Google Scholar]
- Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzi G, Perrera C, Blaser H, Kuster P, Marra G, Davies SL, Ryu GH, Freire R, Hickson ID, Jiricny J, Stagljar I. Direct association of Bloom’s syndrome gene product with the human mismatch repair protein MLH1. Nucleic Acids Res. 2001;29:4378–4386. doi: 10.1093/nar/29.21.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. Biochemical mutants in the smut fungus Ustilago maydis. Genetics. 1949;34:607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M, Siomos MF, Nasmyth K. Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–440. doi: 10.1016/s0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Plank JL, Wu J, Hsieh TS. Topoisomerase IIIalpha and Bloom’s helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proc Natl Acad Sci U S A. 2006;103:11118–11123. doi: 10.1073/pnas.0604873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochart P, Woltering D, Hollingsworth NM. Conserved properties between functionally distinct MutS homologs in yeast. J Biol Chem. 1997;272:30345–30349. doi: 10.1074/jbc.272.48.30345. [DOI] [PubMed] [Google Scholar]
- Richardson C, Stark JM, Ommundsen M, Jasin M. Rad51 overexpression promotes alternative double-strand break repair pathways and genome instability. Oncogene. 2004;23:546–553. doi: 10.1038/sj.onc.1207098. [DOI] [PubMed] [Google Scholar]
- Rockmill B, Fung JC, Branda SS, Roeder GS. The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr Biol. 2003;13:1954–1962. doi: 10.1016/j.cub.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Rockmill BM, Voelkel-Meiman K, Roeder GS. Centromere-proximal crossovers are associated with precocious separation of sister chromatids during meiosis in Saccharomyces cerevisiae. Genetics. 2006;174:1745–1754. doi: 10.1534/genetics.106.058933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Identification of joint molecules that form frequently between homologs but rarely between sister chromatids during yeast meiosis. Cell. 1994;76:51–63. doi: 10.1016/0092-8674(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell. 1995;83:783–791. doi: 10.1016/0092-8674(95)90191-4. [DOI] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog-only pathway. Cell. 1997;90:1123–1135. doi: 10.1016/s0092-8674(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Shinohara A, Shinohara M. Roles of RecA homologues Rad51 and Dmc1 during meiotic recombination. Cytogenet Genome Res. 2004;107:201–207. doi: 10.1159/000080598. [DOI] [PubMed] [Google Scholar]
- van Brabant AJ, Ye T, Sanz M, German IJ, Ellis NA, Holloman WK. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39:14617–14625. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- van Veen JE, Hawley RS. Meiosis: When even two is a crowd. Curr Biol. 2003;13:R831–833. doi: 10.1016/j.cub.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Wang TF, Kung WM. Supercomplex formation between Mlh1-Mlh3 and Sgs1-Top3 heterocomplexes in meiotic yeast cells. Biochem Biophys Res Commun. 2002;296:949–953. doi: 10.1016/s0006-291x(02)02034-x. [DOI] [PubMed] [Google Scholar]
- Watt PM, Hickson ID, Borts RH, Louis EJ. SGS1, a homologue of the Bloom’s and Werner’s syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics. 1996;144:935–945. doi: 10.1093/genetics/144.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LM, Hodges CA, Baart E, Baker SM, Liskay M, Hunt PA. Chromosomal influence on meiotic spindle assembly: abnormal meiosis I in female Mlh1 mutant mice. J Cell Biol. 1999;145:1395–1406. doi: 10.1083/jcb.145.7.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Bachrati CZ, Ou J, Xu C, Yin J, Chang M, Wang W, Li L, Brown GW, Hickson ID. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc Natl Acad Sci U S A. 2006;103:4068–4073. doi: 10.1073/pnas.0508295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Hickson ID. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


