Abstract
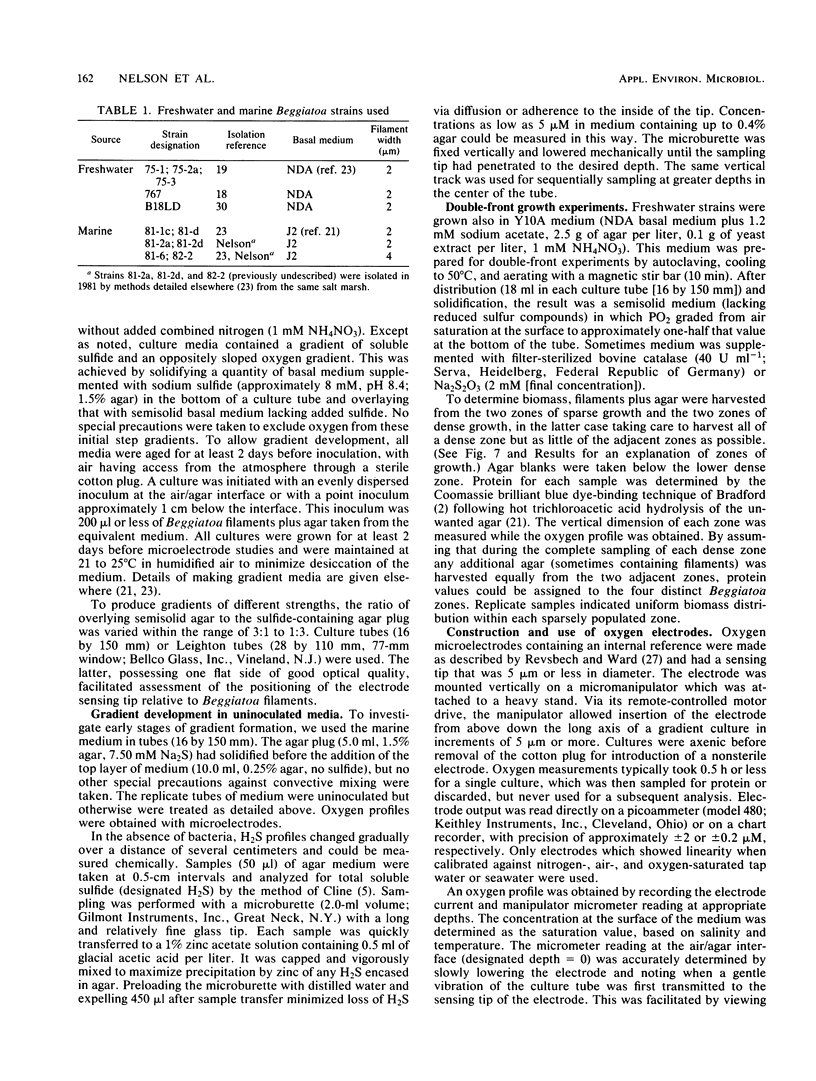
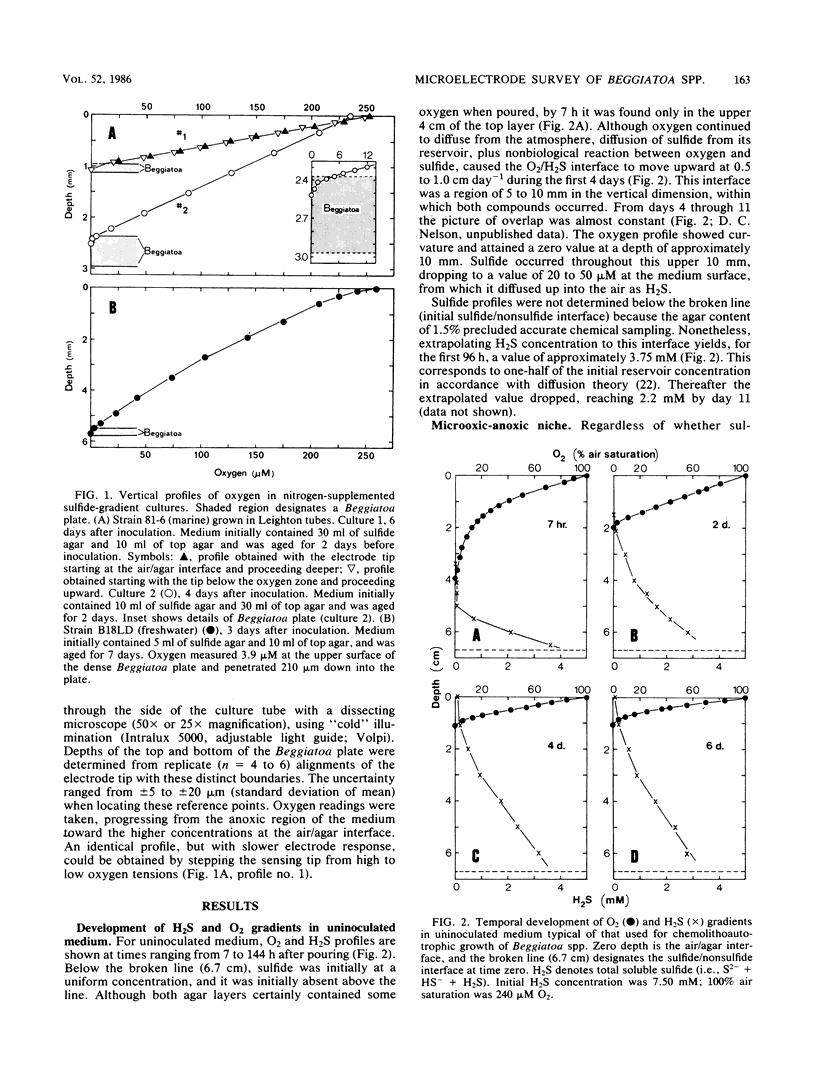
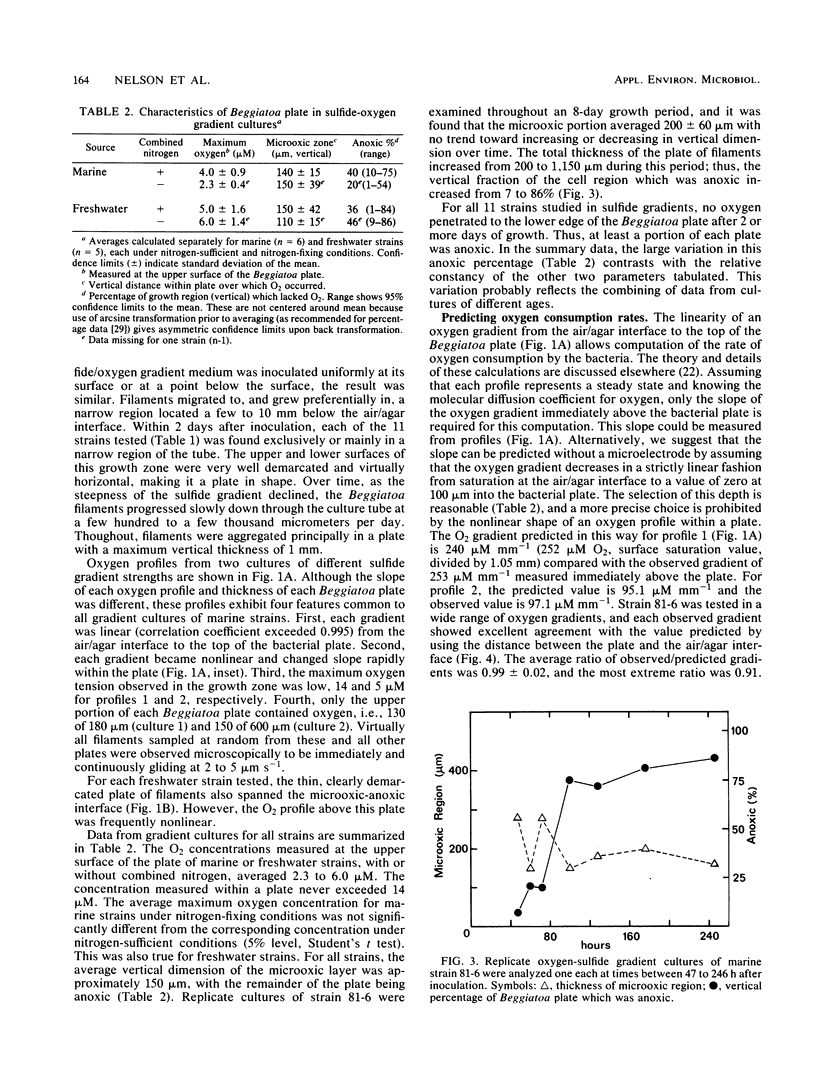
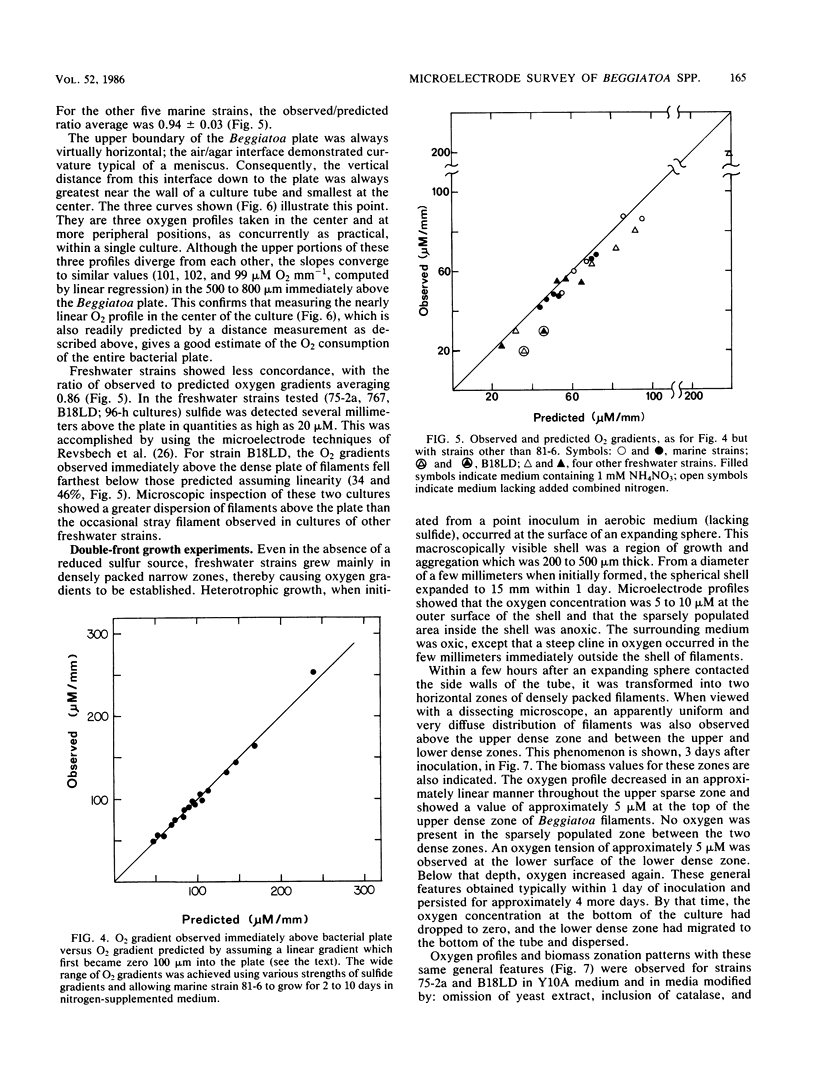
Beggiatoa spp. grow optimally in media containing opposed gradients of oxygen and soluble sulfide, although some strains also require an organic substrate. By using microelectrodes, we characterized oxygen and sulfide gradients during their initial development in uninoculated media and in cultures of marine and freshwater strains. In gradient media, Beggiatoa strains always grew some distance below the air/agar interface as a dense “plate” of constantly gliding filaments with sharply demarcated upper and lower boundaries. Within established plates, the maximum oxygen partial pressure was 0.6 to 6.0% of air saturation and not significantly lower if filaments were fixing nitrogen. Oxygen penetrated only 100 to 300 μm into the plate, and the anoxic fraction increased from less than 10% to approximately 90% during later stages of growth. For lithoautotrophically grown marine strains, the linearity of the oxygen profile above the plate plus its drop to zero therein indicated that oxygen uptake for the entire tube occurred only within the Beggiatoa plate. Consequently, oxygen consumption could be predicted solely from the distance between the air/agar interface and the top of a plate, given the diffusion coefficient for oxygen. By contrast, for freshwater strains grown heterotrophically (with sulfide also in the medium), oxygen profiles were frequently nonlinear because of nonbiological reaction with sulfide which had diffused past the aggregated filaments. For all strains tested, microoxic aggregation also occurred in the absence of sulfide, apparently reflecting a step-up phobic response to oxygen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON S. D., MORITA R. Y. EFFECT OF CATALASE AND CULTURAL CONDITIONS ON GROWTH OF BEGGIATOA. J Bacteriol. 1964 Dec;88:1755–1761. doi: 10.1128/jb.88.6.1755-1761.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- FAUST L., WOLFE R. S. Enrichment and cultivation of Beggiatoa alba. J Bacteriol. 1961 Jan;81:99–106. doi: 10.1128/jb.81.1.99-106.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Harrison D. E. The regulation of respiration rate in growing bacteria. Adv Microb Physiol. 1976;14(11):243–313. doi: 10.1016/s0065-2911(08)60229-5. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., George H. A., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979 Jan;25(1):8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- Jørgensen B. B., Revsbech N. P. Colorless Sulfur Bacteria, Beggiatoa spp. and Thiovulum spp., in O(2) and H(2)S Microgradients. Appl Environ Microbiol. 1983 Apr;45(4):1261–1270. doi: 10.1128/aem.45.4.1261-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller M. M., Nielsen L. P., Jørgensen B. B. Oxygen Responses and Mat Formation by Beggiatoa spp. Appl Environ Microbiol. 1985 Aug;50(2):373–382. doi: 10.1128/aem.50.2.373-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Castenholz R. W. Organic nutrition of Beggiatoa sp. J Bacteriol. 1981 Jul;147(1):236–247. doi: 10.1128/jb.147.1.236-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Castenholz R. W. Use of reduced sulfur compounds by Beggiatoa sp. J Bacteriol. 1981 Jul;147(1):140–154. doi: 10.1128/jb.147.1.140-154.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Jørgensen B. B., Revsbech N. P. Growth Pattern and Yield of a Chemoautotrophic Beggiatoa sp. in Oxygen-Sulfide Microgradients. Appl Environ Microbiol. 1986 Aug;52(2):225–233. doi: 10.1128/aem.52.2.225-233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim E. G. Die Mixotrophie von Beggiatoa. Arch Mikrobiol. 1967;59(1):247–254. [PubMed] [Google Scholar]
- Revsbech N. P., Ward D. M. Oxygen Microelectrode That Is Insensitive to Medium Chemical Composition: Use in an Acid Microbial Mat Dominated by Cyanidium caldarium. Appl Environ Microbiol. 1983 Mar;45(3):755–759. doi: 10.1128/aem.45.3.755-759.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. R., Cannon G. C., Shively J. M., Güde H., Hook L. A., Lane C. M., Larkin J. M. Heterotrophic carbon metabolism by Beggiatoa alba. J Bacteriol. 1981 Nov;148(2):572–583. doi: 10.1128/jb.148.2.572-583.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. R., Larkin J. M. Enumeration, isolation, and characterization of beggiatoa from freshwater sediments. Appl Environ Microbiol. 1978 Nov;36(5):755–770. doi: 10.1128/aem.36.5.755-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



