Abstract
In animal models, allopregnanolone (ALLO) negatively modulates the hypothalamic-pituitary-adrenal (HPA) axis and has been shown to exert analgesic effects. The purpose of this study was to assess the relationship between plasma ALLO immunoreactivity (ALLO-ir), HPA-axis measures, and pain sensitivity in humans. Forty-five African Americans (21 men, 24 women) and 39 non-Hispanic Whites (20 men, 19 women) were tested for pain sensitivity to tourniquet ischemia, thermal heat, and cold pressor tests. Plasma ALLO-ir, cortisol, and β-endorphin concentrations were taken following an extended rest period. Lower concentrations of ALLO-ir were associated with increased pain tolerance to all three pain tests and increased pain threshold to the thermal heat pain task in the non-Hispanic Whites only (rs = -.35 to -.49, ps < .05). Also, only in the non-Hispanic Whites was cortisol associated with thermal heat tolerance (r = +.39, p<.05) and threshold (r = +.50, p < .01) and cold pressor tolerance (r = +.32, p < .05), and was β-endorphin concentrations associated with cold pressor tolerance (r = +.33, p<.05). Mediational analyses revealed that higher cortisol levels mediated the relationship between lower ALLO-ir and increased thermal heat pain threshold in the non-Hispanic Whites only. These results suggest that lower ALLO-ir concentrations are associated with decreased pain sensitivity in humans, especially in non-Hispanic Whites, and that this relationship may be mediated by HPA-axis function.
INTRODUCTION
African Americans experience more clinical pain (Edwards et al. 2001a; McCracken et al. 2001; Riley et al. 2002) and report more pain associated with chronic medical conditions (Edwards et al. 2001b). In the laboratory, studies consistently indicate no ethnic differences in pain onset (i.e. pain threshold) but that African Americans have reduced pain tolerance relative to Caucasians (Chapman & Jones 1944; Woodrow et al. 1972; Edwards & Fillingim 1999; Sheffield et al. 2000; Campbell et al. 2005; Mechlin et al. 2005). Studies of biological mechanisms that may contribute to these ethnic differences are scant (Mechlin et al. 2005).
While there is a well-documented relationship between higher blood pressure (BP) levels and reduced pain sensitivity in Caucasians (Zamir & Shuber 1980; Maixner 1991; Sheps et al., 1992; McCubbin & Bruehl 1994; Bragdon et al., 1997), thought to be mediated by stimulation of mechanoreceptive afferents (i.e., baroreceptors; Randich & Maixner, 1986), a recent report from our laboratory found no evidence for a relationship between BP and pain sensitivity in African Americans, while the expected relationship held for a primarily Caucasian sample (Mechlin et al. 2005). Greater concentrations of stress-induced cortisol are also associated with decreased pain sensitivity in Caucasian samples (al’Absi et al., 2002; Girdler et al., 2005; Mechlin et al., 2005). However, our prior study found no evidence for a relationship involving cortisol and pain sensitivity in African Americans, which was documented in the primarily Caucasian sample (Mechlin et al. 2005).
Taken together, the results of our previous study suggest ethnic differences in the relationship of stress-responsive biological measures, that may act to diminish pain as part of an integrated response during the defense reaction (Maixner 1991), to pain sensitivity. Another stress-relevant neuroendocrine factor that may modulate pain perception in humans, and which constitutes the novel focus of this report, is Allopregnanalone (ALLO). ALLO is an A-ring-reduced metabolite of progesterone that is produced by ovary and adrenals and also de novo in brain (Paul & Purdy 1992). Owing to its lipophilicity, even peripherally produced ALLO readily crosses into brain (Paul & Purdy 1992). ALLO is a potent modulator of GABAA receptors via dose-dependent enhancement of GABA-induced Cl- ion channels (Morrow et al. 1987). ALLO is stress sensitive in both animals (Purdy et al. 1991; Barbaccia et al. 1996), and humans (Girdler et al. 2001). Animal models indicate that stress-induced increases in ALLO negatively modulate hypothalamic-pituitary-adrenal (HPA)-axis activity, thereby facilitating the recovery of physiologic homeostasis in this system following stress (Guo et al. 1995; Patchev et al. 1996).
Stress-induced increases in ALLO may be adaptive since ALLO has analgesic properties when administered in animals (Kavaliers & Wiebe 1987). Furthermore, animal models clearly indicate the involvement of central GABAA receptors in modulating pain sensitivity (Yokoro et al. 2001). To date, no studies have examined ALLO-associated analgesia in humans. The primary aim of this report is to examine the relationship of ALLO to experimental pain sensitivity in humans and determine whether ethnic differences are present. A secondary aim of this report involves examining the inter-relationships of plasma cortisol and β-endorphin, in the relationship of ALLO to pain sensitivity. To our knowledge, the relationship of β-endorphin concentrations to pain sensitivity in different ethnic groups has not been studied.
METHODS
Subjects
Subjects that comprise the present report represent a large sub-sample of the participants included in our recent report on ethnicity, pain sensitivity, and endogenous pain regulatory mechanisms described above (Mechlin et al. 2005). While that prior study compared African Americans with all ‘Other’ ethnic groups, given the evidence that Hispanic populations differ in clinical pain systems (Hernandez & Sachs-Ericsson, 2006), the evidence for ethnic differences between Asian Americans and European Americans in stress-responsive measures (Stoney et al., 2002; Shen et al. 2004), and since the numbers of Hispanics (n = 2) and Asians (n = 6) in our study did not allow for valid analyses, the present report compares African Americans with non-Hispanic Whites only. Of the 107 subjects included in that earlier report, we were able to analyze ALLO immunoreactivity (ALLO-ir) in plasma from 85 subjects who self-reported as African American or non-Hispanic White. Thus, the present report represents new measurements (ALLO and β-endorphin) in a large subset of those subjects on which we previously reported.
The participants were recruited through newspaper advertisements seeking male and female nonsmokers for a study on pain perception. Non-smokers were recruited based on self-report. Smokers were excluded since cigarette smoking has been shown to have analgesic properties (Girdler et al. 2005). The sub-sample of subjects providing ALLO-ir samples was composed of 41 men and 44 women, aged 18 - 47 years. Approximately half (n = 45) of the subjects was African American (21 men, 24 women) while the other half (n = 39; 20 men, 19 women) was non-Hispanic White.
There were no gender or ethnic group differences in age (range 18-47) or diastolic blood pressure (DBP; range 50-87mmHg), or heart rate (range 50-92bpm). There were ethnic differences in body mass index (BMI), since African Americans had higher BMIs than non-Hispanic Whites (28.9 vs. 25.1; F(3, 82) = 7.92, p < .01). Also, men had higher resting systolic blood pressure (SBP) than women (120mmHg vs. 112mmHg; F(3, 82) = 10.85, p < .01). No ethnic × gender interactions were observed for any of the above biological measures.
All subjects were medically healthy, with no more than mildly elevated BP (< 160/90 mmHg) as determined during an initial screening session. Only 4 subjects (2 non-Hispanic Whites and 2 African Americans) had elevated BP, defined as SBP > 135mmHg and/or DBP > 85mmHg. Additionally, subjects were not taking any prescription medication, including oral contraceptives or psychotropics, and none took any over-the-counter medication more than 4 times per month. Consistent with other studies on experimental pain (France et al. 2002; al’Absi et al. 2004; Fillingim et al. 2005) we instructed subjects to refrain from using any analgesic or other over-the-counter medications for 24 hours prior to pain testing. This was verified based on self-report. All women reported regular menstrual cycles. Excluded from participating were subjects with chronic pain conditions (e.g. temporomandibular joint disorder, fibromyalgia, arthritis) and those exhibiting signs of depression or anxiety based on Hamilton rating scales for depression (score > 7) and anxiety (> 9).
The protocol was approved by the institution’s Institutional Review Board and all subjects provided informed, written consent prior to participating. Subjects received $150 compensation.
Procedures
Women were tested three times, once during the early follicular, once during the late follicular, and once during the luteal phase of their menstrual cycles. Cycle phases were subsequently confirmed using serum estradiol and progesterone concentrations. Men were also tested three times, matched for number of days between test sessions. Since ALLO-ir levels are non-detectable in a substantial proportion of women in their follicular phase (Girdler et al. 2001), only luteal phase data are included in the present report. There were no significant differences in the proportion of African American versus non-Hispanic White women whose luteal phase session was the first test session (9 vs. 6, respectively), second test session (5 vs. 9), or last test session (10 vs. 4; χ2(2) = 3.78, p = .15).
One goal of the larger parent study (Mechlin et al., 2005) was to examine stress-induced analgesia (SIA). Thus, pain testing occurred twice; once following a modified Trier Social Stress Test (TSST; Kirschbaum et al. 1993a,b, 1995a,b) and once following a time equivalent rest control period. Half of the subjects received the rest condition first, while the other half received the stress condition first. The order of stress first versus rest first was fully counter-balanced within gender and ethnic groups. Analyses were limited to the pain sensitivity measures obtained following the TSST (see Figure 1) since ALLO-ir and other neuroendocrine factors were assessed during the extended baseline rest period that immediately preceded the TSST. Since no blood samples were taken during the baseline period that preceded the rest control period, measures of pain sensitivity following the TSST were more temporally contiguous with assessment of neuroendocrine factors.
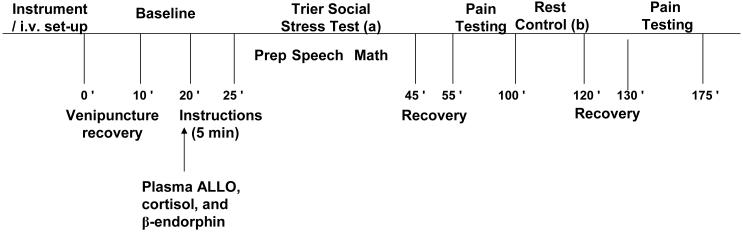
Figure 1. Overview of the Laboratory Testing Protocol.
Schematic diagram representing the sequence of events during laboratory testing. Half of each ethnic and gender group had the order of stress test and then rest control period as indicated above (a followed by b), while the other half had rest control first (b followed by a).
For all subjects, lab testing began between 12pm and 2pm. An intravenous line (i.v.) was then established in an arm vein and once in place, a curtain was drawn to hide the i.v. and arm, and to minimize awareness of blood sampling.
The sequence of laboratory events was as follows (see Figure 1): 1) Instrumentation and acclimation to the testing chamber; 2) i.v. set-up and recovery from venipuncture (10 min); 3) Baseline (10 min quiet rest); 4) TSST (20 min); 5) Recovery (10 min); 6) Pain Testing; These events are described fully below.
Baseline
Immediately following the i.v. setup, a 10 minute recovery from venipuncture ensued, followed by a 10-minute baseline rest period. Blood was sampled at minute 10 of this rest period for baseline ALLO-ir, cortisol and β-endorphin concentrations.
The Trier Social Stress Test (TSST)
The TSST is a stress test that reliably induces large and consistent HPA and cardiovascular responses (Kirschbaum et al.1993a,b, 1995a,b). The TSST involved the following components (see Mechlin et al. 2005 for detailed description of stressor events): 1) Pre-Task Instructions (5 min); 2) Speech Preparation Period (5 min); 3) Job Speech (5 min); and 4) Paced Auditory Serial Addition Test (PASAT; Gronwall 1977) (8.5 min).
Stress Recovery (10 min)
Subjects rested quietly alone. Blood was also sampled for stress-induced increases in cortisol and ALLO-ir at the end of this recovery period1.
Pain Testing Procedures
Immediately after the recovery periods that followed the TSST, subjects were exposed to the three different pain tests. One of three task orders (i.e., 1-tourniquet, thermal, cold; 2- thermal, cold, tourniquet; or 3 - cold, tourniquet, thermal) was randomly assigned to each subject, ensuring that equal numbers of men and women and African Americans and non-Hispanic Whites received each of the three orders. There was a 5-minute recovery period following each pain test.
The Submaximal Effort Tourniquet Procedure
In this procedure, as described previously (Maixner et al. 1990), a tourniquet cuff was positioned on the subject’s arm and the arm placed to the side. Prior to inflating the tourniquet cuff to 200 mmHg (Hokanson E20 Rapid Cuff Inflator), the subject’s arm was raised for 30 seconds to promote venous drainage, and then the cuff was inflated, the experimenter’s stopwatch started, and the arm returned to the side. To promote forearm ischemia, subjects engaged in 20 handgrip exercises at 30% of their maximum force with an inter-squeeze interval of 2 sec. Subjects were instructed to indicate when the sensations in their arm first became painful (pain threshold) and when they were no longer willing or able to tolerate the pain (pain tolerance). A maximum time limit of 20 min was enforced, though subjects were not informed of this limit.
Hand Cold Pressor
The apparatus for the cold pressor consisted of a container filled with ice and water that was maintained at 4° C as recorded immediately prior to initiating the test. The use of a water circulator prevented the water from warming near the subject’s hand. At the onset of the test, subjects were instructed to submerge their hand to the marked line on their wrist and to remain still. Subjects were instructed to indicate to the experimenter when the sensations in their hand first became painful (pain threshold) and to also indicate when they were no longer willing or able to tolerate the pain by saying ‘stop’ (pain tolerance). A maximum time limit of 5 min was imposed, though subjects were not informed of this limit.
Thermal Heat Pain Testing
Thermal heat pain threshold and tolerance were determined by an ascending method of limits using a 1-cm diameter contact thermode with the capability for a rise time of 10° C/ sec (Medoc TSA-II Neurosensory Analyzer). The thermode was controlled by a personal computer, and thermal probe applied to the left volar forearm. During the pain testing, an adapting temperature of 38° C was maintained for 10 sec. Then, the temperature increased directly to 41.5° C and from that point on increased 0.5° C every 5 sec until it reached 53° C or until the subject reached his/her tolerance. To determine thermal pain onset (threshold), subjects were instructed to press a mouse button (which terminated the stimulus) when the thermal percept first became painful. This was repeated three times and averaged to calculate thermal pain thresholds. Then, three series to determine average thermal pain tolerance were conducted by instructing the subject to press a mouse button when they were no long able to tolerate the pain.
Hormone and Neuroendocrine Assays
Plasma ALLO-ir (3α,5α-THP) was assessed by radioimmunoassay (RIA) following extraction and purification by column chromatography as previously described (Janis et al. 1998; Girdler et al. 2001). The 3α,5α-THP antiserum has previously been shown to produce minimal cross reactivity with other circulating steroids (Janis et al. 1998). Cross-reactivity with progesterone (<3%), as well as the stereochemical isomers of 3α,5α-THP is minimal (3α,5β-THP 6.6%; 3β,5α-THP 2.8%; 3β,5β-THP 0.5%). In contrast, the steroid 3α-hydroxy-4-pregnen-20-one binds to the antibody to a greater degree than 3α,5α-THP (169% of 3α,5α-THP). It is unknown, however, whether 3α-hydroxy-4-pregnen-20-one exists in human serum. If the steroid does exist in human serum, then it may contribute to the measurement of ALLO; however, because both ALLO-ir and the pregnen-4 compound are equally efficacious agonists of GABAA receptor mediated Cl--uptake (Morrow et al. 1990), they would be expected to produce similar effects. The intra- and inter-assay coefficients of variation from the assay are 6.2% and 5.9% respectively.
Plasma cortisol was determined using RIA techniques (MP Biomedicals, Inc.). The intra- and inter-assay coefficients of variation from the assay are 4.7% and 7.6%, respectively. The sensitivity of the assay is 0.07 μg/dL, and the specificity high, showing 0.05-2.2% cross-reactivity with most similar compounds.
Plasma β-endorphin was determined following extraction by RIA using a commercial kit (INCSTAR Corp.). The intra- and inter-assay coefficients of variation from the assay are approximately 10% and 15%, respectively, and the assay sensitivity is 3 pmol/L. There is less than 0.01% cross-reactivity with most other peptides. Since β-endorphin is released in a pulsatile manner, and therefore can vary greatly over time, β-endorphin concentrations were averaged across all three test sessions, and this average β-endorphin concentration was used in subsequent analyses.
Data Reduction and Analyses
Our first analytical strategy involved comparing groups for differences that existed in demographic and baseline measures. For each dependent measure, a 2(Gender) × 2 (Ethnicity) analysis of variance (ANOVA) was employed. Chi-square analyses were also employed where indicated to examine ethnic differences in the proportion of subjects falling into various cells.
In order to investigate the relationship between ALLO and pain tolerance, as well as the relationships involving β-endorphin, cortisol, and pain tolerance, a series of Pearson product moment correlational analyses were employed. Since our prior report (Mechlin et al. 2005) documented that ethnic differences in pain sensitivity were evident in both genders, and since ethnic differences existed only for measures of pain tolerance and not pain threshold, to reduce Type I error rates our primary analyses focused exclusively on differences between African Americans and non-Hispanic Whites, collapsing across gender though summary statistics by gender are provided in Table 1.
Table 1.
Mean (±SEM) Biological and Pain Sensitivity Measures as a Function of Ethnicity and Gender
| African American Females (n = 24) | Non-Hispanic White Females (n = 19) | African American Males (n = 21) | Non-Hispanic White Males (n = 20) | |
|---|---|---|---|---|
| Baseline ALLO (ng/mL) A | 1.25 (0.08) | 1.50 (0.10) | 0.36 (0.09) | 0.35 (0.09) |
| Baseline Cortisol (pg/mL)B | 6.85 (0.67) | 5.92 (0.75) | 9.27 (0.72) | 11.01 (0.73) |
| Baseline β-endorphin (pg/mL)B | 6.47 (0.60) | 5.84 (0.69) | 9.61 (0.65) | 9.00 (0.67) |
| Thermal Heat Threshold (°C) B | 42.70 (0.59) | 42.45 (0.69) | 44.39 (0.64) | 45.53 (0.65) |
| Thermal Heat Tolerance (°C) B, C | 46.88 (0.37) | 48.02 (0.44) | 48. 47 (0.41) | 50.26 (0.42) |
| Ischemic Threshold (sec) B | 222 (51) | 239 (62) | 332 (56) | 333 (59) |
| Ischemic Tolerance (sec) B, C | 336 (67) | 431 (78) | 478 (73) | 739 (75) |
| Cold Pressor Threshold (sec) B, C | 6 (4) | 10 (4) | 12 (4) | 28 (4) |
| Cold Pressor Tolerance (sec) B, C | 12 (14) | 42 (16) | 25 (15) | 121 (15) |
Females > Males, p < .0001
Males > Females, p < .05
non-Hispanic Whites > African Americans, p < .01
Results revealed a significant order effect for sensitivity to the cold pressor task (F(1, 83) = 4.15, p < .05), since cold pressor tolerance was lowest for subjects who had the order thermal, cold pressor, tourniquet (mean cold pressor tolerance = 19 sec; standard error of the mean (SEM) = 2), compared with those who had the order cold pressor, tourniquet, and thermal (mean cold pressor tolerance = 39 sec; SEM = 11), and those who had the order tourniquet, thermal then cold pressor (mean cold pressor tolerance = 79 seconds; SEM = 20). Therefore, order of pain testing was partialed out of all correlational analyses involving pain tolerance for all three pain tasks, and was used as a covariate for all ANOVAs involving pain sensitivity.
Where significant inter-correlations involving ALLO-ir, pain tolerance and either cortisol or β-endorphins emerged, we used the approach recommended by Baron and Kenny (1986), including the Sobel test, which takes the standard error of regression coefficients into account, and conducted mediational analyses to test whether the relationship between ALLO-ir and pain tolerance was influenced by either cortisol or β-endorphin concentrations. This analysis generates a student’s t value, an unstandardized regression coefficient (b) which is the value of predicted change in the dependent variable given a one unit change in the independent variable of interest when all other independent variables are held constant, and a standardized regression coefficient (β) is similar to an unstandardized regression coefficient, however, since it is standardized it allows for the comparison between independent variables of their relative contribution to the dependent variable, for each variable in the model. Additionally, the percentage of variance of the dependent variable accounted for by the independent variable and mediator (R 2) is obtained, as well as a z-statistic from the Sobel test, which when significant indicates that the mediator does in fact, carry an influence from the independent variable to the dependent variable.
RESULTS
Baseline Characteristics
As summarized in Table 1, women in the luteal phase of their menstrual cycle had significantly higher levels of ALLO-ir than men (F(1,82) = 119.12, p < .0001). Men had higher concentrations of β-endorphin than women (F(1, 83) = 23.23, p < .0001). As previously reported in the larger sample (Mechlin et al. 2005), overall the subset of men also had greater cortisol concentrations than women (F(1,82) = 25.81, p < .0001), greater pain threshold (Fs(1, 82) = 2.51 to 7.63, ps < .05) and tolerance to all three pain tests (Fs(1, 82) = 4.94 to 12.13, ps < .01) collapsing across ethnic groups (i.e. main effect of gender) even after controlling for order of pain tests. Finally, as previously observed in the larger sample, African Americans had lower tolerance values to all three pain tests relative to non-Hispanic Whites, and this was true for both genders and after controlling for order (main effect of ethnicity: Fs(3, 92) = 3.32 to 10.53, ps< .05). There was no main effect of ethnicity on pain threshold for the thermal or tourniquet tasks (Fs(2, 81) = 1.04 and 0.82, respectively), however there was a difference for cold pressor pain threshold (F(2, 81) = 4.17, p < .01), with African Americans exhibiting a lower pain threshold than non-Hispanic Whites.
For the cold pressor task, significantly more non-Hispanic White subjects than African American subjects reached the 5-minute time limit (7 vs. 0; χ2(1) = 11.34, p < .01). There were no ethnic differences in the proportion of subjects that reached the 20-minute time limit for the tourniquet task (3 vs. 6; χ2(1) = 1.75) or the 53°C limit for the thermal heat pain task (1 vs. 0; χ2(1) = 1.19). When comparing subjects who had rest first vs. stress first, there were no significant differences in thermal pain tolerance (48.4°C vs. 48.3°C, respectively), cold pressor tolerance (62 seconds vs. 35 seconds), tourniquet pain tolerance (524 seconds vs. 426 seconds), baseline ALLO-ir (0.91ng/mL vs. 0.85ng/mL), baseline cortisol (7.63pg/mL vs. 8.76pg/mL), or baseline β-endorphin (7.96pg/mL vs. 7.49pg/mL). Therefore, subsequent analyses did not control for the order of stress vs. rest.
Relationships Involving Neuroendocrine Factors and Pain Sensitivity
As summarized in Table 2, the only significant partial correlations (partialing out order of pain tests) to emerge involving plasma ALLO-ir, β-endorphin or cortisol concentrations and pain tolerance were seen in the non-Hispanic White subjects. ALLO-ir levels were negatively correlated with thermal heat pain tolerance (r = -.47, p < .01), cold pressor pain tolerance (r = -.35, p < .05), and ischemic pain tolerance (r = -.43, p < .01) in the non-Hispanic Whites. We observed a positive partial correlation between plasma β-endorphin concentrations and cold pressor tolerance levels (r = +.38, p<.05), but only in the non-Hispanic Whites, who also showed the expected positive correlation between plasma cortisol and thermal heat pain tolerance (r = +.39, p<.05) and cold pressor pain tolerance (r = +.32, p < .05). The partial correlation coefficients relating ALLO-ir, β-endorphin, or cortisol concentrations to pain tolerance were uniformly non-significant in the African Americans.
Table 2.
Partial Correlations Relating Pain Tolerance to Neuroendocrine Measures by Ethnicity (Controlling for Order of Pain Tests)
| African Americans (n = 45) | ||||||
| Thermal Threshold | Thermal Tolerance | Cold Pressor Threshold | Cold Pressor Tolerance | Ischemic Threshold | Ischemic Tolerance | |
| Baseline ALLO | r = -.24 p = ns |
r = -.22 p = ns |
r = -.29 p = ns |
r = -.17 p = ns |
r = -.18 p = ns |
r = -.19 p = ns |
| Baseline Cortisol | r = +.18 p = ns |
r = +.21 p = ns |
r = -.05 p = ns |
r = +.04 p = ns |
r = +.12 p= ns |
r = +.15 p = ns |
| Baseline β-endorphin | r = +.18 p = ns |
r = +.14 p = ns |
r = +.24 p = ns |
r = +.18 p = ns |
r = +.14 p = ns |
r = +.02 p = ns |
| Non-Hispanic Whites (n = 39) | ||||||
| Thermal Threshold | Thermal Tolerance | Cold Pressor Threshold | Cold Pressor Tolerance | Ischemic Threshold | Ischemic Tolerance | |
| Baseline ALLO |
r = -.49** p < .01 |
r = -.47** p < .01 |
r = -.24 p = ns |
r = -.35* p < .05 |
r = -.24 p = ns |
r = -.43** p < .01 |
| Baseline Cortisol |
r = +.50** p < .01 |
r = +.39* p < .05 |
r = +.24 p = ns |
r = +.32* p < .05 |
r = +.15 p = ns |
r = +.19 p = ns |
| Baseline β-endorphin | r = +.07 p = ns |
r = +.06 p = ns |
r = +.24 p = ns |
r = +.38* p < .05 |
r = +.37* p < .05 |
r = +.22 p = ns |
p < .01
p < .05
In non-Hispanic Whites, thermal heat pain threshold was significantly correlated with both ALLO-ir (r = -.49, p < .01) and cortisol (r = +.50, p < .01), and ischemic pain threshold was correlated with β-endorphin (r = +.37, p < .05). There were no significant partial correlations involving pain threshold and biological measures in African Americans (rs = -.29 to +.24, ps > .05).
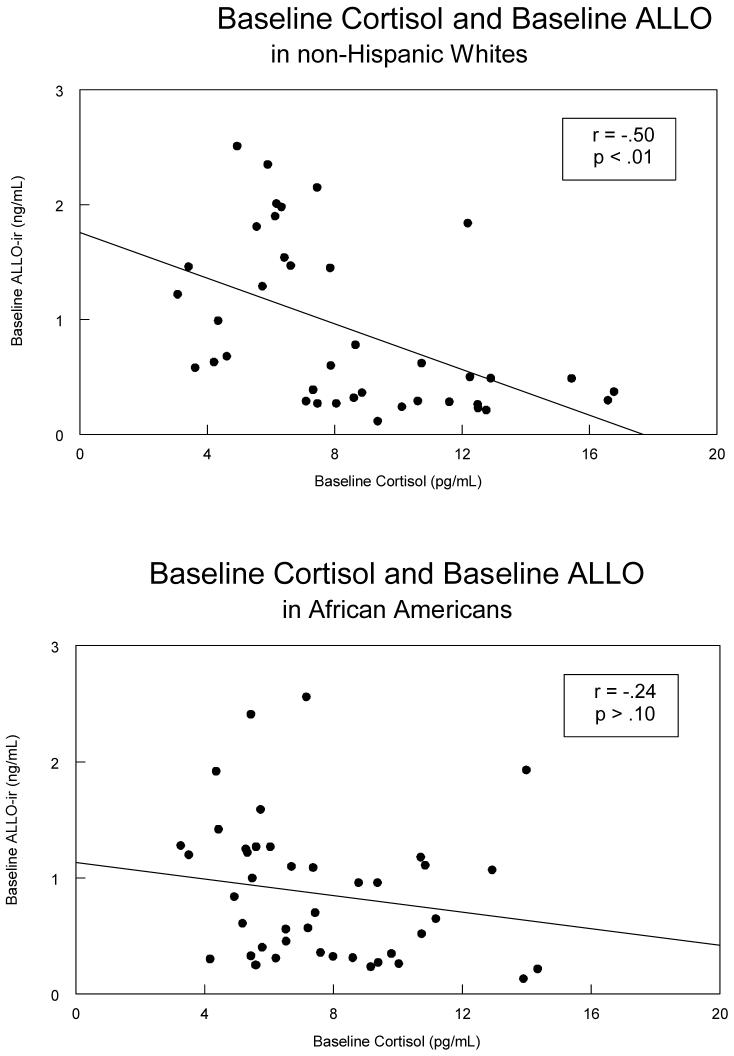
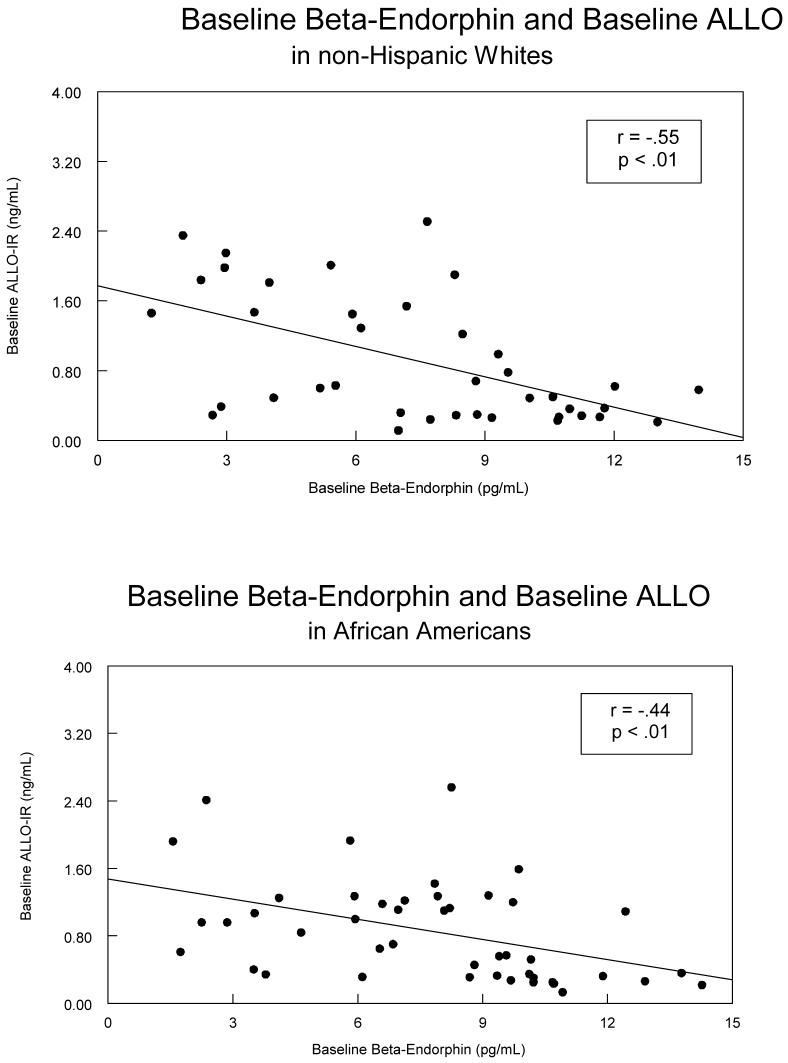
As depicted in Table 3, the expected relationship between ALLO-ir concentrations and cortisol concentrations (i.e., negative) was only significant in non-Hispanic Whites (r = -.50, p < .01; see Figure 2), though the direction of the relationship was similar in the African Americans (r = -.24; Figure 2), but it was not significant. Both groups showed negative correlations involving ALLO-ir and β-endorphin concentrations (rs = -.44 and -.55, ps < .01; see Figure 3), while neither group showed a statistically significant relationship involving plasma cortisol and β-endorphin.
Table 3.
Relationship of Neuroendocrine Factors as a Function of Ethnicity
| African Americans (n = 45) | Non-Hispanic Whites (n = 39) | |||
|---|---|---|---|---|
| Baseline ALLO | Baseline Cortisol | Baseline ALLO | Baseline Cortisol | |
| Baseline ALLO | — | r = -.24 p = ns |
— |
r = -.50** p < .01 |
| Baseline β-endorphin |
r = -.44** p < .01 |
r = -.01 p = ns |
r = -.55** p < .01 |
r = +.30 p = ns |
p < .01
Figure 2.
A scatterplot of the relationship between baseline levels of ALLO-ir and Cortisol separated by ethnicity with non-Hispanic Whites displayed in the top panel, and African Americans displayed in the bottom panel.
Figure 3.
A scatterplot of the relationship between baseline levels of ALLO-ir and β-endorphin with non-Hispanic Whites displayed in the top panel, and African Americans displayed in the bottom panel.
Mediational Analyses
Since only the non-Hispanic Whites showed significant correlations among ALLO-ir, cortisol, β-endorphin, and pain tolerance, mediational analyses were conducted in that sample only (Baron & Kenny 1986). Because baseline cortisol and β-endorphin were not correlated with each other, they were not considered together in multiple mediation models but instead each was examined as a simple mediator of the relationship between ALLO-ir and pain tolerance. Despite the numerous intercorrelations between ALLO-ir, cortisol, β-endorphin, and pain tolerance in non-Hispanic Whites, the only measure to meet strict criteria as a mediator in the relationship between ALLO-ir and pain sensitivity was cortisol.
For non-Hispanic Whites, since baseline ALLO-ir was negatively correlated with baseline cortisol (r = -.50, p < .01), and since both baseline ALLO-ir and baseline cortisol were correlated with thermal heat pain threshold (rs = -.49 and +.50 respectively, ps < .01) ), a series of linear regression analyses were performed (Baron & Kenny 1986) to test whether cortisol served as a statistical mediator of the relationship between ALLO-ir and thermal heat pain threshold. These analyses indicated that the significant regression coefficient relating baseline ALLO-ir to thermal heat pain threshold (t = -2.85, p < .01; b = -1.72; β = -0.43; R2 = 0.16, p < .01) is reduced (t = -1.34, p > .05; b = -0.85; β = -0.21) and in fact no longer significant, when baseline cortisol (t = 2.80, p < .01; b = 0.34; β = 0.44) is added to the model. Thus, higher baseline concentrations of cortisol met criteria for mediating the relationship between lower baseline ALLO-ir and higher thermal heat pain threshold (z = -2.18, p < .05). Baseline cortisol and baseline ALLO-ir concentrations together accounted for 30% of the variance in thermal heat pain tolerance (F(2, 35) = 8.74, p < .001).
When examining the relationship between ALLO-ir, cortisol, and thermal heat pain tolerance; ALLO-ir, cortisol, and cold pressor pain tolerance; and ALLO-ir, β-endorphin, and cold pressor pain tolerance, analyses indicated that cortisol did not meet statistical criteria as a mediator in these relationships involving cortisol and pain sensitivity.
Discussion
The results of our study, which is the first to examine the relationship of plasma ALLO-ir concentrations to pain sensitivity in humans, suggest that there may be ethnic differences in the degree to which ALLO interacts with HPA-axis variables, cortisol and β-endorphin, to influence pain perception.
Higher ALLO concentrations are associated with lower pain tolerance
Contrary to expectations, and inconsistent with animal studies showing that increased concentrations of GABAergic neurosteroids, including ALLO-ir are associated with a decrease in pain sensitivity (Kavaliers & Wiebe 1987), we found that higher ALLO-ir concentrations were associated with increased pain sensitivity (lower pain tolerance), at least in non-Hispanic Whites, and this was true for all three pain tests. One possibility for this discrepancy is that in the animal models, animals are tested for pain sensitivity following the administration of exogenous ALLO (Kavaliers & Wiebe 1987; Wiebe & Kavaliers 1988; Frye & Duncan 1994), while our study examined the relationship of endogenous levels of ALLO-ir and pain perception. Thus, the animal studies may have elicited supraphysiologic concentrations of ALLO. Since there is evidence for a bimodal effect of ALLO concentration on GABAA-regulated mood in humans (Miczek et al. 1993, 1997, 2003; Andréen et al. 2005), we speculate that lower endogenous concentrations of ALLO, such as those seen during the luteal phase, may be associated with enhanced sensitivity to pain, while higher concentrations of ALLO, such as seen during pregnancy may be analgesic. Dose-response studies in humans examining different ALLO-ir concentration profiles and pain sensitivity will be needed to test this hypothesis.
The relationship between ALLO and pain sensitivity may be mediated by HPA-Axis Factors
Another, though not mutually exclusive possibility for finding an inverse relationship between ALLO-ir concentrations and pain tolerance is indicated by our mediational analyses conducted in the non-Hispanic Whites. A number of prior studies have shown that measures reflecting increased activation of the HPA-axis, specifically higher plasma cortisol and higher plasma β-endorphin concentrations, are related to reduced sensitivity to experimental pain procedures, including cold pressor, ischemic, and thermal heat pain (al’Absi et al., 2002; Straneva et al., 2002; Girdler et al., 2005; Mechlin et al., 2005). We also observed significant positive correlations involving greater cortisol and β-endorphin concentrations and greater tolerance to thermal heat and cold pressor pain in the non-Hispanic Whites.2 The results of our mediational analyses extend the literature on HPA-axis activation and pain perception by providing the first evidence in humans for an interrelationship between ALLO and cortisol to influence pain sensitivity. Though we found that lower ALLO-ir concentrations were related to higher pain tolerance to all three pain tests in non-Hispanic Whites, mediational analyses indicated that at least for the thermal pain test, the inverse relationship was mediated by greater plasma cortisol.
While the exact mechanisms by which cortisol mediates the relationship between ALLO and pain sensitivity are unknown, one possibility involves the negative modulation of the HPA-axis by ALLO. Animal models demonstrate that increases in ALLO facilitate the return of HPA-axis activation to homeostasis following stress (Guo et al. 1995; Patchev et al. 1996). Activation of the HPA-axis with the associated pituitary release of β-endorphin and/or increased corticotrophin-releasing hormone (CRH) activity, may be part of an integrated adaptive mechanisms since both are associated with reduced pain sensitivity in humans (Hargreaves et al. 1987; Rosa et al. 1988; Sheps et al., 1992; Lariviere & Melzack 2000; Straneva et al. 2002). It is now well established that CRH acts on a large number of brain structures involved in pain processing, including the locus coeruleus, and that it can act both centrally and peripherally to produce analgesia (see Larivier & Melzack 2000 for review). Thus, since ALLO and cortisol are negatively related (Girdler et al. 2001; Girdler et al. 2006), and ALLO modulates the HPA-axis at multiple levels (Owens et al. 1992; Patchev et al. 1994; Patchev et al. 1996; Calogero et al. 1998), higher circulating ALLO would be expected to be associated with increased pain sensitivity as we documented in the non-Hispanic Whites. Regardless of mechanism, ALLO-associated hyperalgesia in women could contribute to greater experimental pain sensitivity when they are tested in their luteal versus follicular phase of the menstrual cycle (Fillingim et al. 1997; Pfleeger et al. 1997; Riley et al. 1999).
Ethnic differences in the relationship of ALLO to pain sensitivity
Also regardless of mechanism, and in contrast to our findings in the non-Hispanic Whites, our findings that significant interrelationships involving ALLO, cortisol, and pain sensitivity were not observed in African Americans is consistent with our other finding from this same cohort indicating ethnic differences in the relationship between pain sensitivity and stress-responsive biological factors. We had previously suggested (Mechlin et al. 2005) that the ethnic differences in the relationship involving plasma cortisol, NE, and BP and pain sensitivity may contribute to the greater clinical pain severity (Mechlin et al. 2005) experienced by African Americans. We have now extended those findings to include ethnic differences in the relationships between both ALLO and β-endorphin and pain sensitivity. At the same time, it must be acknowledged that many of the observed correlation coefficients in African Americans involving pain tolerance and ALLO as well as the HPA-axis variables were similar in direction to those observed in the non-Hispanic Whites, though they did not reach conventional levels of significance (Table 2). While it is probable that these correlation coefficients would have reached statistical significance if larger numbers of African Americans were included, low statistical power to detect significant relationships cannot be the sole or even primary explanation for the ethnic differences in the pattern of effects since our African American sample was larger than the non-Hispanic White sample and there was no evidence for ethnic differences in the variability of the neuroendocrine or pain sensitivity measures. Still, these findings should be replicated in larger samples of African American and non-Hispanic Whites. If confirmed, these results may have implications for the treatment of clinical pain in African Americans.
Limitations
There are also several limitations of the study, which should be addressed. The primary limitation to this report stems from the fact that the subjects represent a large subset of those previously on which we previously reported, where we observed the absence of relationships involving plasma cortisol, NE, and BP and pain sensitivity in African Americans. Thus, while the current report extends our observations to include the absence of relationships involving plasma ALLO-ir and β-endorphin with pain sensitivity in African Americans, the possibility exists that these findings are unique to this particular cohort of African Americans for whatever reason. Replication of these general findings in other cohorts of African Americans will be needed before any interpretations regarding ethnic differences in endogenous pain regulatory mechanisms can or should be made. Second, we relied exclusively on baseline and not post-stress samples and this may account for the unexpected inverse relationship we observed between ALLO and pain tolerance in humans. Third, although the intercorrelations involving ALLO-ir, cortisol, and β-endorphin were significant in the non-Hispanic Whites, the large range of values, particularly for cortisol and β-endorphin, may limit the predictability of these measures. Fourth, another limitation to our study relates to the use of laboratory testing procedures to make inferences regarding clinical pain. For example, although both male and female experimenters were randomly used, only non-White Hispanic experimenters administered the pain tests. Although the effect of the experimenter’s ethnicity on pain tolerance is equivocal (Zatzick & Dimsdale 1990; Weisse et al. 2005), the possibility does exist that the ethnicity of the experimenter or other non-specific effects of a laboratory environment could have influenced our findings. Finally, while one strength of our study was the use of multiple pain tests, this strength was balanced by our finding of an order effect involving sensitivity to the cold pressor test. Though we did statistically control for the order effects in all analyses, the reason for these effects remain unexplained. Regardless of the reason for this finding, it suggests the possibility for carryover effects from one procedure to another in human clinical research that should be carefully conducted and controlled for in subsequent studies.
Conclusion
Despite the limitations to our study, the novel focus of the relationship of ALLO to pain sensitivity in humans, and the interactions involving the HPA-axis are worth underscoring and may serve as important early observations involving unfamiliar pain regulatory mechanisms in humans. Our results suggest that lower physiologic concentrations of plasma ALLO are associated with increased pain threshold and tolerance in non-Hispanic Whites, and that for thermal heat pain threshold the relationship is mediated by higher cortisol concentrations. The fact that we did not observe any significant relationships involving ALLO-ir, cortisol or β-endorphins and pain sensitivity in African Americans adds to our prior report documenting ethnic differences in the relationship of plasma NE, cortisol, and BP to pain sensitivity (Mechlin et al. 2005), though, these findings need to be replicated in separate cohorts of African Americans and non-Hispanic Whites.
Acknowledgments
This study was supported by NIH grants NIDA R01 DA1375 and GCRC RR00046. The authors are grateful to Dot Faulkner for her assistance with manuscript preparation.
Footnotes
Relationships involving ALLO-ir, cortisol, and pain sensitivity as a function of ethnicity did not differ in any appreciable way when analyses were conducted using post-stress samples (post-stress samples of β-endorphin were not obtained). However, preliminary data from our laboratory indicated that the post-stress sample obtained for ALLO-ir was delayed and did not capture the peak ALLO response to stress (Girdler et al., unpublished data). Thus, in order to reduce further Type I error rates, to increase the generalizability of our findings to the existing literature on neuroendocrine factors and pain perception, and because the ALLO response was not sampled when at peak (Girdler et al. unpublished data), the present report focuses exclusively on the relationship of resting baseline samples to pain sensitivity.
It may be important to note that the relationship between baseline cortisol and increased thermal heat and cold pressor pain tolerance in non-Hispanic Whites in the present report was not found in our prior report (Mechlin et al., 2005) from the larger sample. One distinct possibility for this discrepancy concerns the influence of the menstrual cycle on the HPA-axis (Kirschbaum et al., 1999; Kowalczyk et al., 2006) since all samples included in the present report came from women in the luteal phase of their menstrual cycle, while the prior report, which focused on the first test session only, included women in various stages of their cycles.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- Andréen L, Sundström-Poromaa I, Bixo M, Andersson A, Nyberg S, Böckström T. Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology. 2005;30:212–224. doi: 10.1016/j.psyneuen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bragdon EE, Light KC, Girdler SS, Maixner W. Blood Pressure, gender, and parental hypertension are factors in baseline and poststress pain sensitivity in normotensive adults. Int J Behav Med. 1997;4:17–38. doi: 10.1207/s15327558ijbm0401_2. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in response to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Palumbo MA, Bosboom AMJ, Burrello N, Ferrara E, Palumbo G, Petraglia F, D’Agata R. The neuroactive steroid allopregnanolone suppresses hypothalamic gonadotropin-releasing hormone release through a mechanism mediated by the gamma-aminobutyric acidA receptor. J Endocrinol. 1998;158:121–125. doi: 10.1677/joe.0.1580121. [DOI] [PubMed] [Google Scholar]
- Chapman WP, Jones CM. Variations in cutaneous and visceral pain sensitivity in normal subjects. J Clin Invest. 1944;23:81–91. doi: 10.1172/JCI101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001a;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001b;94:133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W, Girdler SS, Light KC, Harris MB, Sheps DS, Mason GA. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychsom Med. 1997;59(5):512–520. doi: 10.1097/00006842-199709000-00008. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- France CR, France JL, al’Absi M, Ring C, McIntyre D. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain. 2002;99:459–463. doi: 10.1016/s0304-3959(02)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duncan JE. Progesterone metabolites, effects at the GABAA receptor complex, attenuate pain sensitivity in rats. Brain Res. 1994;643:194–203. doi: 10.1016/0006-8993(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Mechlin MB, Light KC, Morrow AL. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology. 2006;43:331–336. doi: 10.1111/j.1469-8986.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- Girlder SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Guo AL, Petraglia F, Criscuolo M, Ficarra G, Nappi RE, Palumbo MA, Trentini GP, Purdy RH, Genazzani AR. Evidence for a role of neurosteroids in modulation of diurnal changes and acute stress-induced corticosterone secretion in rats. Gynecol Endocrinol. 1995;9:1–7. doi: 10.3109/09513599509160184. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Mueller GP, Dubner R, Goldstein D, Dionne RA. Corticotropin-releasing factor (CRF) produces analgesia in humans and rats. Brain Res. 1987;422:154–157. doi: 10.1016/0006-8993(87)90550-6. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Sachs-Ericsson N. Ethnic differences in pain reports and the moderating role of depression in a community sample of Hispanic and Caucasian participants with serious health problems. Psychosom Med. 2006;68(1):121–128. doi: 10.1097/01.psy.0000197673.29650.8e. [DOI] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Kavaliers M, Wiebe JP. Analgesic effects of the progesterone metabolite, 3α-hydroxy-5α-pregnan-20-one, and possible modes of action in mice. Brain Res. 1987;415:393–398. doi: 10.1016/0006-8993(87)90228-9. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993a;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmcol Biochem Behav. 1993b;44:527–31. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychsom Med. 1995a;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995b;57:468–74. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain. 2006;7(3):151–160. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84:1–12. doi: 10.1016/S0304-3959(99)00193-1. [DOI] [PubMed] [Google Scholar]
- Maixner W. Interactions between cardiovascular and pain modulatory systems: physiological and pathophysiological implications. J Cardiovasc Electrophysiol. 1991;2:S3–S12. [Google Scholar]
- Maixner W, Gracely RH, Zuniga JR, Humphrey CB, Bloodworth GR. Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol. 1990;259:R1156–R1163. doi: 10.1152/ajpregu.1990.259.6.R1156. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Matthews AK, Tang TS, Cuba SL. A comparison of blacks and whites seeking treatment for chronic pain. Clin J Pain. 2001;17:249–255. doi: 10.1097/00002508-200109000-00011. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Bruehl S. Do endogenous opioids mediate the relationship between blood pressure and pain sensitivity in normotensives? Pain. 1994;57:63–67. doi: 10.1016/0304-3959(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychsom Med. 2005;67:948–956. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, DeBold JF. Alcohol, benzodiazepine-GABAA receptor complex and aggression: ethological analysis of individual differences in rodents and primates. J Stud Alcohol Suppl. 1993;11:170–179. doi: 10.15288/jsas.1993.s11.170. [DOI] [PubMed] [Google Scholar]
- Miczek KA, DeBold JF, van Erp AM, Tornatzky W. Alcohol, GABAA-benzodiazepine receptor complex, and aggression. Recent Dev Alcohol. 1997;13:139–171. doi: 10.1007/0-306-47141-8_9. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–257. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM. Characterization of steroid interactions with the gamma-aminobutyric acid receptor-gated chloride ion channel: Evidence for multiple steroid recognition sites. Mol Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Ritchie JC, Nemeroff CB. 5α-Pregnane-3α,21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: comparison with alprazolam. Brain Res. 1992;573:353–355. doi: 10.1016/0006-8993(92)90788-b. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotrophin-releasing hormone-induced anxiety and alters the release and gene expression of corticotrophin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pfleeger M, Straneva PA, Fillingim RB, Maixner W, Girdler SS. Menstrual cycle, blood pressure and ischemic pain sensitivity in women: A preliminary investigation. Int J Pyschophysiol. 1997;27(2):161–166. doi: 10.1016/s0167-8760(97)00058-5. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations in gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4453–4457. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Maixner W. The role of sinoaortic and cardiopulmonary baroreceptor reflex arcs in nocicpetion and stress-induced analgesia. Ann NY Acad Sci. 1986;467:385–401. doi: 10.1111/j.1749-6632.1986.tb14642.x. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3rd, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3rd, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD. Racial/ethnic differences in the experience of chronic pain. Pain. 2002;100:291–298. doi: 10.1016/S0304-3959(02)00306-8. [DOI] [PubMed] [Google Scholar]
- Rosa C, Ghione S, Mezzasalma L, Pellegrini M, Basile Fasolo C, Giaconi S, Gazzetti P, Ferdeghini M. Relationship between pain sensitivity, cardiovascular reactivity to cold pressor test and indexes of activity of the adrenergic and opioid system. Clin Exp Hypertens A. 1988;10(Suppl 1):383–390. doi: 10.3109/10641968809075994. [DOI] [PubMed] [Google Scholar]
- Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS. Race and sex differences in cutaneous pain perception. Psychosom Med. 2000;62:517–523. doi: 10.1097/00006842-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Shen BJ, Stroud LR, Niaura R. Ethnic differences in cardiovascular responses to laboratory stress: A comparison between Asian and White Americans. Int J Behav Med. 2004;11(3):181–186. doi: 10.1207/s15327558ijbm1103_7. [DOI] [PubMed] [Google Scholar]
- Sheps DS, Bragdon EE, Gray TF, 3rd, Ballenger M, Usedom JE, Maixner W. Relation between systemic hypertension and pain perception. Am J Cardio. 1992;70(16):3F–5F. doi: 10.1016/0002-9149(92)90181-w. [DOI] [PubMed] [Google Scholar]
- Stoney CM, Hughes JW, Kuntz K, West SG, Thornton LM. Cardiovascular stress responses among Asian Indian and European American women and men. Ann Behav Med. 2002;24(2):113–121. doi: 10.1207/S15324796ABM2402_08. [DOI] [PubMed] [Google Scholar]
- Straneva PA, Maixner W, Light KC, Pedersen CA, Costello NL, Girdler SS. Menstrual Cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychol. 2002;21(4):358–367. [PubMed] [Google Scholar]
- Weisse CS, Foster KK, Fisher EA. The influence of experimenter gender and race on pain reporting: Does racial or gender concordance matter? Pain Med. 2005;6(1):80–87. doi: 10.1111/j.1526-4637.2005.05004.x. [DOI] [PubMed] [Google Scholar]
- Wiebe JP, Kavaliers M. Analgesic effects of the putative FSH-suppressing gonadal seroid 3α-hydroxy-4-pregnen-20-one: Possible modes of action. Brain Res. 1988;461:150–157. doi: 10.1016/0006-8993(88)90733-0. [DOI] [PubMed] [Google Scholar]
- Woodrow KM, Friedman GD, Siegelaub AB, Collen MF. Pain tolerance: differences according to age, sex and race. Psychsom Med. 1972;34:548–556. doi: 10.1097/00006842-197211000-00007. [DOI] [PubMed] [Google Scholar]
- Yokoro CM, Pesquero SMS, Turchetti-Maia RMM, Francischi JN, Tatsuo MAKF. Acute phenobarbital administration induces hyperalgesia: Pharmacological evidence for the involvement of supraspinal GABA-A receptors. Braz J Med Biol Res. 2001;34:397–405. doi: 10.1590/s0100-879x2001000300015. [DOI] [PubMed] [Google Scholar]
- Zamir N, Shuber E. Altered pain perception in hypertensive humans. Brain Res. 1980;201:471–474. doi: 10.1016/0006-8993(80)91055-0. [DOI] [PubMed] [Google Scholar]
- Zatzick DF, Dimsdale JE. Cultural variations in response to painful stimuli. Psychosom Med. 1990;52(5):544–57. doi: 10.1097/00006842-199009000-00007. [DOI] [PubMed] [Google Scholar]