Abstract
Atrazine is a widely used herbicide applied to corn, sugar and other crops as a broad leaf weed inhibitor. Using the Balb/c mouse model, we have determined that prenatal/lactational exposure to atrazine alters adult immune function. Pregnant Balb/c dams were exposed subcutaneously for 21 days via time release pellets to 700 µg per day of atrazine beginning between days 10 and 12 of pregnancy. Prenatal/Lactational exposure caused no overt physical malformations in the offspring and had no effect on the number of litters carried to term or the litter size. Upon reaching early adulthood (approximately 3 months of age), the state of their immune system was evaluated. There were no changes in body weight or in the organ to body weight ratio of the spleen. Additionally, no changes were observed in the number of CD8+ T cell, CD4+ T cell, or B220+ B cell subpopulations in the spleen. T cell function was assessed by measuring proliferation and cytolytic activity after in vitro allogeneic stimulation. Male mice which had been prenatally/lactationally exposed to atrazine had an increase in both T cell proliferation and cytolytic activity. The humoral immune response was assessed after immunization with heat killed Streptococcus pneumoniae (HKSP). There was a significant increase in the number of HKSP‐specific IgM secreting B cells in the spleen of prenatal/lactational exposed male mice. Inasmuch as atrazine is a widespread environmental contaminant, this immunopotentiation raises concerns that it may potentiate clinical diseases, such as autoimmune disease and hypersensitivity, and needs to be carefully monitored and studied.
Keywords: Atrazine, Herbicide, Immunotoxicity, B cell response, T cell response, Developmental, Mouse
Introduction
The ubiquitous use of herbicides in modern agriculture and the resulting exposure of the population have been a continued health concern for the last half century. Atrazine is the second most commonly applied herbicide in the United States (US) with over 75 million pounds applied annually (Short and Colborn, 1999). Atrazine and its primary metabolite, deethylatrazine, are the most commonly encountered ground and surface water contaminants in the US and can persist in those water supplies for months (Koskinen and Clay, 1997). Human exposure to atrazine is understandably extensive. Biomarker studies have detected nanomolar equivalents of atrazine in the urine of applicators during the spraying season (Hines et al., 2003). Separate studies detected atrazine metabolites in urine of 80% of applicators during the spraying season (Perry et al., 2000, 2001). Atrazine has also been found in the urine of children of nonagricultural families demonstrating nonoccupational exposures (Adgate et al., 2001). Exposure via groundwater also occurs at a higher than expected rate. The EPA sets the “maximum allowable contribution of water containing pesticide (atrazine) residues permitted in diet” at 12.5 parts per billion (ppb) for infants and pregnant women and 23 ppb for children between the ages of 1 and 6 (EPA, 2003). A survey of 39 community drinking water sources found amounts of atrazine in excess of these levels in 34 of the water sources (EPA, 2003). These data indicate that chronic exposure of small children and pregnant women to excessive levels of atrazine may occur in communities serviced by these water sources. Furthermore, these communities were located in or near agriculturally active areas, thus presenting a potential for aggregate exposures through occupational or other environmental routes. The exposure limit set for pregnant women is not based on direct research of a mammalian gestational model but is rather inferred from studies of early life exposure models (EPA, 2003). Prenatal atrazine exposure represents a potential risk to public health and needs to be assessed.
Atrazine in particular has been demonstrated to be both a reproductive and immunotoxic substance in animal models. Prenatal/Lactational atrazine exposure has been shown to affect the health and development of the offspring in several rat models. In some strains of rats, Holtzman (HLZ), Sprague–Dawley (SD), Long–Evans (LE) and Fischer 344, atrazine is directly toxic to the reproductive system and interferes with successful pregnancies (Cummings et al., 2000). In Wistar rats, maternal atrazine exposure alters the development of the offspring by delaying the onset of puberty (Stoker et al., 2002). Stoker et al. (1999) reported that exposing lactating Wistar rats to atrazine inhibited the suckling‐induced prolactin surge. The absence of this prolactin surge caused the offspring to have a lifelong deregulation of prolactin production (Stoker et al., 1999), and prolactin is a potent immunomodulator (Gerlo et al., 2005; Sun et al., 2004).
The direct immunotoxic potential of acute atrazine exposure of adult animals has been assessed. Acute exposures to atrazine via intraperitoneal injection induced short‐lived but significant decreases in the numbers of hematopoietic progenitors in murine bone marrow (Mencoboni et al., 1992). Other studies demonstrated that a single oral dose of atrazine causes transient suppression of IgM production and T cell proliferation in adult mice (Fournier et al., 1992). Fourteen‐day oral atrazine exposure has a transient negative effect on the cell make up of spleens and thymuses in juvenile C57Bl/6 mice, decreasing the levels of CD4+ lymphocytes and MHC‐II+ cells (Filipov et al., 2005). Karrow et al. (2005) also report numerous immunotoxic effects on B6C3F1 mice after orally administering atrazine for 14 days, in particular, they noted an increase in the mixed lymphocyte reaction (MLR) and cytotoxic T lymphocyte (CTL) response at their lowest tested dose (25 mg/kg/day).
The developing immune system is more sensitive than the adult immune system to xenobiotic exposure (Blaylock et al., 1992; Holladay, 1999; Theus et al., 1992). The toxic effects of atrazine in adult‐exposed animals do not forecast a probable outcome when animals are exposed prenatally. Prenatal exposure to atrazine often causes substantially different effects in the adult offspring from the effects noted in adult‐exposed animals (Laws et al., 2003; Rayner et al., 2004). The effect of prenatal and lactational exposure to atrazine on the immune system has been explored in Sprague–Dawley rats. In this model, atrazine was administered by oral gavage beginning at day 10 of gestation and continued until the pups were weaned on postnatal day 23 (Rooney et al., 2003). Male, but not female, offspring assessed 2 weeks after weaning had a decrease in the magnitude of the delayed type hypersensitivity response and the IgM antibody response to sheep red blood cell (SRBC) immunization (Rooney et al., 2003). A potential link between decreased prolactin levels during lactation and lactational atrazine exposure was tested by using an inhibitor of prolactin secretion, bromocryptine. Bromocryptine was administered to pregnant dams from day 10 of gestation to day 10 of lactation and did not mimic the immunotoxic effects of an identical atrazine exposure regime (Rooney et al., 2003). It was concluded that the immunosuppression observed in prenatally exposed adult male Sprague–Dawley rats was not the result of an atrazine‐mediated disruption of prolactin.
The present study tested the hypothesis that prenatal/lactational exposure to atrazine would effect the development of the immune system and alter adult immune function in a mouse model. The results demonstrate that male, but not female, mouse offspring were affected by the prenatal atrazine exposure at 3 months of age. Our data show an increase number of IgM producing cells after immunization with our model antigen (HKSP). Cytotoxic lymphocyte function and the proliferative response of splenocytes following ex vivo allogeneic stimulation were increased in prenatally exposed male offspring. This study demonstrates that in‐direct early life exposure to atrazine significantly affects the adult immune system in experimental animals.
Methods
Animals
Specific pathogen‐free male and female Balb/c mice between 6 and 8 weeks of age were purchased from Charles River Laboratories (Wilmington, MA). Eight‐week‐old female C57Bl/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were housed in sterilized microisolator cages in the university vivarium, and facility sentential animals were regularly screened for specific pathogenic agents. Mice were kept on a 12‐h light–dark cycle and allowed to acclimate to the facility for at least 1 week prior to the initiation of any experiments. Food and water were provided ad libitum. These studies were conducted in accordance with all federal and institutional guidelines for animal use and were approved by the West Virginia University Institutional Animal Care and Use Committee. The Balb/c mice were bred by placing male mice (> 8 weeks of age) with a pair of female Balb/c mice for 3 days. The female mice were housed two per cage for the duration of the pregnancy. Pups were counted 1 week after birth and were sexed and weaned at 4 weeks of age. At weaning, the animals were ear punched, housed 2–4 per cage and sorted to provide a cage group of individuals from different dams. Male and female offspring were evaluated for effects on the immune system at 3 months of age.
Atrazine
Atrazine (98% purity) was purchased from Chemservice (West Chester, PA) and shipped directly to Innovative Research of America (Sarasota, FL). Innovative Research of America produced 21‐day time‐release pellets containing a total of 14.7 mg atrazine which released 700 µg/day. A properly implanted pellet releases at zero order kinetics, that is, the same amount of atrazine will be released every minute of every hour of every day until the end of the 21 days after implantation (per the quality assurance of the manufacturer). The beginning weight of the dams was approximately 20 g, therefore, the rate of exposure was 35 mg/kg body weight. The dose decreased as weight increased with pregnancy, and so by the time the mice gave birth, the daily dose dropped to approximately 23 mg/kg/day. A placebo pellet of the same size and composition was used as a control.
Atrazine administration
Each experiment was initiated when one male mouse was placed in a cage with two female mice with a total of 60 breeding cages prepared at the beginning of each experiment. The male was removed after 3 days, and all females were assumed pregnant following the cohabitation period. Mice were not checked for vaginal plugs. Ten days after the removal of the male from the cage, an atrazine‐time‐release pellet or a placebo pellet (described above) was inserted subcutaneously, using a steel trochar as described by the pellet manufacturer (Innovative Research of America; Sarasota, FL). The actual date of dosing initiation (i.e., d10, d11, d12) for each litter was calculated based on the date of litter delivery, assuming a 21‐day gestation period. We did not find a significant difference in the immune responses between offspring from dams which received the atrazine pellet on day 10, day 11, or day 12 of gestation. The progeny were weaned at 4 weeks of age. Offspring were assayed during the third month‐of‐life, and the data between the groups of progeny (1) offspring of dams that received an atrazine pellet 10 days after the removal of the male (atrazine offspring), and (2) offspring of dams that received a placebo pellet (control offspring) 10 days after the removal of the male were compared. In the first experiment, it was unknown whether the atrazine released from the pellet would interfere with the reproductive health of the dams as was noted in some strains of rats (Cooper et al., 1996). To potentially compensate for any reduction in pregnancy rates or litter size; twice as many breeding mice were implanted with the atrazine pellet than the placebo pellet. There was not an observed atrazine effect on pregnancy rates or litter size (Fig. 1). Because there were fewer placebo‐treated dams than atrazine‐treated dams, there were insufficient numbers of the placebo offspring in the first iteration of these experiments to meet the number of offspring required to establish significant effect with the assays used. Thus, in assays from the first experiment, approximately half of the controls were age‐matched offspring from dams that did not receive a pellet. Comparison of data from the age‐matched control group and the placebo offspring group determined that there was no significant difference between the groups. In the second experiment, equal numbers of dams were implanted with atrazine or placebo pellets, and only mice whose mother received a placebo pellet were used as the control offspring.
Fig. 1.
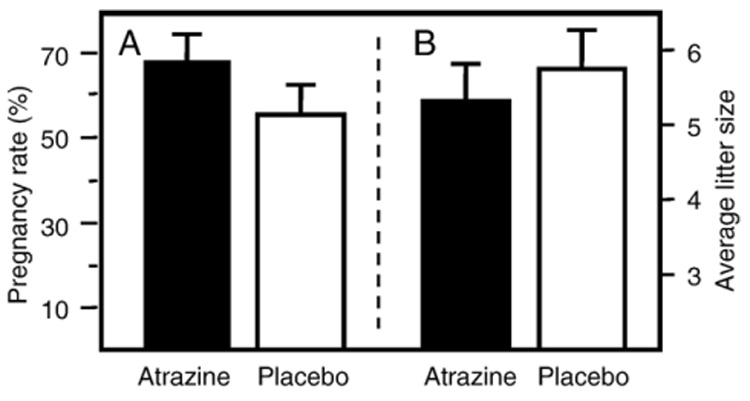
The effects of atrazine exposure on mouse reproduction. (A) Pregnancy rate of a total of 71 breeding females receiving an atrazine pellet and a total of the 49 breeding females receiving a placebo pellet. (B) The average litter size of a total of 48 breeding females receiving an atrazine pellet and a total of 25 breeding females receiving a placebo pellet. Data are the average of data obtained from two experiments.
Cell culture
Unless otherwise noted, all cell cultures were maintained at 37 °C in 5% CO2 in RPMI 1640 plus supplements (referred to as complete RPMI or cRPMI). The RPMI supplements were 10% heat‐inactivated fetal bovine serum (Hyclone, Logan UT), 2 mM of l‐glutamine (Medtech Inc., Herndon VA), 50 mM HEPES (Sigma Chemical Co. St. Louis, MO), 49 µM β‐mercaptoethanol (Sigma Chemical Co.) and 100 µg/ml of streptomycin and 100 U/ml penicillin (Hyclone).
CTL assay
To generate effector cells cultures spleens of atrazine and control offspring were surgically removed, and a single cell suspension was produced by crushing the spleen between two frosted glass slides. The cells were washed with cRPMI three times and suspended in cRPMI at a concentration of 5 × 105 cells per ml. Stimulator cells were prepared in an identical manner from the spleens of female C57Bl/6 mice. The stimulator cells were rendered incapable of mitosis by exposure to 20 Gy of gamma radiation from a cesium source. The Balb/c splenocytes (effectors) were activated by co‐culturing with the allogenic C57Bl/6 splenocytes (stimulators) in a total volume of 10 ml in a 25‐cm² tissue culture flask (Costar, Corning NY) at a one to one (1∶1) ratio and then incubated for 5 days at 37 °C with 5% CO2.
The lytic potential of ex vivo activated T cells was assessed using EL‐4 lymphoma cells as targets (a gift from Dr. Kenneth Landreth, WVU). The EL‐4 cells were harvested, washed and resuspended in 50 µl of cRPMI. The EL‐4 target cells were incubated for 1 h at 37 °C with 200 µBq of chromium‐51 (51Cr) (PerkinElmer, Boston MA). After the incubation, the 51Cr‐labeled EL‐4 cells were washed once and incubated for 20 min in a 37 °C water bath in order to allow leakage of excess chromium from the cells. Effector cells were harvested, washed once and resuspended at 2 × 108 cells/ml. Effector cells generated from the splenocytes of atrazine and control Balb/c offspring were added to V‐bottom 96‐well microplates in a volume of 100 µl at a concentration of 2 × 107, 1 × 107, 5 × 106, 2.5 × 106, 1.25 × 106 or 6.25 × 105 cells per well. EL‐4 target cells (2 × 105 in 100 µl) were added to all wells, resulting in effector to target (E∶T) ratios that ranged from 100∶1 to 3.12∶1. A total of two wells were set up for each effector to target ratio. Two control cell wells consisting of only labeled target cells, i.e., without effector cells present, were also incubated in parallel. Plates were incubated for 4 h at 37 °C in 5% CO2 and then centrifuged at 1500 × g for 7 min. Supernatants were collected, and the chromium content (counts per minute) was determined using a gamma‐counter (PerkinElmer). To determine the maximum amount of 51Cr contained in the labeled target cells, target cell control wells were lysed using 100 µl of 0.1% Triton X‐100. The amount of 51Cr spontaneously released was determined by harvesting supernatants from unlysed target cells. Specific cell lysis was determined as follows.
ELISpot analysis
Multiscreen 96‐well plates (Millipore, Bedford MA) were coated with 50 µl of a 10 µg/ml solution of the same batch of HKSP used to immunize the mice or purified phosphorylcholine conjugated to BSA (PC) (Biosearch Technologies, Novato, CA). The plates were incubated overnight at 4 °C. The next day, the plates were washed once with 200 µl/well of sterile PBS. Following the wash, 200 µl of RPMI 1640 supplemented with 20% FBS was added to each well to block nonspecific binding, and the plates were incubated for 2 h at 37 °C. Next the plates were washed twice with sterile PBS and 1 × 106, 2 × 105 or 1 × 105 spleen cells were added per well in a volume of 100 µl cRPMI and incubated for 4 h at 37 °C and 5% CO2. A total of two wells per sample were set up for each cell concentration. The plates were washed vigorously three times with a PBS + 0.05% Tween 20 solution. Once washed, alkaline phosphatase‐conjugated isotype‐specific polyclonal goat anti‐mouse immunoglobulin antibody was added to each well, and the plates were incubated overnight at 4 °C. The plates were vigorously washed three times with a PBS + 0.05% Tween 20 solution, and 100 µl/well of SIGMAFAST (Sigma‐Aldrich Chemical Co. St. Louis, MO) was added to each well. SIGMAFAST (5‐bromo‐4‐chloro‐3‐indolyphosphate/nitro blue tetrazolium) is an alkaline phosphatase substrate and was prepared according to manufacturers directions. The reaction was stopped by extensive washing with water once significant color development had been observed. The spots were counted using a dissecting microscope. Each spot represents a single antigen‐specific antibody secreting B cell. The total number of B cells per well was calculated using the ratio of B cells in the spleen determined by flow cytometry (described below).
Flow cytometry
Single cell suspensions of spleen cells were prepared, as described above, and stained, according to the suppliers instructions, with fluorescently conjugated antibodies specific for the mouse T cell markers CD4 and CD8 and the mouse B cell marker B220. Fluorescein isothiocyanate (FITC) conjugated rat anti‐mouse CD4 (clone L3T4), phycoerythrin (PE) conjugated rat anti‐mouse CD8α (clone 53–6.7) and Cytochrome‐C (Cy)‐conjugated rat anti‐mouse CD45R/B220 (clone RA3‐6B2) were obtained from BD Pharmingen (Franklin Lakes, NJ). One million spleen cells were incubated with purified rat immunoglobulin and purified mouse immunoglobulin (BD Pharmingen) for 30 min on ice to prevent nonspecific binding of the specific antibodies to the cells. Next, the cells were washed with ice‐cold PBS containing 2% FBS (Hyclone) and 0.2% sodium azide (Sigma‐Aldrich Chemical Co). The cells were incubated with buffer containing the appropriate antibody reagent for 30 min and fixed in 0.4% paraformaldehyde. FITC‐anti‐CD4 and the Cy‐anti‐B220 were used at a concentration of 0.5 µg/106 cells, and the PE‐anti‐CD8 antibody was used at a concentration of 1 µg/106 cells. The cells were then analyzed using a Becton Dickinson Facscalibur (San Jose, CA) and Cell Quest Pro software (San Jose, CA).
Heat‐killed Streptococcus pneumoniae (HKSP) preparation and immunization
An avirulent, unencapsulated strain of S. pneumoniae (strain R36A) was grown to mid‐log phase in Todd‐Hewitt broth plus 0.05% yeast extract and stored at −70 °C. Prior to immunization, an aliquot of R36A culture was plated onto blood agar plates, and a few characteristic colonies were selected and suspended in 200 ml Todd‐Hewitt broth plus 0.05% yeast extract. Bacteria were grown at 37 °C until they reached an optical density absorbance reading of 0.4 at 650 nm. The bacteria were standardized to a final concentration of 1 × 109 CFU/ml in PBS based on colony counts performed by standard techniques. The HKSP vaccine was prepared by heat killing the bacterial suspension at 60 °C for 10 h. Sterility was confirmed by culture, and the HKSP stock was stored at −20 °C in 1 ml aliquots. Mice were immunized intraperitoneally (i.p.) with 0.2 ml (the equivalent of 2 × 108 CFU) of the vaccine, and the number of antibody secreting cells (ASC) was determined 2 weeks postimmunization by ELISpot analysis.
Mixed lymphocyte response
Atrazine‐ and control‐exposed Balb/c splenocytes were co‐cultured with irradiated C57Bl/6 splenocytes. The effector and stimulator cells were mixed at a one to one (1∶1) ratio in a total volume of 10 ml containing 1 × 106 cells per ml (5 × 105 cells/ml of each cell population) in a 25‐cm² tissue culture flask (Costar) and incubated for 4 days at 37 °C with 5% CO2. After 4 days in culture, the cells were harvested, washed in cRPMI and counted. The cells were plated at 2.5 × 104 cells per well in sterile flat bottom 96‐well plates (Costar). Tritiated thymidine (³H‐Thy) (PerkinElmer) was added to each well at a concentration of 0.01 µCi/well. The plates were incubated for 18 h at 37°C and 5% CO2. The ³H‐thymidine addition time point was chosen because T cell precursors are largely recruited on days 2–3 poststimulation, and daughter cell accumulation and proliferation is best quantified approximately 5 days after T cell stimulation (Chen et al., 2003). The cells were then lysed using sterile water, and the nucleic acids were collected via glass fiber filter paper purchased from Brandel, Inc. (Gaithersburg, MD). The amount of ³H‐Thy incorporated into the DNA of proliferating cells was determined using a Wallac‐Pharmacia (PerkinElmer) liquid scintillation counter.
Spleen weights
Animals were weighed prior to being euthanized. The spleens were removed, blotted and weighed. The spleen weight was calculated as a ratio to body weight in order to compensate for difference in body mass that may affect the organ weight.
Statistical analysis
The number of animals required per test group to provide sufficient statistical power to obtain a significant effect was determined using a power analysis and SigmaStat software. Briefly, the type one error (alpha) was set to 0.05, and the desired power was set to 0.95. Values for standard deviation and the difference between treatment groups were taken from unpublished ELISpot data generated from the offspring of dams which were exposed orally to atrazine or a vehicle. The necessary sample size was determined to be 11 mice. Since the dam was the subject of atrazine exposure, each group of offspring analyzed included at least one offspring from 11 different litters. The ‘n’ used for statistical analysis was set to the number of offspring from a unique litter. In some assays, more than one mouse from a single litter was included in the graphic representation of the sample set, and if this is the case, it is noted in the figure legend. The desired sample size determined from previously generated ELISpot data was also applied to the assessment of T cell function because previous experiments did not assess T cell function. Retroactive power analysis using the standard deviations from the initial analyses determined that a sample size less than 11 could be used to establish statistical significance, and the size of the experimental group was reduced accordingly. With the exception of the assessment of T cell cytolytic function, statistical significance was determined by comparing the data of the atrazine‐treated groups against the data obtained from the control groups using a t test. Because the chromium release assays included 6 different effector‐to‐target ratios and 2 treatment groups, an analysis of variance (ANOVA) was used to test the significance of the observed changes. It is known that differences in the effector to target ratios will affect the amount of chromium released, thus our main interest in the ANOVA was the difference between the two treatment groups. In the ANOVA associated with the treatment effect, there was 1 degree of freedom, and thus, a post hoc test following the ANOVA was not necessary.
Results
Reproductive toxicity
Atrazine causes postimplantation loss of the fetus in some strains of rats and prevents proper ovulation (Cummings et al., 2000). Prior to this study, it was unknown if atrazine would interfere with the ability of the Balb/c mouse to carry a litter to term. The percent of pregnant dams was determined by comparing the number of dams birthing litters to the total number of dams matched with males. There was no significant difference between atrazine‐ and placebo‐exposed dams in the number of litters carried full‐term or the size of these litters (Fig. 1). There was also no change in the gender distribution of the litters from atrazine‐treated dams compared to placebo‐treated dams (data not shown).
Body and spleen weights
The weight and total cellularity of the spleen can sometimes provide clues about the toxic effects of chemicals. At 3 months of age, there were no significant treatment‐related differences in body weights between unvaccinated atrazine‐exposed males (25.9 ± 0.36 g) and control males (26.5 ± 0.36g). There was also no difference between the body weight of atrazine‐treated females (22.2 ± 0.24 g) and control females (21.9 ± 6.4 g) at 3 months of age. Spleen cellularity was not significantly different between atrazine‐exposed and control groups as shown in Table 1. The spleen weight of unvaccinated prenatally/lactationally exposed males or females was not significantly different when compared to control animals (data not shown). The average spleen weight for treated males was 97 ± 4 mg, and for control males, it was 102 ± 2 mg. The average spleen weight for treated females was 115 ± 2 mg, and for control females, it was 119 ± 6 mg. The spleen‐to‐body weight ratios of treated animals were not significantly different from those of controls (data not shown).
Table 1.
Effect of prenatal/lactational atrazine exposure on spleen cell phenotype at 3 months of age
| Phenotypea | Malesb |
Femalesb |
||
|---|---|---|---|---|
| Unimmunized | Immunizedc | Unimmunized | Immunizedc | |
| CD4+: Atrazine | 1.7 ± 0.2 | 1.8 ± 0.1 | 2.2 ± 0.2 | 2.5 ± 0.3 |
| CD4+: Control | 1.8 ± 0.3 | 1.6 ± 0.2 | 1.9 ± 0.3 | 2.6 ± 0.3 |
| CD8+: Atrazine | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1* | 1.2 ± 0.2 |
| CD8+: Control | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.3 ± 0.1 |
| B220+: Atrazine | 3.3 ± 0.3 | 3.8 ± 0.2 | 4.0 ± 0.3 | 4.6 ± 0.5 |
| B220+: Control | 3.8 ± 0.3 | 3.7 ± 0.5 | 3.6 ± 0.6 | 4.4 ± 0.4 |
| Totald: Atrazine | 6.8 ± 1.0 | 8.8 ± 1.6 | 11.6 ± 4.6 | 9.8 ± 1.9 |
| Totald: Control | 7.1 ± 1.1 | 8.5 ± 1.9 | 10.7 ± 3.0 | 9.7 ± 1.3 |
Spleen cells were stained with anti‐CD4 (T helper cell), anti‐CD8 (cytotoxic T cell) and anti‐B220 (B cell) and analyzed by flow cytometry.
All values are average number of cells of the indicated phenotype standardized to 107 cells and expressed with standard deviation. The data are representative of a single experiment the n of the atrazine and control groups were 14 and 16 animals respectively.
Spleen cells from animals immunized with HKSP.
Total splenocyte number includes monocytes and other cell types not enumerated through flow cytometry.
P < 0.05.
Splenocyte phenotype
To determine if prenatal/lactational exposure to atrazine altered the phenotype of splenocytes, the numbers of CD4+ T cells, CD8+ T cells and B cells were determined by flow cytometry (Table 1). Statistical comparison between immunized atrazine offspring and control offspring and between unimmunized atrazine offspring and control offspring of the same sex were performed to determine if prenatal atrazine exposure altered the spleen cell phenotypic distribution. There was no statistically significant change in the distribution of specific subpopulations for most groups; however, there was a small but statistically significant (P < 0.05) increase (a 26% increase over control animals) in the number of CD8+ T cells in unimmunized atrazine offspring females at 3 months of age (Table 1). There was no significant difference in the numbers of each phenotype in the spleens between the atrazine offspring and control offspring regardless of gender or immunization regimen (data not shown).
The B cell response
The antibody response to i.p. HKSP vaccination has previously been found to peak between 10 and 14 days (Wu et al., 2002; Wuorimaa et al., 2001). Male atrazine‐exposed offspring had 78.7 ± 4.5 HKSP‐specific IgM antibody secreting cells (ASC) per 106 B220+ cells compared to 58.6 ± 3.0 ASC per 106 B220+ cells for male control offspring. The number of responding B cells was normalized to 106 B cells to account for any variability in overall spleen size. This was a significant increase in the number of IgM secreting B cells responding to the HKSP vaccination (Fig. 2). Female atrazine offspring of the same age were found to have 51 ± 4.8 ASC per 106 B220+ cells compared to 67 ± 8.5 ASC per 106 B220+ cells for female control offspring (Fig. 2). This difference was not significantly different. The B cell response of age‐matched controls was not significantly different from placebo‐exposed progeny (data not shown). Phosphorylcholine is the dominant polysaccharide antigen in the HKSP vaccine. Therefore, we also performed the ELISpot assay specific for the anti‐PC responding B cells on a separate group of mice bred and dosed under the same conditions as those discussed above. Following an HKSP vaccination, we observed a B cell response to PC analogous to that observed using a total HKSP‐specific ELISpot assay.
Fig. 2.
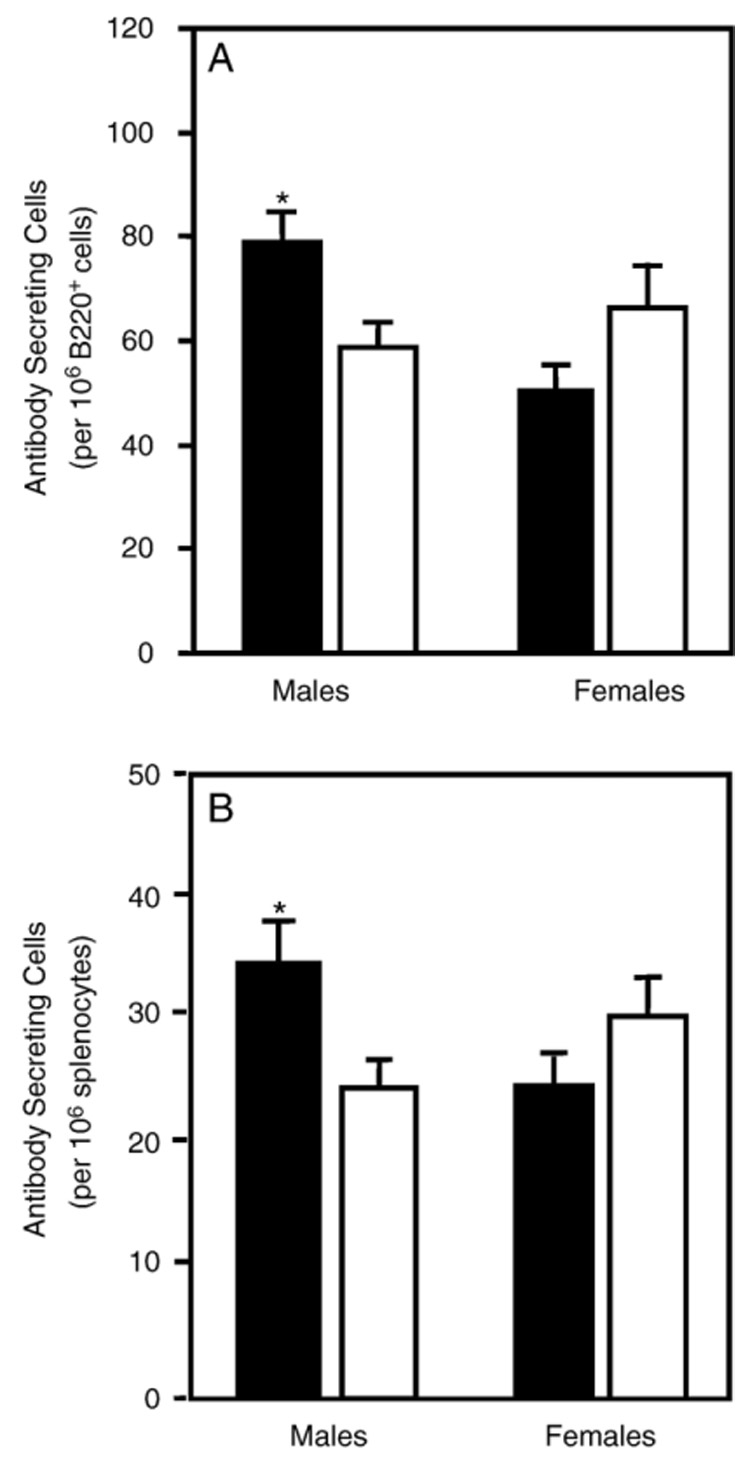
Number of anti‐HKSP antibody secreting splenocytes in 3‐month‐old offspring treated prenatally/lactationally with atrazine (solid bars) or placebo (open bars). The spleens of animals were removed 14 days after immunization and the number of HKSP‐specific B cells quantified by the ELISpot assay. The data are presented as the number of antibody secreting cells (spots) normalized to 106 B220+ cells (A; enumerated by flow cytometry) and the number of antibody secreting cells normalized to 106 splenocytes (B) for each sex. Error bars represent the SEM; *indicates that the values obtained for atrazine offspring were significantly (P < 0.05) different from those obtained for control offspring. The data are representative from 1 of 2 experiments (male treated, n = 14 representing offspring from 13 different dams; male controls, n = 16 representing offspring from 12 different dams; female treated, n = 14 from 12 different dams; female controls n = 15 from 14 different dams).
Antibody ELISAs were also preformed using serum samples collected from HKSP vaccinated mice. Serum antibody titers to HKSP, pneumococcal surface protein A (PspA, the dominant T‐dependent antigen) and PC were determined by ELISA at 7 and 14 days following immunization. Serum antibody titers of atrazine‐treated offspring were not significantly different from the control offspring or provided equivocal results, i.e., one experimental group of offspring showed a significant difference, while in the other experimental group, there was no statistically significant difference.
Cell‐mediated response
To test the effect of atrazine on the cell‐mediated immune response of the progeny, the ability of splenocytes from atrazine‐ and placebo‐exposed mice to respond to an allogeneic stimulation was assessed using two different assays. Splenocytes were isolated from unimmunized prenatally/lactationally exposed Balb/c mice and stimulated using irradiated splenocytes from female C57Bl/6 mice. The ability of these alloreactively stimulated lymphocytes to proliferate was assessed by measuring the incorporation of ³H‐thymidine into newly synthesized DNA following a 96‐h co‐culture with alloreactive stimulator cells and an 18‐h incubation with ³H‐thymidine, as previously described. The ³H‐thymidine incorporation increases observed at time points after 72 h of MLR are largely due to the result of CD4+ T cell proliferation (Cantor and Boyse, 1975; Mason and Simmonds, 1988). In this assay, the male atrazine offspring T cell proliferation was significantly increased compared to control offspring (atrazine offspring 67 ± 5 × 10³ cpm, control offspring 46 ± 4 × 10³ cpm) (Fig. 3). There was no significant difference in the allogeneic‐induced cell proliferation in female mice which had been exposed to atrazine (91 ± 14 × 10³ cpm) compared to control mice (109 ± 7 × 10³ cpm) (Fig. 3).
Fig. 3.
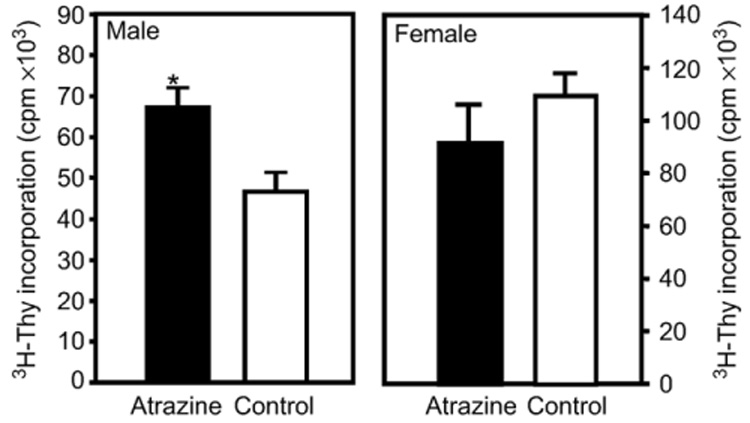
The mixed lymphocyte reaction in 3‐month‐old offspring. Splenocytes harvested from 3‐month‐old offspring of dams that were treated with atrazine or placebo as described in the Methods section were stimulated by co‐culturing with irradiated C57Bl/6 splenocytes. Four days after allogeneic stimulation, the cells from co‐cultures were extracted washed in cRPMI and the level of ³H‐Thy quantified by liquid scintillation methods. Error bars represent the SEM; *indicates that the values obtained for atrazine offspring were significantly (P < 0.05) different from those obtained for control offspring. Data representative of 2 experiments (male treated, n = 14 comprised of offspring from 13 different dams; male control, n = 16 comprised of offspring from 12 different dams; female treated, n = 6 comprised of offspring from 6 different dams; female control, n = 6 comprised of offspring from 6 different dams).
The ability of the T cells to lyse alloreactive target cells, i.e., the CTL response, was also assessed following alloreactive stimulation. These data were statistically analyzed by comparing the lytic curves between the atrazine and control offspring by ANOVA. There was a significant increase in killing capacity of the splenocytes from atrazine offspring as compared to the control offspring (Fig. 4). The CTL response of controls and atrazine‐treated animals was assessed by comparing the difference in the number of effectors required to cause a set percentage of lysed targets, as described previously (Bevan and Hunig, 1981; Sheil et al., 1987). Thirty percent lysis was chosen because a valid E∶T ratio could be determined for both the atrazine and control offspring at this value. At 30% specific lysis, the control offspring required ≈25 effectors per target, while only ≈6 effectors from the atrazine offspring per target were required to cause 30% specific lysis (Fig. 4). There was no significant difference in the 3‐month‐old female mice (data not shown).
Fig. 4.
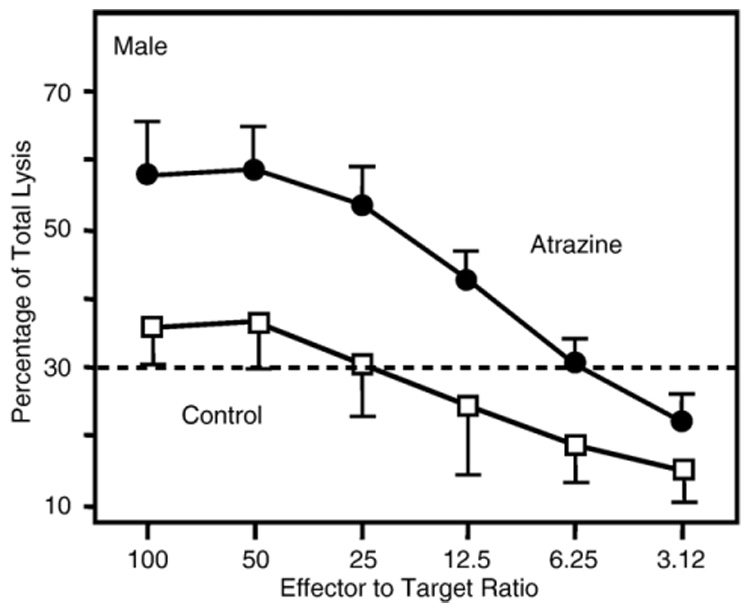
Cytotoxic lymphocyte response in 3‐month‐old offspring. Splenocytes harvested from 3‐month‐old offspring of dams that were treated with atrazine as described in the Methods were stimulated by co‐culturing with irradiated C57Bl/ 6 splenocytes. The effector and stimulator cells were mixed at a one to one (1∶1) ratio and then incubated for 5 days. After 5 days, specific lysis was determined by incubating the stimulated effector cells with 51Cr‐labeled EL‐4 target cells at ratios varying from 100∶1 to 3.12∶1 effector:target cell for 4 h and then assaying the supernatant for 51Cr‐released. Error bars represent the SEM; ANOVA indicates that the values obtained for atrazine offspring were significantly (P < 0.05) different from those obtained for control offspring (atrazine treated, n = 14 comprised of offspring from 13 different dams; control, n = 16 comprised of offspring from 12 different dams).
Discussion
Atrazine is the second most heavily used herbicide in the United States (http://www.epa.gov/oppbead1/pestsales/01pestsales/usage2001_2.html#3_6) and the most common groundwater contaminant (Koskinen and Clay, 1997). Mammalian atrazine exposure in animal models results in reproductive and immune system toxicity (Cooper et al., 1996; Fournier et al., 1992; Karrow et al., 2005; Rooney et al., 2003). The present study investigated whether prenatal/lactational exposure to atrazine results in a persistent change of the immune response or an immunoteratological effect in mice. Evidence generated using the Balb/c mouse model indicates that prenatal/lactational exposure to atrazine altered the functional immune response of adult males that were approximately 3 months of age. Environmental atrazine could affect the fetus either through drinking water, occupational or a combination of maternal exposure modalities. The purpose of the present study, however, was to expose the fetus to atrazine via the mother and not to address different routes of maternal exposure as factors affecting offspring development.
This exposure regiment caused no overt physical malformations in the offspring and had no effect on the number of litters carried to term or the litter size (Fig. 1). In the offspring, no changes in body weight, the spleen to body weight ratio or gross changes in the cell subpopulation phenotypes of the spleen were observed. A small but significant change in the CD8+ subpopulation in the spleens of unimmunized females was observed, but it was not associated with any observed change in immune function. Functional immune assays demonstrated increases in the magnitude of the immune response of prenatally/lactational‐exposed male animals following stimulation of immune effector cells. Specifically, the number of antigen‐specific IgM splenic ASC was increased in prenatally atrazine male mice compared to control animals following immunization with HKSP (Fig. 2). However, serum levels of anti‐HKSP IgM antibodies at day 7 or 14 following immunization did not show a significant change between control and atrazine‐treated offspring regardless of gender. Intuitively one would expect a correlation between the number of IgM producing HKSP‐specific B cells responding to immunization in the spleen and the amount of HKSP‐specific IgM antibody in the serum. This was not the case. A possible explanation for this includes that an enumeration of ASCs via ELISpot does not measure the quantity of antibody produced by each cell. It is conceivable that the amount of antibody produced by each responding cell was decreased following a prenatal/lactational atrazine exposure. Also, it has been recently demonstrated that the number of specific ASCs in the bone marrow, but not in the spleen, correlates more strongly with the serum antibody titers (Salazar et al., 2005), and bone marrow ASCs were not enumerated in our study.
The CD4+ T cell proliferation response to in vitro allogeneic stimulation was increased (Fig. 3), as was the CD8+ T cell cytotoxic potential of male mouse splenocytes following in vitro allogeneic stimulation (Fig. 4). The proliferation and function of HKSP antigen‐specific CD4+ and CD8+ T cells were not directly assessed in vaccinated animals. It is possible that the increased CD8+ cytotoxicity occurred without a concomitant increase in the number of CD8+ T cell splenocytes, and the CD8+ T cells from prenatally/lactationally exposed animals were either more efficient killers or that a greater number of CD8+ cells were activated following alloreactive stimulation than those from control animals.
While both CD8+ and CD4+ T cells are stimulated via alloreactive major histocompatibility complex (MHC) I and the MHC II molecules respectively, the CD4+ T cells proliferate more vigorously in response to nonspecific TCR‐MHC interactions (Cantor and Boyse, 1975; Mason and Simmonds, 1988; Popma et al., 2000). Therefore, our data would suggest that prenatal/lactational atrazine exposure affects CD4+ T cell function. CD4+ T cell support is also a potent modulator of the CD8+ lytic response and the B cell response (Cantor and Boyse, 1975; Romagnani et al., 1985). Thus, the effect observed in all three assays could be the result of an effect on CD4+ T cells alone or a direct effect on multiple cell types.
Rooney et al. (2003) reported a gender‐specific decrease in primary anti‐sheep erythrocyte antibody production and delayed‐type hypersensitivity induction in male Sprague–Dawley rats following a maternal exposure to atrazine. Our study has addressed the effects of prenatal/lactational atrazine exposure on development of the murine immune system using a similar but not identical experimental design. The dosing regimes of the two experimental models differ in several noteworthy respects. First, atrazine administration in the Rooney et al. (2003) study was preformed orally via a daily gavaging of the animals. This allowed for adjustment of the dose to maintain a consistent dose to body weight ratio. Our study used a time release pellet, and thus, the dose to body weight ratio fluctuated with changes in the dams’ body weight. Second, in the Rooney et al. (2003) study, dams were dosed from day 10 of gestation until the pups were weaned. In our study, the dose provided by the time release pellet was exhausted 21 days following insertion, at day 10 of lactation. The extended maternal exposure during the suckling period in the Rooney et al. (2003) study may have affected some later developmental event that was unaffected by our dosing paradigm.
The two studies also employed different animal models, and the active immune response was elicited using different antigens. Rooney et al. (2003) employed the Sprague–Dawley rat model and elicited an immune response using sheep red blood cells. Our study employed the Balb/c mouse, and we used the HKSP vaccine to elicit an active immune response. In addition to the obvious differences in animal species, the Balb/c is purported to have a characteristic cytokine response which biases the immune function towards a Th2 type response (Boom et al., 1990). However, neither study investigated cytokine profiles of stimulated effector cells or the IgG subtypes produced following immunization, and thus, no conclusions can be drawn about the effects of prenatal/lactational atrazine exposure on an animal with this reported Th2 bias. Since the studies also differed in the antigens used, the type of antigenic stimulation may also explain why the observed effects on antibody production differed between the two studies.
It is also not clear whether the effects reported by either study were mediated by either a direct effect of atrazine on the developing fetus or a postnatal effect due an alteration in the milk composition or a combination of the two. The possibility of a direct effect due to atrazine being passed via lactations does not appear to be a likely explanation for at least two reasons. One, recent attempts to screen for the transfer of atrazine to the breast milk have met with limited success, finding the level of atrazine equal to or below the limits of detection and quantification limits (Balduini et al., 2003). Two, atrazine is known to induce aromatase (CYP19) (Sanderson et al., 2000, 2001), and it would be expected that the presence of atrazine in the dam milk would cause an increase in CYP19 levels in the offspring. However, tissue from Long–Evans rats suckled by dams receiving 100 mg/kg of atrazine had a decrease in the level of CYP19 mRNA as compared to controls (Rayner et al., 2004). These data suggest that atrazine is not transferred to the offspring via the milk.
Data suggesting that the atrazine‐induced effect may be related to changes in milk composition (other than the presence of atrazine) come from multiple sources (Rayner et al., 2004; Stoker et al., 1999). A possible candidate for a milk‐borne factor that could modulate the immune response is prolactin, a potent cytokine. Stoker et al. (1999) reported that the atrazine induced an inhibition of the suckling‐induced prolactin surge, and that this had a long‐term effect on the inflammatory response in the male offspring. However, Rooney et al. (2003) tested this hypothesis by administering bromocryptine, a prolactin inhibitor, to inhibit the lactational prolactin surge and observed that an inhibition of the prolactin surge by bromocryptine did not mimic the effects of a 35 mg/kg/day pre‐ and postnatal oral dose of atrazine. Thus, the identity of any change(s) in the milk composition remains elusive.
Interpretation of the data described herein indicates a male‐specific immunopotentiation of the immune response following prenatal/lactational atrazine exposure. These data and the interpretation differ from previous work by Rooney et al. (2003) which approached the problems of maternal atrazine exposure using a rat model and oral exposure and found an immunosuppressive effect. Inasmuch as atrazine is a widespread environmental contaminant, immunopotentiation also raises concerns. Such an effect may potentiate clinical diseases, such as allergies and autoimmune disease, and need to be carefully monitored and studied.
Acknowledgments
The authors would like to acknowledge and thank Cheryl Walton for her technical assistance and Yan Ma and the West Virginia University Department of Statistics for their assistance with the statistical analysis. We would also like to acknowledge the technical support of Dr. C. Cunningham and the West Virginia University Health Sciences Center Flow Cytometry Core Facility. We thank Dr. Robert Luebke for reviewing the manuscript and providing constructive feedback on its content and style. This study was supported by NIOSH grant (OH07686) and NIH grants (RR16440 [flow cytometry] and ES010953 [JBB]).
References
- Adgate JL, Barr DB, Clayton CA, Eberly LE, Freeman NC, Lioy PJ, Needham LL, Pellizzari ED, Quackenboss JJ, Roy A, Sexton K. Measurement of children’s exposure to pesticides: analysis of urinary metabolite levels in a probability‐based sample. Environ. Health Perspect. 2001;109(6):583–590. doi: 10.1289/ehp.01109583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduini L, Matoga M, Cavalli E, Seilles E, Riethmuller D, Thomassin M, Guillaume YC. Triazinic herbicide determination by gas chromatography‐mass spectrometry in breast milk. J. Chromatogr., B. Analyt. Technol. Biomed. Life Sci. 2003;794:389–395. doi: 10.1016/s1570-0232(03)00455-0. [DOI] [PubMed] [Google Scholar]
- Bevan MJ, Hunig T. T cells respond preferentially to antigens that are similar to self H‐2. Proc. Natl. Acad. Sci. U. S. A. 1981;78:1843–1847. doi: 10.1073/pnas.78.3.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock BL, Holladay SD, Comment CE, Heindel JJ, Luster MI. Exposure to tetrachlorodibenzo‐p‐dioxin (TCDD) alters fetal thymocyte maturation. Toxicol. Appl. Pharmacol. 1992;112:207–213. doi: 10.1016/0041-008x(92)90189-y. [DOI] [PubMed] [Google Scholar]
- Boom WH, Liebster L, Abbas AK, Titus RG. Patterns of cytokine secretion in murine leishmaniasis: correlation with disease progression or resolution. Infect. Immun. 1990;58:3863–3870. doi: 10.1128/iai.58.12.3863-3870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H, Boyse EA. Functional subclasses of T‐lymphocytes bearing different Ly antigens: I. The generation of functionally distinct T‐cell subclasses is a differentiative process independent of antigen. J. Exp. Med. 1975;141:1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Chang ML, Muench MO. A kinetic study of the murine mixed lymphocyte reaction by 5,6‐carboxyfluorescein diacetate succinimidyl ester labeling. J. Immunol. Methods. 2003;279:123–133. doi: 10.1016/s0022-1759(03)00236-9. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Goldman JM, Parrish MB, Tyrey L. Effect of atrazine on ovarian function in the rat. Reprod. Toxicol. 1996;10:257–264. doi: 10.1016/0890-6238(96)00054-8. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Rhodes BE, Cooper RL. Effect of atrazine on implantation and early pregnancy in 4 strains of rats. Toxicol. Sci. 2000;58:135–143. doi: 10.1093/toxsci/58.1.135. [DOI] [PubMed] [Google Scholar]
- EPA‐Enviornmental Protection Agency. Interim Reregistration Eligibility Decision for Atrazine. Washington, DC: 2003. [Google Scholar]
- Filipov NM, Pinchuk LM, Boyd BL, Crittenden PL. Immunotoxic effects of short‐term atrazine exposure in young male C57BL/6 mice. Toxicol. Sci. 2005;86:324–332. doi: 10.1093/toxsci/kfi188. [DOI] [PubMed] [Google Scholar]
- Fournier M, Friborg J, Girard D, Mansour S, Krzystyniak K. Limited immunotoxic potential of technical formulation of the herbicide atrazine (AAtrex) in mice. Toxicol. Lett. 1992;60:263–274. doi: 10.1016/0378-4274(92)90284-q. [DOI] [PubMed] [Google Scholar]
- Gerlo S, Verdood P, Hooghe‐Peters EL, Kooijman R. Modulation of prolactin expression in human T lymphocytes by cytokines. J. Neuroimmunol. 2005;162:190–193. doi: 10.1016/j.jneuroim.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Deddens JA, Striley CA, Biagini RE, Shoemaker DA, Brown KK, Mackenzie BA, Hull RD. Biological monitoring for selected herbicide biomarkers in the urine of exposed custom applicators: application of mixed‐effect models. Ann. Occup. Hyg. 2003;47:503–517. doi: 10.1093/annhyg/meg067. [DOI] [PubMed] [Google Scholar]
- Holladay SD. Prenatal immunotoxicant exposure and postnatal autoimmune disease. Environ. Health Perspect. 1999;107 Suppl. 5:687–691. doi: 10.1289/ehp.99107s5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrow NA, McCay JA, Brown RD, Musgrove DL, Guo TL, Germolec DR, White KL., Jr Oral exposure to atrazine modulates cell‐mediated immune function and decreases host resistance to the B16F10 tumor model in female B6C3F1 mice. Toxicology. 2005;209:15–28. doi: 10.1016/j.tox.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Koskinen WC, Clay SA. Factors affecting atrazine fate in north central U.S. soils. Rev. Environ. Contam. Toxicol. 1997;151:117–165. doi: 10.1007/978-1-4612-1958-3_4. [DOI] [PubMed] [Google Scholar]
- Laws SC, Ferrell JM, Stoker TE, Cooper RL. Pubertal development in female Wistar rats following exposure to propazine and atrazine biotransformation by‐products, diamino‐S‐chlorotriazine and hydroxyatrazine. Toxicol. Sci. 2003;76:190–200. doi: 10.1093/toxsci/kfg223. [DOI] [PubMed] [Google Scholar]
- Mason DW, Simmonds SJ. The autonomy of CD8+T cells in vitro and in vivo. Immunology. 1988;65:249–257. [PMC free article] [PubMed] [Google Scholar]
- Mencoboni M, Lerza R, Bogliolo G, Flego G, Pannacciulli I. Effect of atrazine on hemopoietic system. In Vivo. 1992;6:41–44. [PubMed] [Google Scholar]
- Perry M, Christiani D, Dagenhart D, Tortorelli J, Singzoni B. Urinary biomarkers of atrazine exposure among farm pesticide applicators. Ann. Epidemiol. 2000;10:479. doi: 10.1016/s1047-2797(00)00150-2. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Christiani DC, Mathew J, Degenhardt D, Tortorelli J, Strauss J, Sonzogni WC. Urinalysis of atrazine exposure in farm pesticide applicators. Toxicol. Ind. Health. 2001;16:285–290. doi: 10.1177/074823370001600705. [DOI] [PubMed] [Google Scholar]
- Popma SH, Krasinskas AM, McLean AD, Szeto WY, Kreisel D, Moore JS, Rosengard BR. Immune monitoring in xenotransplantation: the multiparameter flow cytometric mixed lymphocyte culture assay. Cytometry. 2000;42:277–283. doi: 10.1002/1097-0320(20001015)42:5<277::aid-cyto4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Rayner JL, Wood C, Fenton SE. Exposure parameters necessary for delayed puberty and mammary gland development in Long–Evans rats exposed in utero to atrazine. Toxicol. Appl. Pharmacol. 2004;195:23–34. doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Romagnani S, Giudizi GM, Maggi E, Almerigogna F, Biagiotti R, Del PG, Mazzetti M, Alessi A, Vercelli D, Ricci M. Synergy of B cell growth factor and interleukin 2 in the proliferation of activated human B cells. Eur. J. Immunol. 1985;15:1158–1164. doi: 10.1002/eji.1830151203. [DOI] [PubMed] [Google Scholar]
- Rooney AA, Matulka RA, Luebke RW. Developmental atrazine exposure suppresses immune function in male, but not female Sprague–Dawley rats. Toxicol. Sci. 2003;76:366–375. doi: 10.1093/toxsci/kfg250. [DOI] [PubMed] [Google Scholar]
- Salazar KD, de la RP, Barnett JB, Schafer R. The polysaccharide antibody response after Streptococcus pneumoniae vaccination is differentially enhanced or suppressed by 3,4‐dichloropropionanilide and 2,4‐dichlorophenoxyacetic acid. Toxicol. Sci. 2005;87:123–133. doi: 10.1093/toxsci/kfi244. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Seinen W, Giesy JP, van den BM. 2‐Chloro‐s‐triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol. Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, van den BM. Effects of chloro‐s‐triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ. Health Perspect. 2001;109:1027–1031. doi: 10.1289/ehp.011091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheil JM, Bevan MJ, Lefrancois L. Characterization of dual‐reactive H‐2Kb‐restricted anti‐vesicular stomatitus virus and alloreactive cytotoxic T cells. J. Immunol. 1987;138:3654–3660. [PubMed] [Google Scholar]
- Short P, Colborn T. Pesticide use in the U.S. and policy implications: a focus on herbicides. Toxicol. Ind. Health. 1999;15:240–275. doi: 10.1191/074823399678846736. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Robinette CL, Cooper RL. Maternal exposure to atrazine during lactation suppresses suckling‐induced prolactin release and results in prostatitis in the adult offspring. Toxicol. Sci. 1999;52:68–79. doi: 10.1093/toxsci/52.1.68. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Guidici DL, Laws SC, Cooper RL. The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol. Sci. 2002;67:198–206. doi: 10.1093/toxsci/67.2.198. [DOI] [PubMed] [Google Scholar]
- Sun R, Li AL, Wei HM, Tian ZG. Expression of prolactin receptor and response to prolactin stimulation of human NK cell lines. Cell Res. 2004;14:67–73. doi: 10.1038/sj.cr.7290204. [DOI] [PubMed] [Google Scholar]
- Theus SA, Tabor DR, Soderberg LS, Barnett JB. Macrophage tumoricidal mechanisms are selectively altered by prenatal chlordane exposure. Agents Actions. 1992;37:140–146. doi: 10.1007/BF01987903. [DOI] [PubMed] [Google Scholar]
- Wu ZQ, Shen Y, Khan AQ, Chu CL, Riese R, Chapman HA, Kanagawa O, Snapper CM. The mechanism underlying T cell help for induction of an antigen‐specific in vivo humoral immune response to intact Streptococcus pneumoniae is dependent on the type of antigen. J. Immunol. 2002;168:5551–5557. doi: 10.4049/jimmunol.168.11.5551. [DOI] [PubMed] [Google Scholar]
- Wuorimaa T, Kayhty H, Eskola J, Bloigu A, Leroy O, Surcel HM. Activation of cell‐mediated immunity following immunization with pneumococcal conjugate or polysaccharide vaccine. Scand. J. Immunol. 2001;53:422–428. doi: 10.1046/j.1365-3083.2001.00882.x. [DOI] [PubMed] [Google Scholar]


