Abstract
Recombinant poxviruses encoding tumor-associated antigens (TAA) are attractive as candidate cancer vaccines. Their effectiveness, however, will depend upon expression of the TAA in appropriate antigen-presenting cells. We have used a murine model in which the TAA is β-galactosidase (β-gal) and a panel of recombinant vaccinia viruses (rVV) in which β-gal was expressed under early or late promoters at levels that varied over 500-fold during productive infections in tissue culture cells. Remarkably, only those rVV employing early promoters were capable of prolonging the survival of mice bearing established tumors expressing the model TAA. Late promoters were ineffective regardless of their determined promoter strength. The best results were obtained when β-gal was regulated by a strong early promoter coupled to a strong late promoter. When a variety of cell types were infected with the panel of viruses in vitro, dendritic cells were found to express β-gal only under the control of the early promoters even though late promoters were intrinsically more active in other cell types. Furthermore, in a functional assay, dendritic cells infected in vitro with rVV encoding β-gal regulated by an early promoter activated β-gal-specific cytotoxic T lymphocytes, whereas similar rVV with a late promoter-regulated gene did not. These data indicate that promoter strength per se is not the most critical quality of a recombinant poxvirus-based tumor vaccine and that the use of promoters capable of driving the production of TAA in “professional” antigen presenting cells may be crucial.
Identification of tumor-associated antigens (TAA) recognized by T lymphocytes makes it possible to test the function of recombinant immunogens in the treatment of cancer (1–5). Optimization of the design and delivery of these immunogens is facilitated by a growing understanding of the molecular signals involved in the activation and proliferation of T lymphocytes and a knowledge of how antigens are processed and presented for recognition. Among potential vectors that could be given to patients with cancer (or those at high risk of developing cancer) are poxviruses. The relative safety, stability, and effectiveness of vaccinia virus, one member of the poxvirus family, was demonstrated in the smallpox eradication program. These viruses are easy to manipulate genetically and capable of accommodating large amounts of heterologous DNA (6). Poxviruses replicate in the cytoplasm, encode their own transcriptional machinery, and are nononcogenic and nonintegrating (7).
The production of heterologous RNAs are driven by viral promoters, placed upstream of and adjacent to the inserted gene sequence (8), that fall into three classes: early, intermediate and late (9). Early promoters are active immediately after infection because the necessary enzymes are brought into the cells within the infectious virus particles. In contrast, the factors and DNA template required for intermediate and late transcription are synthesized de novo. Accordingly, inhibitors of DNA replication such as cytosine arabinoside (araC), suppress intermediate and late gene expression, but do not negatively affect the synthesis of early proteins. Many genes under the control of late promoters are highly expressed as exemplified by the abundant structural proteins of the virus. Abutment of early and late transcriptional regulatory sequences can result in gene expression throughout the replication cycle of vaccinia virus (10).
Based on an understanding of promoter sequences acquired by mutagenesis studies, it is now possible to construct vaccinia viral promoters that are active before or after viral DNA replication and that vary in strength (11, 12). Whereas it may appear intuitive that the enhanced expression of antigens under the control of the more powerful late promoters will induce strong immunity, in some cases, early promoters elicited a greater cytotoxic T lymphocyte (CTL) response than late promoters perhaps because of vaccinia’s interference directly or indirectly with major histocompatibility complex (MHC) class I presentation by late times (13–15). An alternative possibility considered here is that vaccinia virus infection may be abortive in “professional” antigen-presenting cells (APC) so that only the early phase of the replication cycle occurs.
We have previously shown that the administration of recombinant vaccinia virus (rVV) expressing β-galactosidase (β-gal) in combination with interleukin 2 (IL-2) can reduce the number of pulmonary nodules and prolong the survival in mice bearing tumors that have been stably transfected with β-gal gene (16). To investigate the influence of promoter type and strength on the antitumor effect of recombinant poxvirus-based vaccines and expression in “professional” APC, we used a panel of different rVV expressing a model TAA, β-gal, under the control of a variety of early, late, or early/late promoters.
MATERIALS AND METHODS
Cell Lines.
CT26.WT, the β-gal expressing CT26.CL25, the C3–4 murine BALB/c hybridoma transduced with lacZ gene, P815 mastocytoma, and EL4 thymoma and their respective lacZ transfectants P13.1 and E22 have been described (17–20). B-SC-1 cells (CCL26; American Type Culture Collection) and Hela S3 (CCL2.2; American Type Culture Collection) were used to prepare all vaccinia virus stocks.
rVV.
All rVV used in this study were generated by insertion of the foreign genes into the vaccinia virus thymidine kinase gene by homologous recombination and plaque purified (21). Crude virus stocks were prepared in HeLa S3 or B-SC-1 cells as described (22). Viral concentrations were determined by plaque titration on B-SC-1 cells. rVV used in a single experiment were titered at the same time to maximize accuracy. Preparation of rVV expressing the influenza A/PR/8/34 nucleoprotein (V69-NP) was previously described (23). In the HPV16-E6Vac, Escherichia coli lacZ was under the control of the natural p7.5 early/late promoter element from plasmid pSC65 (S. Chakrabarti, J. Sisler, and B.M., unpublished data); this construct was named VJS6 for simplicity. Wild-type vaccinia virus strain WR was kindly provided by J. Yewdell and J. Bennink (Bethesda, MD). β-gal activity was determined using a Promega assay.
Peptides.
The synthetic peptide, TPHPARIG (amino acids 876–884 of β-gal; ref. 24), and LPYLGWLVF (amino acids 35–43 of the P1A tumor antigen; ref. 25) were synthesized by Peptide Technologies (Washington, DC) to a purity of >99% as determined by HPLC and amino acid analysis.
Evaluation of CTL Responses.
Eight- to 12-week-old female BALB/c mice (Animal Production Colonies, Frederick Cancer Research Facility, Frederick, MD) were immunized with gold particles coated with a DNA expression vector (pCMV/β-gal) using the helium-driven “gene gun” (26). Three weeks later, splenocytes were harvested, homogenized to a single cell suspension, and then cultured in RE2 medium (Biofluids) containing 0.1 mM nonessential amino acids, 1 mM sodium pyruvate (Biofluids), 5 × 10−5 μM 2-mercaptoethanol (GIBCO/BRL), and 0.5% mouse serum (Harlan Sera-lab, Accurate Chemicals). For the in vitro stimulation, the β-gal peptide (1 μg/ml) or rVV-infected dendritic cells (DC) were added to the cultures. After 7 days, effectors were harvested and tested in a 6-h 51Cr release assay using 2 × 106 target cells incubated with 200 μCi (1 Ci = 37 GBq) Na51CrO4 (51Cr) for 90 min as described (16).
Isolation of DC and B Cell Populations.
DC were derived from the spleens of 8- to 12-week-old BALB/c or C57BL/6 mice. Splenocyte preparations were depleted of red blood cells by hypotonic lysis (ACK buffer; Biofluids), then resuspended in R2E medium supplemented either with 10% heat inactivated fetal calf serum or 0.5% mouse serum and allowed to adhere for 2 h at 37°C in 5% CO2. Nonadherent cells were removed and the remaining cells were incubated overnight (18–24 h) with recombinant murine granulocyte/macrophage colony-stimulating factor (1 ng/ml; Boehringer Mannheim). Detached cells were collected and further enriched by centrifugation (1,850 × g for 15 min) over 50% Percoll cushion (Sigma). B cells were recovered from the nonadherent cell population collected after the initial 2-h adherence step and subjected to depletion with mAbs Gr-1 and CD90 (10 μg/107 cells, PharMingen) plus complement (1:10 dilution, Cederlane Laboratories/Accurate Chemicals). For each preparation, cells were evaluated cytofluorometrically using the following antibodies: 33D1 (TIB 227; American Type Culture Collection), CD80 (B7–1), CD86 (B7–2), CD90 (Thy-1.2), CD45R/B220, anti-H-2 Dd or Db, I-Ad or I-Ab, CD11b/Mac-1 (PharMingen).
Human DC were prepared from nonadherent peripheral blood mononuclear cells after a 3-h incubation in MEM (Biofluids) supplemented with 10% human AB serum (Applied Biosystems) and IL-3 (200 units/ml final concentration). DC were cultured for 5–7 days in the presence of medium containing IL-4 (2000 units/ml) and granulocyte/macrophage colony-stimulating factor (2000 units/ml) and tested for the presence of CD86, CD80, and CD40 (all >95%; PharMingen).
RESULTS
Expression of β-Gal After in Vitro Infection with rVV.
To evaluate the influence of the level of β-gal expression on the generation of an immune response, a panel of rVV was used. Viruses expressing β-gal under the control of early (E1–E5) or late (L1–L6) promoters were previously constructed by single nucleotide substitutions or complex modifications of the sequence of the early portion of the 7.5-kDa natural early/late or the 28-kDa late promoter, respectively (11, 12). Three additional viruses were used as controls: the lacZ negative virus V69-NP (V69), which encodes the nucleoprotein from influenza virus PR8 under the control of the natural 7.5-kDa early/late (23), HPV16-E6Vac (VJS6) containing the natural 7.5-kDa early/late promoter driving β-gal (J. Sisler and B.M., unpublished data; refs. 16 and 27), and VSC-56 which employs a synthetic “super” early/late designated PSYNTHETIC E/L (S E/L) promoter to drive lacZ (S. Chakrabarti, J. Sisler, and B.M., unpublished data; ref. 28).
The strongest late promoters enabled the synthesis of 20- to 30-fold more β-gal than any of the early promoters (Table 1). Similar levels of β-gal activity were detected in two different cell lines after infection with the same rVV (compare experiments 1–3 and experiment 4 A and B). The β-gal activities of all of the viruses employing late promoters, but not early promoters, were abrogated by araC, an inhibitor of DNA replication, as expected (Table 1, experiments 1–3). Early expression by early/late promoters can be evaluated only indirectly by the level of β-gal produced in the presence of araC. As much or more β-gal was produced by S E/L rVV as by the strongest early promoter (E5) in the presence of araC.
Table 1.
β-Gal production after in vitro infection with rVV containing different viral promoters
| rVV | Code | Exp. 1
|
Exp. 2
|
Exp. 3
|
Exp. 4
|
||||
|---|---|---|---|---|---|---|---|---|---|
| −araC | +araC | −araC | +araC | −araC | +araC | A | B | ||
| None | − | 0 | 0 | 0 | 0 | 0.2 | 0.1 | 0 | 0 |
| V69-NP | V69 | 0 | 0 | 0 | 0 | 0.1 | 0.2 | 0 | 0.1 |
| 177 | E1 | 0.5 | 0.2 | 0.3 | 1.2 | ND | ND | 0.4 | 0.7 |
| 131 | E2 | 1.6 | 3.6 | 3.8 | 3 | ND | ND | 3 | ND |
| 105 | E3 | 3.4 | 9.7 | 7 | 8 | 5 | 7.6 | 8 | 8.5 |
| 342 | E4 | 11 | 8.5 | 14 | 30 | 16 | 15 | 9.9 | 17 |
| 360 | E5 | 13 | 35 | 18 | 16 | 21 | 27 | 17.6 | 22 |
| 438 | L1 | 1.8 | 0 | ND | ND | ND | ND | 1.6 | 2.8 |
| 23 | L2 | 3.8 | 0 | ND | ND | ND | ND | 5.3 | 5.5 |
| 443 | L3 | 97 | 0 | ND | ND | ND | ND | 80 | 130 |
| 455 | L4 | 231 | 0.1 | ND | ND | 310 | 1 | 219 | 320 |
| 441 | L5 | 365 | 0.2 | ND | ND | ND | ND | 521 | 695 |
| HPV16-E6Vac | VJS6 | 48 | 17 | 64 | 20 | 65 | 15 | ND | 70 |
| VSC-56 | S E/L | ND | ND | 570 | 29 | 460 | 28 | ND | 510 |
Duplicate wells containing 2 × 105 BS-C-1 (experiments 1–3) or CT26.WT (experiment 4) were cultured for 24 h in medium alone or medium containing different rVV at a multiplicity of infection (moi) of 10. Cell extracts obtained after three cycles of freezing/thawing were incubated with the substrate [o-nitrophenyl β-d-galactopyranoside (ONPG), and the hydrolysis of the ONPG to o-nitrophenol was detected with a spectrophotometer. Values are expressed as enzyme units (×10−4)/50,000 cells. Note that A and B under experiment 4 are independently performed repeats of the experiment using CT26.WT as a target cell.
Protection Experiments Using Promoters of Different Type and Strength.
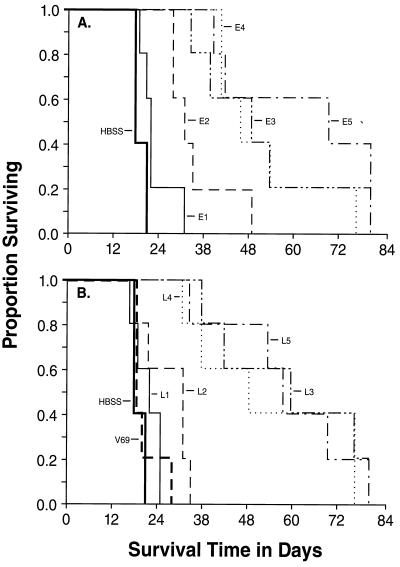
To ascertain the capacities of the rVV to mediate protective immunity to subsequent challenge with the β-gal expressing CT26.CL25, an immunization-challenge experiment was performed. All of the rVV contructs were tested against the non-β-gal expressing CT26.WT tumor and none of them was found alter its growth rate or lethality (data not shown). The E1 and L1 viruses were not significantly different than the negative Hanks’ balanced salt solution (HBSS) or V69 controls in the protection of mice from challenge with the CT26.CL25 tumor. The E2 and L2 constructs were marginally more effective than the negative controls (P = 0.024 for E2 and P = 0.017 for L2). The threshold of efficacy was reached in the form of significant and consistent protection from challenge with CT26.CL25 when the E3, E4, and E5 or L3, L4 or L5 containing viruses were used (P < 0.001) (Fig. 1). The threshold appeared to be higher for late promoters since E3 and L2 had similar strength, but only the former provided protection. Nevertheless, apparently protected mice eventually died of their tumors, a likely consequence of the emergence of β-gal-negative variants, as observed (16).
Figure 1.
Vaccination with rVV mediating the production of high levels of β-gal induce protective immunity against a β-gal-positive tumor. Five BALB/c mice were injected with medium alone (HBSS) or medium containing 5 × 106 pfu of different thymidine kinase− rVV expressing E1–E5 (A) and L1–L5 (B). The negative control (V69) did not express β-gal. After 3 weeks, mice were challenged i.v. with 0.5 ml of medium containing 105 cells of CT26.CL25. From the day of tumor challenge, mice were checked daily for survival. On day 82 following the tumor inoculation, all the mice still surviving showed signs of advanced disease and were euthanized.
Only rVV Constructs Employing Early Promoters To Drive β-Gal Expression Are Effective in the Treatment of Established Disease.
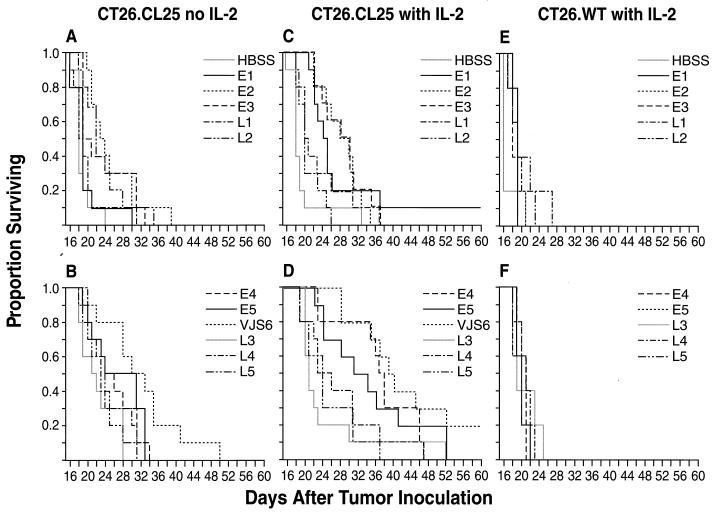
We previously found that pulmonary metastases resulting from injection of CT26.CL25 tumor cells 3 or 6 days earlier can be treated by inoculation of VJS6 if it is followed by a short cycle of recombinant IL-2 (rIL-2) (16). Although previous studies with a limited set of viruses indicated that high levels of antigen expression were better than low levels in the active immunotherapy setting, the promoter type was not explored. To address this issue more comprehensively, mice bearing 3-day-old tumors (original inoculum, 105 tumor cells) were treated with a single dose (5 × 106 pfu) of 1 of the 11 viruses shown in Fig. 2.
Figure 2.
Early expression of β-gal is essential for the therapy of established pulmonary metastases. BALB/c mice were inoculated intravenously with 0.5 ml of HBSS containing the following tumor cells: 105 CT26.CL25 (A–D) and 105 CT26.WT (E and F). Three days after tumor injection, they received a single i.v. injection of the different rVV shown in the figure. Where indicated (with IL-2, C–F), rIL-2 (100,000 Cetus units/mouse, twice a day) was administered i.p. starting 12 h after rVV injection and continued for 3 days. Mice were checked every day for survival. Data are the sum of two different, independent experiments in which at least five mice were included in each group.
A partial therapeutic effect was seen with some rVV administered alone, especially in the case of VJS6 (Fig. 2B). None of the different rVV influenced the survival of mice injected with the CT26.WT tumor cell line (Fig. 2 E and F). Unexpectedly, upon the provision of exogenous rIL-2, the only viruses capable of mediating a significant prolongation of survival were those employing early promoters to drive the lacZ gene, whereas no significant prolongation in median survival was seen when late promoters were used (Fig. 2 C and D). As in the prevention experiments, a clear threshold effect was observed, but the cutoff did not extend to the viruses containing late promoters and started at E4 and included E5 and VJS6 (these rVV all had a P < 0.0001 as compared with the group receiving rIL-2 alone, Fig. 2D). The performance of VJS6, the virus capable of mediating the highest early expression of β-gal, suggested that this had been a good choice for our earlier studies (16).
A rVV Containing a Synthetic Early/Late “Super” Promoter Had the Greatest Therapeutic Impact on Pulmonary Metastases.
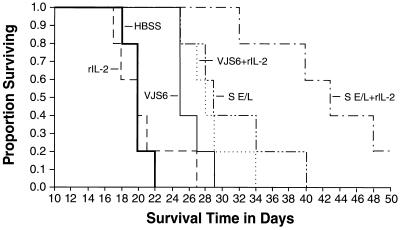
The function of VJS6, which employs a strong naturally occurring early promoter that is coupled with a relatively weak late promoter, led us to explore the additional augmentation of early expression combined with strong late expression. Indeed, the function of an experimental virus employing a totally synthetic early late “super” promoter (S. Chakrabarti and B.M., unpublished data; ref. 28) was remarkable (Fig. 3, S E/L compared with IL-2 alone, P = 0.0018).
Figure 3.
A rVV containing a “super”, synthetic early/late promoter has the best therapeutic effect on pulmonary metastases. Five BALB/c mice per group were injected i.v. with 105 CT26.CL25 cells. Three days later they received a single i.v. injection of HBSS alone or containing 5 × 106 pfu/mouse of different rVV, as indicated. rIL-2 (100,000 Cetus units, twice a day) was administered i.p. starting 12 h after rVV injection and continued for 3 days. Mice were followed every day for their survival. An independent repeat of this experiment gave similar results.
Correlation of Therapeutic Efficacy with Antigen Expression in DC.
To explain why late promoters could not be used to restimulate a CTL response in vitro, investigators have postulated that serine protease inhibitors produced by vaccinia viruses interfered with antigen processing (15, 29). However, recent evidence using vaccinia deletion mutants is inconsistent with this hypothesis (30). Other explanations include the negative effects of vaccinia virus on class I expression (29). Based on recent evidence of the requirement for costimulatory signals in the activation of T lymphocytes and the role of DC in providing these costimulatory signals, we hypothesized that virus-mediated expression in DC could explain our results. Specifically, we explored the possibility that “professional” APC may also be “professional” in handling virus infection, and that viral expression in these infected cells was the critical parameter in determining the efficacy of a recombinant poxvirus-based vaccine.
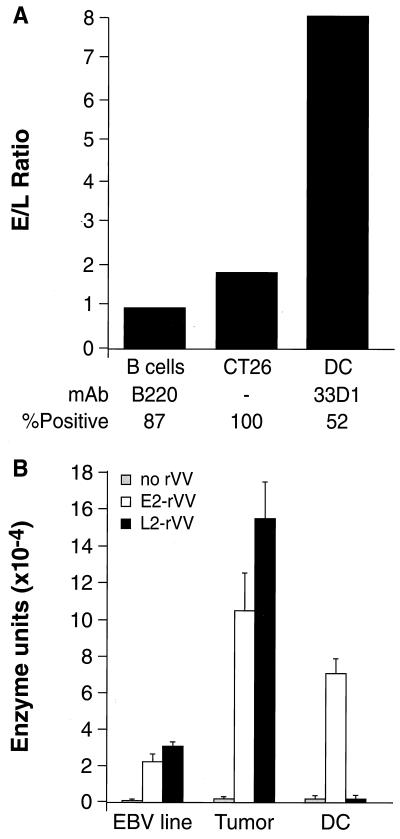
To examine the relative expression of our model antigen under the control of either early or late promoters, we infected primary cultures of DC with the E2 and L2 viruses, previously shown to express equivalent levels of β-gal after infection of tumor cell lines (Table 1). Indeed, the E2/L2 ratio in infected CT26.WT cells was between 1 and 2 in three different experiments (mean 1.8, Fig. 4A). A similar ratio was observed in simultaneously infected fresh B cell population (ratio of 1.0). Most importantly, production of β-gal under the control of the E2 early promoter was 10-fold higher than that driven by L2 in DC. This discrepancy was also observed when DC were prepared from C57BL/6 mice as well as when a different pair of rVV was used (E5 and L5, data not shown).
Figure 4.
Professional APC do not allow the expression of late proteins during in vitro infection with rVV. (A) Duplicate wells (106/well) of CT26.WT tumor cells or different populations of APC enriched from mouse spleens were infected with 30 multiplicity of infection of either E2 or L2 rVV. After 2 h, cells were washed to remove unbound virus and incubated in fresh medium for further 24 h. At the end of incubation period, cell were collected and lysed, and the intracellular content of β-gal was evaluated using an enzymatic assay. Data are presented as the ratio of early/late expression of β-gal for each cell population. Figures at the bottom represent the percentage of cells positive for the indicated antibodies. The percentage of DC was estimated to be around 70% by the use of other markers (although specific, the mAb 33D1 only stains a subpopulation of splenic DC). B cells, B lymphocytes. (B) Preparation of human DC were infected and processed as above and compared with a human Epstein–Barr virus (EBV)-induced lymphoblastoid cell lines (EBV line) and human melanoma cell lines (Tumor). The percentage of DC was estimated to be approximately 95% by fluorescence-activated cell sorter analysis (>95% for CD86, CD80, and CD40). Values express the β-gal enzyme units × 10−4/3 × 105 cells and are means of two different, independent experiments.
In view of the clinical application of our findings, it was important to establish if this state of nonpermissiveness to vaccinia virus replication was shared by human DC. Whereas the performance of E2 or L2 rVV was similar in Epstein–Barr virus transformed B lymphocytes, no detectable β-gal was produced in human DC with L2 rVV, while high levels of β-gal were produced after infection with E2 rVV (Fig. 4B).
Functional Consequences of Poor Late Promoter Activity in DC.
To explore the functional consequences of promoter activity in DC, we focused on the capability of DC to mediate the activation of T lymphocytes ex vivo (31, 32). Splenocytes from naïve mice or mice that had been previously immunized with a plasmid DNA containing the lacZ gene were sensitized in vitro for 7 days with DC infected with viruses employing either early or late gene expression. Mouse serum was used in the culture medium to minimize background activity (33, 34).
Splenocytes stimulated in vitro with vaccinia-infected DC mounted a strong primary immune response against vaccinia-infected CT26 target cells, confirming the function of the DC and verifying that all splenocyte populations were capable of antigen-specific immune reactivity (Fig. 5A). Note that cultures derived from immunized mice and stimulated with rVV-infected DC showed a comparable antivaccinia response, indicating that DC were equivalently infected with the E5 and L3 viruses. Stimulation of the splenocytes from the immune mice with the β-gal peptide resulted in weak but specific cytotoxicity against CT26 cells pulsed with the same peptide (Fig. 5A).
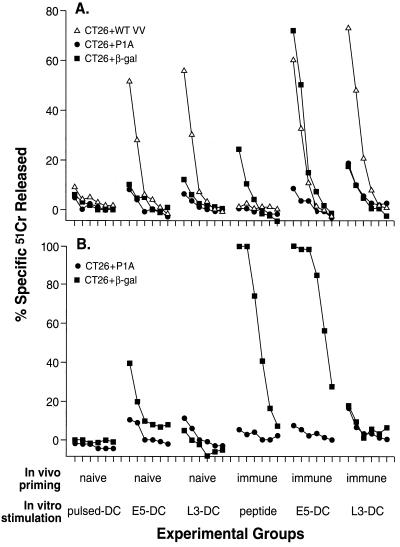
Figure 5.
DC are not able to present epitopes of proteins expressed in the late phase of infection with rVV. Using a helium-driven Accell gene delivery system, BALB/c mice were immunized one time in the epidermis with four shots of 0.25 mg of gold delivering a total of 0.05 μg of plasmid DNA expressing β-gal (immune). Control mice were not immunized (naïve). After 3 weeks, the spleens were aseptically removed and cultured in complete medium containing 0.5% mouse serum and the following stimulators: DC pulsed for 2 h with 10 μg of β-gal peptide (pulsed-DC); DC infected for 2 h with E5 (E5-DC) or L3 (L3-DC) rVV; 1 μg/ml of the β-gal peptide in culture medium (peptide). The characterization of DC gave the following results: B7–1 = 79.2%, B7–2 = 85.5%, CD18 = 97%, Thy-1.2 = 16.2%, B220 = 22.3%, I-Ad= 96.6%. Responders to stimulator ratio was 10:1. IL-2 (5 Cetus units/ml) was added 24 h later and cultures were incubated for 7 days. Cytotoxic activity was then tested in a 6-h 51Cr release assay against CT26.WT tumor cell line alone (data not shown), pulsed with β-gal peptide (CT26 plus β-gal), pulsed with an irrelevant peptide (CT26 plus P1A), or infected (CT26 plus WT VV) during the isotope labeling with crude VV preparation (A). A part of the cultures was further restimulated with β-gal-positive irradiated tumor cells (C3–4 cell line) and syngeneic splenocytes for 7 days before testing in a 6-h 51Cr release assay (B). The effector-to-target cell ratio was 50:1 and then 1:3 dilutions.
Most importantly, only the DC infected with the rVV expressing the antigen under the control of the early promoter (E5) successfully stimulated a β-gal specific cytolytic response (Fig. 5A) despite the fact that production of β-gal in tumor cell lines was 4 to 7-fold higher when using the L3 virus than when using the E5 virus (see Table 1).
To explore whether T lymphocyte activation occurred at subdetectable levels that could be observed with continued expansion, cultures were restimulated with an irradiated, β-gal-expressing tumor cell line called C3–4. Anti-β-gal cytolytic activity was detectable in cultures of naïve spleens that were initially stimulated with E5-infected but not L3-infected DC (Fig. 5B). Restimulation of immune splenocyte cultures resulted in CTL activity against the relevant target only when E5-infected DC, or peptide-stimulated controls were used as in vitro activators. These experiments demonstrated the inability of DC to present immunogenic determinants from a protein produced during the late stage of vaccinia infection.
DISCUSSION
Clinical trials are currently underway to test the possible application of recombinant poxviruses encoding TAA, the ultimate goal being the stimulation of strong antitumor responses (35). Previous animal studies done in this laboratory and elsewhere using rVV as antitumor vaccines have not addressed optimal promoter type or strength. The present studies thus have significant clinical relevance and indicate that the use of a completely synthetic, strong promoter with early activities is optimal for use in the active immunotherapy of established disease. These findings may be relevant to the elicitation of antiviral, as well as antitumor, immune responses.
DC are commonly thought to be the most important elements for the priming of naïve mice although the contribution of macrophages (Mø) cannot be disregarded. This is especially true for the presentation of antigens to naïve CD8+ lymphocytes (36). However, several papers support the the notion that the replicative cycle of vaccinia virus is also aborted in primary cultures of Mø, either of human or rodent origin (37, 38). We were able to independently confirm these data using highly enriched preparation of splenic Mø (data not shown).
The response of professional APC to viral infection has not been subjected to extensive investigations. However, studies on the interaction between influenza virus and APC suggest that the nonpermissiveness observed with vaccinia virus might be a general feature of DC. After overnight incubation, human monocytes and DC showed similar level of infection with influenza virus strain PR8 (A/Puerto Rico/8/34), but a clear cytopathic effect was visible only in monocyte cultures suggesting that infection of DC was nonproductive and nontoxic. Moreover, virus infected DC were 30- to 50-fold more effective than Mø in supporting T cell proliferation (39). In a different study, DC were collected from mediastinal lymph nodes of mice 2 days after intranasal administration of the X-31 (H3N2) influenza strain. The titration revealed that only 1 out of 84,000 DC contained the virus, but as few as 10,000 cells were sufficient to stimulate the proliferation of T cell hybridoma recognizing a H-2Db-restricted viral peptide. These results suggest that more DC carrying antigenic material were present than the amount estimated by titration in eggs (40).
The mechanisms underlying this nonpermissive state for virus replication are currently unclear but it may facilitate the stimulation of a naïve immune system by allowing increased time for a rare, recirculating naïve T lymphocyte, either a Th or a CTL, to encounter an infected APC. Inhibition of viral replication would minimize the potential danger of transmitting the infection to the T lymphocytes during the cell–cell contact with the APC. Moreover, inhibition of viral replication would delay the cytopathic effect, thereby retarding the loss in the ability to efficiently present immunogenic determinants.
Although the data on the nonpermissive state of APC came from in vitro studies, it is reasonable to assume that the same condition is occurring after infection of mice with rVV. The detection of β-gal in splenic DC or Mø after inoculation of rVV has posed several technical difficulties due to the paucity of both APC among the splenocytes. However, using an ELISA assay, the content of β-gal protein found in the whole spleen 1 day after i.v. inoculation of E5 and L5 rVV was increased only after infection with the latter rVV (3.5-fold above the background; data not shown). Although these data do not allow any conclusion of differential expression in spleen subpopulations, at least they indicate that the difference in production of β-gal among late and early expressors observed after in vitro infection is reflecting an analogous situation in vivo.
If DC and Mø are not able to present epitopes from protein synthesized late during the vaccinia infection cycle, how can late promoters allow the generation of an immune reactivity against β-gal (Fig. 1 and data not shown)? One or several mechanisms may have developed to assure recognition of protein controlled by late promoters: (i) DC and Mø could undergo changes in their state of permissivity after activation or differentiation in response to cytokines secreted by activated lymphocytes, (ii) presentation through the fully permissive B cells could occur, and (iii) Mø could ingest and then present the β-gal released by the bystander infected cells following the cytopathic action of the virus or the killing of virus-infected cells (cross-priming).
Several pieces of evidence confirmed the existence of a class I MHC-restricted presentation of exogenous proteins. Mø and Mø cell lines can present class I MHC restricted epitopes through nonconventional pathways involving phagocytosis of particulate form of the antigen (41, 42) or a macropinocytosis of soluble proteins (43). Cross-priming by TAA released from dying tumor cells and ingested by infiltrating monocytes/Mø is thought to play an important role in initiating an antitumor response (44). Our study suggests that even if the cross-priming is occurring, it is not sufficient to limit the growth of the tumor and only direct presentation of TAA by professional APC can trigger a therapeutic antitumor response and allow tumor eradication. At present, it is unclear whether the efficacy of early promoters is due to the induction of a “faster” immune response, a response rapid enough to eliminate the tumor cells before they reach an untreatable stage, or if the presentation of early proteins through professional APC elicits a qualitatively different response. It is clear that defining these aspects is of paramount importance for the design of effective vaccination protocols for cancer patients.
Acknowledgments
We thank J. Berzofsky and K. Irvine for expert advice, P. Spiess and D. Jones for help with the animal experiments, M. Blalock for the assistance with graphics, and D. Surman and N. Cooper for tissue culture preparations. This work was supported in part by the Strong Children’s Research Center, University of Rochester School of Medicine, Rochester, NY (M.W.).
ABBREVIATIONS
- APC
antigen-presenting cells
- rVV
recombinant vaccinia virus
- TAA
tumor-associated antigen
- DC
dendritic cells
- Mø
macrophages
- araC
cytosine arabinoside
- CTL
cytotoxic T lymphocyte
- MHC
major histocompatibility complex
- β-gal
β-galactosidase
- IL
interleukin
- pfu
plaque-forming units
- E. early
L, late
- rIL-2
recombinant IL-2
- HBSS
Hanks’ balanced salt solution
References
- 1.De Plaen E, Lurquin C, Van Pel A, Mariamé B, Szikora J-P, Wölfel T, Sibille C, Chomez P, Boon T. Proc Natl Acad Sci USA. 1988;85:2274–2278. doi: 10.1073/pnas.85.7.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox A L, Skipper J, Chen Y, Henderson R A, Darrow T L, Shabanowitz J, Engelhard V H, Hunt D F, Slingluff C L., Jr Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Sakaguchi K, Appella E, Yannelli J R, Adema G J, Miki T, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Rivoltini L, Topalian S L, Miki T, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moss B. Science. 1991;252:1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- 7.Moss B. In: Field’s Virology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott–Raven; 1996. pp. 2637–2672. [Google Scholar]
- 8.Mackett M, Smith G L, Moss B. J Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss B. In: Transcription: Mechanisms and Regulation. Conaway R C, Conaway J W, editors. New York: Raven; 1994. pp. 185–205. [Google Scholar]
- 10.Cochran M A, Puckett C, Moss B. J Virol. 1985;54:30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison A J, Moss B. J Mol Biol. 1989;210:749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- 12.Davison A J, Moss B. J Mol Biol. 1989;210:771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- 13.Coupar B E H, Andrew M E, Both G W, Boyle D B. Eur J Immunol. 1986;16:1479–1487. doi: 10.1002/eji.1830161203. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, McIndoe A, Davies H, Sun X-Y, Crawford L. Virology. 1991;181:203–210. doi: 10.1016/0042-6822(91)90485-t. [DOI] [PubMed] [Google Scholar]
- 15.Townsend A, Bastin J, Gould K, Brownlee G, Andrew M, Coupar B, Boyle D, Chan S, Smith G. J Exp Med. 1988;168:1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronte V, Tsung K, Rao J B, Chen P W, Wang M, Rosenberg S A, Restifo N P. J Immunol. 1995;154:5282–5292. [PMC free article] [PubMed] [Google Scholar]
- 17.Brattain M G, Strobel-Stevens J, Fine D, Webb M, Sarrif A M. Cancer Res. 1980;40:2142–2146. [PubMed] [Google Scholar]
- 18.Irvine K R, McCabe B J, Rosenberg S A, Restifo N P. J Immunol. 1995;154:4651–4657. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Bronte V, Chen P W, Gritz L, Panicali D, Rosenberg S A, Restifo N P. J Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 20.Rammensee H G, Schild H, Theopold U. Immunogenetics. 1989;30:296–302. doi: 10.1007/BF02421334. [DOI] [PubMed] [Google Scholar]
- 21.Chakrabarti S, Brechling K, Moss B. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earl P L, Moss B. In: Current Protocols in Molecular Biology. Ausubel F M, Kinston R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. New York: Greene/Wiley Interscience; 1991. pp. 16.18.1–16.18.10. [Google Scholar]
- 23.Smith G L, Levin J Z, Palese P, Moss B. Virology. 1987;160:336–345. doi: 10.1016/0042-6822(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 24.Gavin M A, Gilbert M J, Riddell S R, Greenberg P D, Bevan M J. J Immunol. 1993;151:3971–3980. [PubMed] [Google Scholar]
- 25.Van den Eynde B, Lethe B, Van Pel A, De Plaen E, Boon T. J Exp Med. 1991;173:1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irvine K R, Rao J B, Rosenberg S A, Restifo N P. J Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 27.Rao J B, Chamberlain R S, Bronte V, Carroll M W, Irvine K, Moss B, Rosenberg S A, Restifo N P. J Immunol. 1996;156:3357–3365. [PMC free article] [PubMed] [Google Scholar]
- 28.Blasco R, Moss B. Gene. 1995;158:157–162. doi: 10.1016/0378-1119(95)00149-z. [DOI] [PubMed] [Google Scholar]
- 29.Andrew M E, Boyle D B. In: Recombinant Poxviruses. Binns M M, Smith G L, editors. Boca Raton, FL: CRC; 1992. pp. 207–234. [Google Scholar]
- 30.Blake N W, Kettle S, Law K M, Gould K, Bastin J, Townsend A R, Smith G L. J Gen Virol. 1995;76:2393–2398. doi: 10.1099/0022-1317-76-9-2393. [DOI] [PubMed] [Google Scholar]
- 31.Steinman R M. Annu Rev Immunol. 1996;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 32.Inaba K, Metlay J P, Crowley M T, Witmer-Pack M, Steinman R M. Int Rev Immunol. 1996;6:197–206. doi: 10.3109/08830189009056630. [DOI] [PubMed] [Google Scholar]
- 33.Porgador A, Gilboa E. J Exp Med. 1995;182:255–260. doi: 10.1084/jem.182.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young J W, Inaba K. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg S A. Cancer J Sci Am. 1995;1:90–100. [PubMed] [Google Scholar]
- 36.Sprent J, Schaefer M. Immunol Rev. 1990;117:213–234. doi: 10.1111/j.1600-065x.1990.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 37.Broder C C, Kennedy P E, Michaels F, Berger E A. Gene. 1994;142:167–174. doi: 10.1016/0378-1119(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 38.Natuk R J, Holowczak J A. Virology. 1985;147:354–372. doi: 10.1016/0042-6822(85)90138-2. [DOI] [PubMed] [Google Scholar]
- 39.Bhardwaj N, Bender A, Gonzalez N, Bui L K, Garret M C, Steinman R M. J Clin Invest. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton-Easton A, Eichelberger M. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovacsovics-Bankowski M, Rock K L. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 42.Reis e Sousa C, Germain R N. J Exp Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norbury C C, Hewlett L J, Prescott A R, Shastri N, Watts C. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 44.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]