Abstract
Tissue remodeling following injury involves TGF-β-mediated fibroblast contraction. While these cells are embedded in a fibronectin (FN)-rich matrix, the role of FN-cell interactions in this process is not fully understood. To explore the role of FN matrix presentation, we analyzed the effect of TGF-β on fibroblasts adhered to FN-coated polyacrylamide gel (PAG). Surprisingly, under these conditions TGF-β triggered cell rounding/contraction. This was accompanied by increased Rho activation and MLCK phosphorylation and was reversed by inhibition of Rho kinase. Although fibroblasts are known to bind to fibronectin’s RGD and synergy sites, their relative contribution to cell function is not clear. MLC phosphorylation was reduced and cell contraction was reversed when FN’s synergy site was blocked, indicating that contraction requires signals from the synergy site in addition to TGF-β mediated Rho activation. Thus, regulating the FN synergy site therapeutically may provide a mechanism for modulating contractile forces during tissue repair.
Keywords: Myofibroblast, fibronectin, TGF-β, contractility, wound healing, Rho GTPase, extracellular matrix
Introduction
TGF-β promotes myofibroblast generation and regulates extracellular matrix (ECM) production during wound healing. However, it is unclear how signals from this growth factor are integrated with signals propagated from the ECM to alter myofibroblast contraction during wound healing [1]. The ECM provides a structural, chemical, and mechanical substrate that is essential in development, growth, and the host response to pathogenic signals. Transmembrane receptors termed integrins, that bind ECM components, provide a mechanism for integrating the dynamic interaction of environmental cues with intracellular events, orchestrating functions including organogenesis, regulation of gene expression, cell proliferation, differentiation, migration, and death. [2]. Integrins are noncovalently associated heterodimeric transmembrane receptors composed of α and β subunits, with α subunits ranging from 120 to 180kDa, whereas β subunits are 90 to 110 kD. Integrin subunits consist of a large extracellular domain (700 to1100 aa), a single transmembrane segment, and most have short cytoplasmic tails, ranging from 20 to 60 aa that mediate intracellular signaling [3]. ECM proteins ligated by integrins transduce extracellular signals to intracellular networks that modulate cell behaviors.
Fibronectin (FN) is a multifunctional ECM protein that is ubiquitously expressed during embryogenesis and in healing wounds, tumors and inflammatory sites. Because it binds several ligands (e.g. cells, collagen, fibrin, heparin) and self-assembles to form fibrils, FN is thought to provide an extracellular scaffold supporting a network of interactions as well as subserving important structural roles in remodeling tissues [4]. During wound healing, plasma FN (pFN) is deposited abundantly as a consequence of blood plasma extravasation [5,6]. In addition, alternatively spliced forms of “cellular” FN, produced by wound fibroblasts, are deposited in forming granulation tissue [7–9]. The central portion of FN is comprised of structural motifs, termed FN Type III repeats. Among these are the cell-binding domain comprised of the synergy site in III8,9 and the RGD in III10 which function together to mediate cell adhesion [4]. While the synergy site contributes to cell adhesion via integrins (i.e., α5β1), variations in the presentation of the synergy site may modulate cellular functions, integrin usage and cell migration [10,11].
In the work reported here, we tested the impact of the FN on cell contraction using a polyacrylamide gels (PAG) system to which pFN was coupled but on which extensive FN matrix assembly did not occur. We observed that fibroblasts spread on all ECM-PAGs tested in the absence of TGF-β. Surprisingly, fibroblasts on pFN-PAGs, but not gelatin- or collagen-PAGs, rounded markedly in the presence of TGF-β. The rounding was reversible with a Rho kinase inhibitor (Y-27632), was accompanied by Rho activation and myosin light chain (MLC) phosphorylation, was reversed by removal of TGF-β and was not attributable to cell death. When PAGs were made with a recombinant FN fragment that includes the synergy site (III8,9,10 ), fibroblasts contracted. When an anti-synergy site Mab was added to pFN-PAGs in the presence of TGF-β, rounding was inhibited. We conclude that our work has revealed a novel role for the synergy site within FN in which it modulates TGF-β-mediated Rho activation and the contractile state of fibroblasts.
Materials and methods
Reagents
Unless otherwise indicated, all reagents were from Sigma Chemical Co. (St Louis, MO). Others were purchased as follows: fetal bovine serum (FBS, Hyclone: Logan, UT), human pFN (Molecular Innovations: Southfield, MI), Type I collagen (Vitrogen Cohesion: Palo Alto, CA), recombinant human TGF-β (R&D Systems: Minneapolis, MN), protease inhibitor cocktail (Roche: Mannheim, Germany), monoclonal anti-ERK1/2 (Upstate: Charlottesville, VA), monoclonal anti-synergy site (HFN7.1, Neomarkers: Fremont, CA), , polyclonal anti-MLC (Cell Signaling Technology: Beverly, MA), polyclonal anti-Rho (Santa Cruz: Santa Cruz, CA), HRP- secondary antibodies (Calbiochem: San Diego, CA). Alexa-Fluor 594- phalloidin and Hoechst 33342 (Molecular Probes: Eugene, OR), Sulfo-succinimidyl 6-(4′-azido-2′-nitrophenylamino) Hexanoate (Sulfo-SANPAH) and SuperSignal West Pico (Pierce: Rockford, Il), Y27632 (Chemicon: Temecula, CA), Dulbecco’s Minimal Essential Medium (DMEM), penicillin-streptomycin, Dulbecco’s Phosphate Buffered Saline (DPBS), L-glutamine, and Trypsin-EDTA (Gibco-Invitrogen: Carlsbad, CA). Recombinant GST-tagged Rhotekin was a gift from Dr. Martin Schwartz (University of Virginia, Charlottesville, VA). Recombinant GST-FN10 and GST-FNIII8,9,10 were prepared as described previously [12,13]. Polyclonal anti-phospho-Ser19/Thr18 MLC was a gift from Dr. Peter Vincent (Albany Medical College, Albany, NY)
Cell culture
Human foreskin fibroblasts (HFF) and other human fibroblasts were grown in Dulbecco’s Minimal Essential Medium supplemented with 10% FBS, and 100 U/mL penicillin-streptomycin. All experiments were performed in modified serum free media (DMEM supplemented with Hams F12, 0.5 μM insulin, 5 μg/mL transferrin, 2 mM L-glutamine, 0.2 mM ascorbate, and 100 U/mL penicillin-streptomycin). For Rho activity assays, cells were serum starved for 24 h prior to the day of the experiment and cells were cultured in serum free media.
Polyacrylamide gel (PAG) substrate preparation
Flexible PAG substrates with a thickness of 70 μm were prepared on 25-mm square glass coverslips and derivitized with either 10 μg/mL pFN or gelatin using Sulfo-SANPAH exactly as described by Pelham and Wang [14]. Routinely, substrates used in this study were 8% acrylamide/0.2% N,N-methylene-bis-acrylamide with a measured Young’s modulus of ~22 kPa. HFF-2s were cultured in serum free media in the presence or absence of 10ng/mL TGF-β and at designated times analyzed for morphology, extracted for analysis of Rho activity or myosin light chain phosphorylation. For some experiments, soluble pFN, either pre-treated or not, with Sulfo-SANPAH in solution was non-covalently bound (1 h, 37°C) to gelatin-PAG. In some experiments, pFN-PAGs were pre-incubated with mAb HFN7.1 (10 μg/mL; 37°C, 5% CO2, 1 h) in serum free media. Cells were then plated on HFN7.1 blocked pFN-PAGs with or without TGF-β as indicated.
Immunofluorescence and microscopy
Cells were washed with DPBS, fixed with 4% paraformaldehyde/PDBS, permeabilized with 0.5% Triton X-100, and blocked with 2% BSA for 1 h. Cells were stained for F-actin (Phalloidin) or nuclei (Hoechst 33342). Photographs were taken using phase optics or epifluorescence illumination on a Nikon Eclipse TE2000 U using a SPOT camera (Diagnostic Instruments, Sterling Heights, MI).
Cell adhesion assay
Wells of 96-well flat-bottomed microtiter plates were coated (10 μg/mL, 4°C, overnight) with either BSA, soluble pFN or with gelatin (10 μg/ml in PBS) to which soluble pFN was non-covalently bound (1 h, 37°C). Wells were washed (PBS), blocked (3% BSA/PBS, room temperature, 15 min) and a cell suspension (Hoechst 33342-stained HFF, 15 min) in serum-free DMEM was added (200 μl/well of 50,000 cells/ml). Plates were centrifuged (top side up) at 10 x g for 5 min, incubated (30 min, 37°C). Non-adherent cells were removed by centrifugation (top side down) at 50 x g for 5 min, followed by three rinses with DPBS. Cell adhesion was quantified using a SpectraMax Gemini EM microplate fluorescence plate reader (Molecular Devices, Sunnyvale, CA) using 360 nm excitation/480 nm emission filters.
Rho pull-down assay
Rho activation assays were performed as described [15]. In brief, cells were washed with ice-cold PBS, promptly treated with ice cold lysis buffer (50 mM Tris-HCl pH7.5, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, 1 mM NaF, 1 mM sodium orthovanadate, and protease inhibitor cocktail), rapidly lysed and centrifuged at 13,000 rpm for 2 min at 4°C and the supernatants were applied to immobilized Rhotekin-agarose (45 min, 4°C). The beads were washed with ice cold wash buffer (50 mM Tris-HCl pH7.5, 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 1 mM NaF, 1 mM sodium orthovanadate, and protease cocktail inhibitors) and taken up in SDS-PAGE sample buffer. SDS-PAGE and western blotting were employed to quantitate active Rho (Rhotekin-bound) and total Rho from a normalized volume of cell lysate. Blots were treated successively with anti-Rho antibody, HRP-conjugated secondary antibodies and SuperSignal West Pico chemi-luminescent substrate. Rho activity was expressed as a percentage of total Rho with bound GTP, was determined by quantitating the amount of Rhotekin-bound Rho versus the total Rho in each lysate.
Myosin light chain phosphorylation assay
Cells treated with the indicated conditions were lysed in 200 μl of boiling-hot Laemmli sample buffer supplemented with 1 mM each of NaF and sodium orthovanadate and subjected to SDS-PAGE and western blotting (PVDF membrane). Duplicate blots were treated successively with either phospho-Ser19/Thr18 MLC antibody or a non-phosphospecific MLC antibody, an appropriate HRP-conjugated secondary antibody, followed by SuperSignal West Pico Chemiluminescent substrate.
Results and Discussion
We sought to develop a system for analyzing the impact of the cell-binding domain of FN on fibroblast contraction in the absence of extensive FN matrix assembly. We employed a well-characterized system in which various ECM molecules are coupled covalently to polyacrylamide gels (PAG, [14]). Human fibroblasts (i.e., adult dermal, fetal lung and neonatal foreskin) spread normally within 2 h on gelatin-PAG (not shown), collagen-PAG (not shown) or pFN-PAG (Fig. 1A). We also observed comparable adhesion of human fibroblasts to FN-derivitized surfaces alone or on FN-gelatin-derivitized surfaces (Fig. 1B). Importantly, we also observed that fibroblasts which spread on pFN-PAG elaborated a dramatically reduced FN matrix compared to those on pFN-glass (Fig. 1C, D).
Fig. 1.
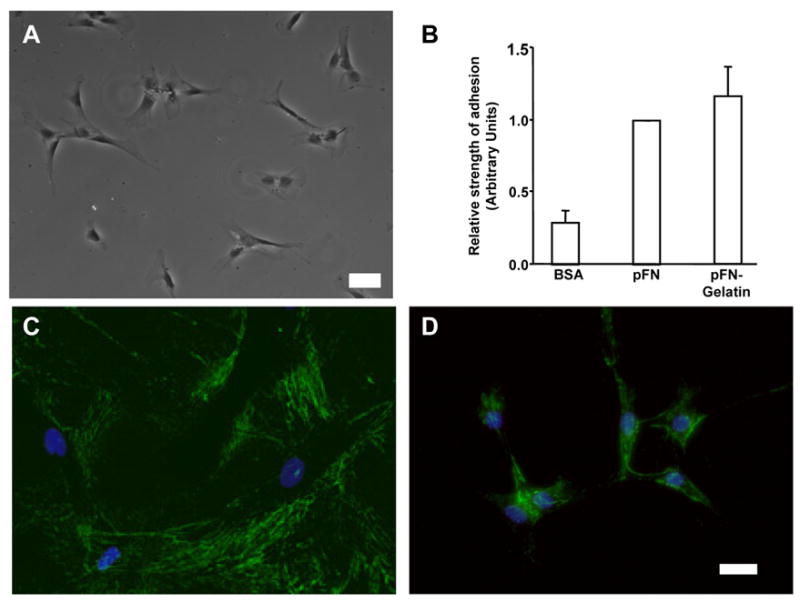
FN matrix assembly, cell morphology and adhesion. A - Phase contrast image of cells on FN-PAG in the absence of TGF-β (Bar: 100 μm), showing morphology of typical of fibroblasts on ECM-PAG. B - Adhesion assay using HFFs cells. Wells were coated with respective proteins (10 μg/ml) and cell adhesion performed using centrifugation (50 x g, 5 min) to remove cells. Data are shown as the mean ± SE from six independent experiments. Adhesion strength is comparable on pFN, or pFN-gelatin coated surfaces. C, D - HFFs cultured (1 day) on pFN-coverslips (C) or pFN-PAGs (D) were stained for FN by indirect immunofluorescence. Note that significantly less FN is present in assembled matrix on pFN-PAG than on pFN-glass. Bar: 25 μm.
TGF-β induces fibroblasts on pFN-PAG, but not gelatin- or collagen-PAG, to round
We next tested how fibroblasts on pFN-PAG responded to treatment with TGF-β, an important signal for inducing fibroblasts contraction. When TGF-β was included in cultures of primary human fibroblasts (i.e., adult dermal, fetal lung, foreskin) on pFN-PAG, cells initially spread (Fig. 2B) but rounding commenced within 6–15 h (Fig. 2) and was accompanied by dense phalloidin staining (Fig. 2F, inset). Fibroblasts cultured with TGF-β on either collagen-PAG or gelatin-PAG remained spread (not shown). In contrast to cells on pFN-PAG, fibroblasts seeded on coverslips cross-linked either with collagen, gelatin, or pFN, did not round in the presence or absence of TGF-β (not shown). Although in the foregoing experiments, TGF-β was added when cultures were initiated, we also observed that fibroblast rounding occurred when TGF-β was added up to 4 days after establishing cultures on pFN-PAG and could be reversed up to 3 days post treatment by the removal of TGF-β. TGF-β treatment increased Smad-3 phosphorylation equally on all PAGs whether cross-linked with pFN, collagen, or gelatin demonstrating that the rounding phenotype requires both FN and TGF-β (data not shown). The viability of round fibroblasts remained high (greater than 98%) for up to five days after addition of TGF-β. Caspase-3 activity was not increased above control levels demonstrating that the rounding process was not a consequence of initiation of apoptotic pathways. Our data also indicated that the rounding was not a function of the cells’ ability to ligate pFN because cell adhesion and spreading occurred on pFN-PAG at levels comparable to those of gelatin-PAG (Fig. 1).
Fig. 2.
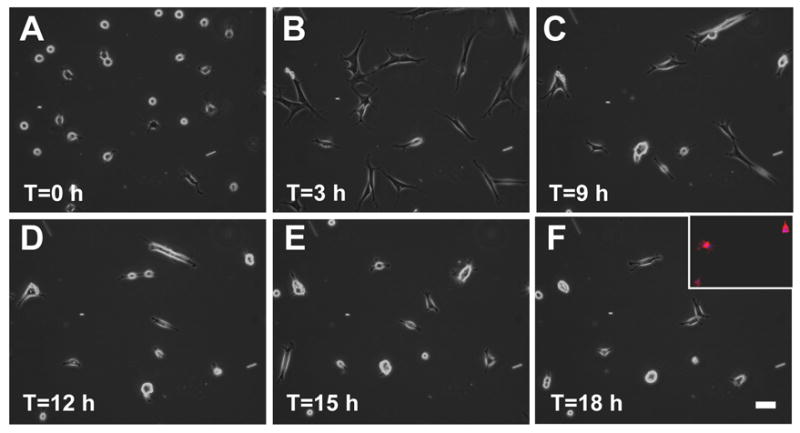
Fibroblasts undergo spreading before they round on pFN-PAG in the presence of TGF-β. Time-lapse, phase contrast images of human fibroblasts on pFN-PAG at designated times after addition of TGF-β (10 ng/mL). Inset (F): human fibroblasts on pFN-PAG in the presence of TGF-β (10 ng/mL); actin cytoskeleton was and visualized by phalloidin staining for actin. Bar: 50 μm.
In summary, fibroblasts seeded on pFN-PAG do not elaborate an extensive fibrillar network and promptly adopt a rounded phenotype when treated with TGF-β; they remain spread on both collagen- or gelatin-PAG in the presence or absence of TGF-β or on rigid surfaces coupled either with pFN, collagen, or gelatin. Our data suggest that a FN-specific signaling mechanism modulates TGF-β-dependent fibroblast rounding (henceforth termed “contractile”) activity.
The synergy/cell binding domain in FN III8–10 supports fibroblast contraction
Given the absence of an extensive FN matrix in our experimental system, we hypothesized that presentation to fibroblasts of the cell-binding domain residing in FN Type III repeat 8–10 (FNIII8,9,10) that includes both the RGD sequence and the synergy sites might be critical for the observed rounding. We tested short, recombinant FNs to determine whether or not FNIII8,9,10 was sufficient to mediate the cell contraction that we observed. Fibroblasts spread on FNIII8,9,10-PAG in the absence of TGF-β, and contracted on this short, recombinant FN in the presence of TGF-β (Fig. 3A,B) as they did on intact pFN (Fig. 2C,F); fibroblasts seeded on FNIII8,9,10 cross-linked glass (or plastic) remained spread in the presence or absence of TGF-β treatment (data not shown). As a complementary approach, we also pre-incubated pFN-PAGs with HFN7.1, a monoclonal antibody that binds to the synergy site in FNIII9 [16,17]. In contrast to control cultures, we observed that fibroblast contraction was inhibited and the cells retained a spread morphology in the presence of TGF-β (Fig. 3C,D). These data support a mechanism in which a site within III8,9,10 is required in the TGF-β-dependent contraction that we observed on compliant pFN-PAGs.
Fig. 3.
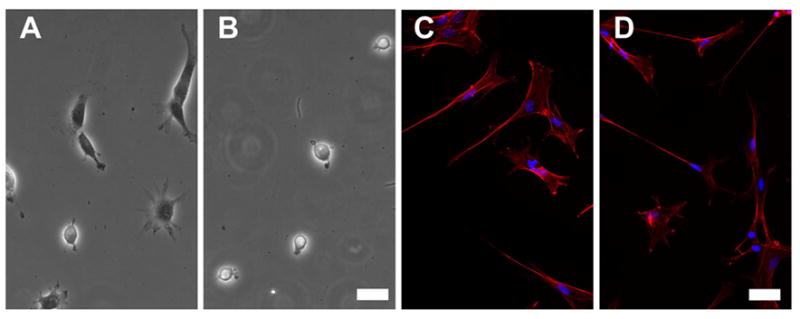
A site within III8,9,10 in pFN is required to promote rounding in the presence of TGF-β. A, B - PAGs prepared with recombinant III8,9,10 PAG were cultured with fibroblasts either without (A) or with (B) TGF-β (10 ng/mL). PAGs were prepared with purified pFN and preincubated with anti-synergy site antibody (HFN7.1) on which fibroblasts were cultured without (C) or with (D) TGF-β (10 ng/mL). Fibroblasts were stained with Alexa 594-phalloidin. Bar: 50 μm.
Fibroblast contraction on pFN-PAG and TGF-β activates Rho GTPase and requires ROCK
Because Rho activity is known to regulate cell contractility, we tested whether or not the round phenotype observed was a consequence of Rho and ROCK activation. Y27632, a pharmacological inhibitor of Rho kinase (ROCK), was used to assess its participation in fibroblast rounding. TGF-β-dependent fibroblast rounding was inhibited by concentrations of Y27632 as low as 0.1 μM (Fig. 4A,B). Moreover, Y27632 could reverse fibroblast contraction and elicit the spread morphology when added at any time up to 72 h after TGF-β addition (data not shown). The reversibility of the rounding underscored the viability of the round cells and indicated that a ROCK-dependent pathway is required for the observed contraction.
Fig. 4.
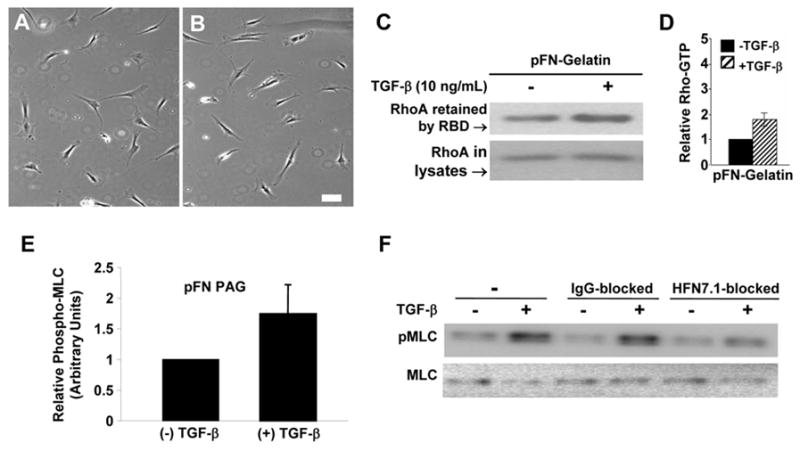
The Rho-ROCK pathway participates in cell contraction induced by TGF-β on pFN-PAG. A, B - HFF cells were grown on pFN-PAG treated with 0.1mM Y27632 in the absence (A) or presence (B) of 10ng/mL TGF-β. Bar, 50 μm. C - Representative western blot for Rho GTPase activity from HFF cells grown on plates coated with gelatin to which pFN was added in the absence or presence of TGF-β (10 ng/mL, 24 h). D - Quantitative analysis by densitometry of the results from pooled experiments (n=4). Bars represent mean ± SEM. E - Quantitation of total Phospho-MLC protein of HFF2 cells grown on pFN-PAG in the absence or presence of 10 ng/mL TGF-β. F - Representative experiment showing western blot of MLC phosphorylation of HFF cells grown with or without TGF-β (10 ng/mL) on pFN-gelatin-PAG pre-treated in the absence or presence of either mouse IgG or the anti-synergy site Mab, HFN7.1.
To measure directly the activation of Rho, we performed Rho pull down assays on fibroblast extracts. To attain sufficient lysate volumes, we modified our approach by coupling plastic dishes directly with pFN (not shown) or with gelatin followed by pFN (Fig. 4C,D) pretreated with Sulfo-SANPAH to simulate conditions on PAGs. There was a slightly lower, but statistically insignificant, baseline Rho activation when cells were cultured on Sulfo-SANPAH pretreated pFN compared to non-crosslinked FN on gelatin (24 h) in the absence of TGF-β (not shown). Importantly, we observed a marked increase in Rho activation when TGF-β was added to fibroblasts on pFN-gelatin, indicating that TGF-β activates Rho in these cells (Fig. 4C,D). Rho pull down assays were also done with cells on pFN-PAG, with and without TGF-β, and activation of Rho to levels comparable to those in Fig. 4C were again observed (not shown). These data suggest that the vigorous fibroblast rounding observed on pFN-PAG in the presence of TGF-β involves the FN synergy site and occurs as a consequence of cell contraction mediated by TGF-β-dependent activation of the Rho-ROCK pathway.
A site within FN III8,9,10 modulates TGF-β mediated MLC phosphorylation
We also observed that TGF-β stimulates MLC phosphorylation when fibroblasts undergo TGF-β-dependent contraction on pFN-PAG (Fig. 4E). Taken with our ROCK inhibition studies (above), these data are consistent with a signaling mechanism in which a Rho-ROCK-dependent pathway activates MLC-dependent contraction. When we pre-incubated pFN-PAG with HFN7.1, TGF-β-mediated-MLC phosphorylation was reduced (Fig. 4F). Therefore, the observed dependence upon the cell-binding domain for the full contractile phenotype (Fig. 3) was also reflected in MLC phosphorylation (Fig. 4). These data build on the observations that Rho activation, leading to MLC phosphorylation, requires cell adhesion to the cell-binding domain of FN [18,19].
Our data suggest a mechanism in which the presentation of the cell-binding domain within FN regulates TGF-β-dependent, Rho-mediated fibroblast contractility. Recent data suggests that FNIII repeats are quite flexible, revealing cryptic sites when either cleaved proteolytically or stretched mechanically [20,21]. We and others speculate that matrix remodeling during wound healing - through proteolysis or mechanical mechanisms - modulates the presentation to fibroblasts of the cell-binding domain within FN [22]. In a pathogenic setting the exuberant contractile state of fibroblasts, driven by activated Rho, could further distort the FN cell-binding domain resulting in a “feed-forward” mechanism that perpetuates the chronic fibrotic state. Regulation of fibroblast contractility is a critical aspect of the contraction phase of connective tissue remodeling and there are presently few therapeutic options for modulating wound contracture [23]. The present study provides the basis for a previously unrecognized therapeutic approach in which targeting the synergy site could modulate fibroblast contraction.
Acknowledgments
We are grateful to Dr. M.A. Schwartz for helpful discussions and to Dr. P.A. Vincent (Albany Medical College) for a gift of anti-phospho-MLC and help in optimizing the analysis of MLC phosphorylation. We also thank Debbie Moran for care in preparation of the manuscript. This work was supported by NIH GM-56442 (L. Van De Water) and NIH CA-69612 (P. McKeown-Longo). T. Meckmongkol is supported by NIH-NHLBI training grant T32-HL-07194.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 3.Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 4.Hynes RO. Fibronectins. Springer-Verlag; New York: 1990. [Google Scholar]
- 5.Clark RAF. The Molecular and Cellular Biology of Wound Repair. Plenum Press; NY: 1996. [Google Scholar]
- 6.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, Van De Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ffrench-Constant C, Van De Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989;109:903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh P, Reimer CL, Peters JH, Stepp MA, Hynes RO, Van De Water L. The spatial and temporal expression patterns of integrin α9β1 and one of its ligands, the EIIIA segment. J Invest Dermatol. 2004;123:1176–1181. doi: 10.1111/j.0022-202X.2004.23485.x. [DOI] [PubMed] [Google Scholar]
- 10.Mao Y, Schwarzbauer JD. Accessibility to the fibronectin synergy site in a 3D matrix regulates engagement of α5β1 versus αvβ3 integrin receptors. Cell Commun Adhes. 2006;13:267–277. doi: 10.1080/15419060601072215. [DOI] [PubMed] [Google Scholar]
- 11.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein RM, Zheng M, Ambesi A, Van De Water L, McKeown-Longo PJ. Stimulation of extracellular matrix remodeling by the first type III repeat in fibronectin. J Cell Sci. 2003;116:4663–4674. doi: 10.1242/jcs.00778. [DOI] [PubMed] [Google Scholar]
- 13.Hocking DC, Smith RK, McKeown-Longo PJ. A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol. 1996;133:431–444. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoen RC, Bentley KL, Klebe RJ. Monoclonal antibody against human fibronectin which inhibits cell attachment. Hybridoma. 1982;1:99–108. doi: 10.1089/hyb.1.1982.1.99. [DOI] [PubMed] [Google Scholar]
- 17.Bowditch RD, Halloran CE, Aota S, Obara M, Plow EF, Yamada KM, Ginsberg MH. Integrin αIIbβ3 (platelet GPIIb-IIIa) recognizes multiple sites in fibronectin. J Biol Chem. 1991;266:23323–23328. [PubMed] [Google Scholar]
- 18.Ren XD, Wang R, Li Q, Kahek LA, Kaibuchi K, Clark RA. Disruption of Rho signal transduction upon cell detachment. J Cell Sci. 2004;117:3511–3518. doi: 10.1242/jcs.01205. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Clark RA, Mosher DF, Ren XD. Fibronectin’s central cell-binding domain supports focal adhesion formation and Rho signal transduction. J Biol Chem. 2005;280:28803–28810. doi: 10.1074/jbc.M501421200. [DOI] [PubMed] [Google Scholar]
- 20.Hynes RO. The dynamic dialogue between cells and matrices: implications of fibronectin’s elasticity. Proc Natl Acad Sci USA. 1999;96:2588–2590. doi: 10.1073/pnas.96.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Ann Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 22.Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell-fibronectin matrix interactions during tissue repair. J Invest Dermatol. 2006;126(Suppl):73–78. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- 23.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]


