Abstract
Esterification of glycyrrhetinic acid (GA) with dehydrozingerone (DZ) resulted in a novel cytotoxic GA-DZ conjugate. Based on this exciting finding, we conjugated eleven different DZ analogs with GA or other triterpenoids, including oleanoic acid (OA) or ursolic acid (UA). In an in vitro anticancer assay using nine different human tumor cell lines, most of the GA-DZ conjugates showed significant potency. Particularly, compounds 5, 29, and 30 showed significant cytotoxic effects against LN-Cap, 1A9, and KB cells with ED50 values of 0.6, 0.8, and 0.9 μM, respectively. Similar conjugates between DZ and OA or UA were inactive suggesting that the GA component is critical for activity. Notably, although GA-DZ conjugates showed potent cytotoxic activity, the individual components (GA and DZ analogs) were inactive. Thus, GA-DZ conjugates are new chemical entities and represent interesting hits for anticancer drug discovery and development.
Keywords: Glycyrrhetinic acid, Dehydrozingerone, Conjugation, Cytotoxicity
1. Introduction
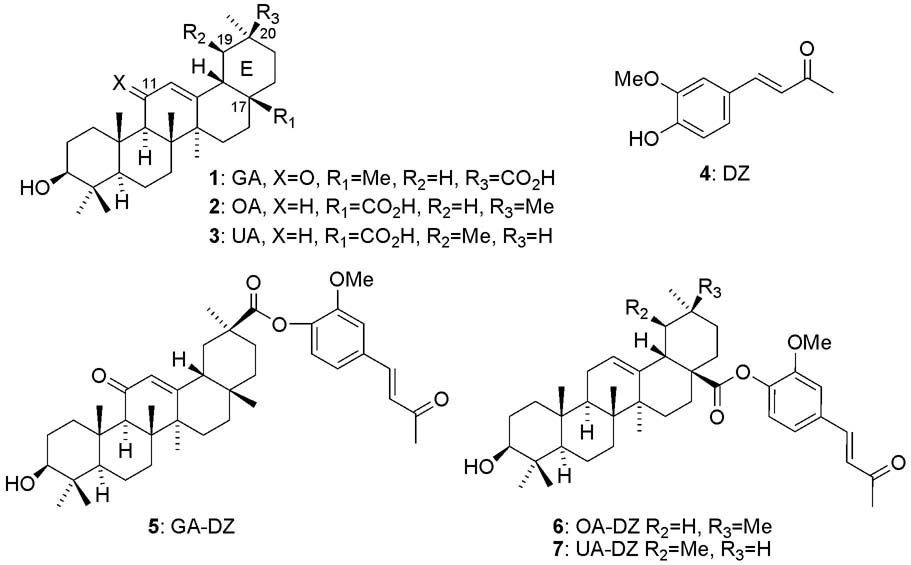
Triterpenoids, including glycyrrhetinic acid (GA) (1), oleanoic acid (OA) (2), and ursolic acid (UA) (3), are widely distributed in the plant kingdom worldwide and show various pharmacological activities. Structurally, OA and UA have a carboxylic acid on the C17 position rather than the C20 position as in GA and also do not have a ketone at C11 as does GA. GA (1) is the main constituent in the roots of the medicinal plant licorice (Glycyrrhiza glabra L), which is broadly used as a flavoring and sweetening agent in food products. The wide-ranging biological activities of GA include anti-inflammatory,1 anti-viral,2 anti-allergic,3,4 and antitumor promoting effects.5 OA (2) and its regioisomer UA (3) also have interesting biological activities.6 In addition, a well known phenolic natural product, dehydrozingerone (DZ) (4), possesses anti-flammatory, antioxidant, and antitumor promoting activities.7
Conjugation of two bioactive compounds is now accepted as an effective strategy for designing ligands, inhibitors, and other drugs.8 Our group has implemented the conjugation approach in drug discovery, which has led to promising results with varying compound classes.9 Therefore, based on the diverse bioactivities of the above-mentioned terpenoids as well as DZ, we initiated a structure activity relationship (SAR) study of terpenoid-DZ conjugation. Herein, we report the syntheses of terpenoid-DZ analogs and their unique cytotoxic activities.
2. Results and discussion
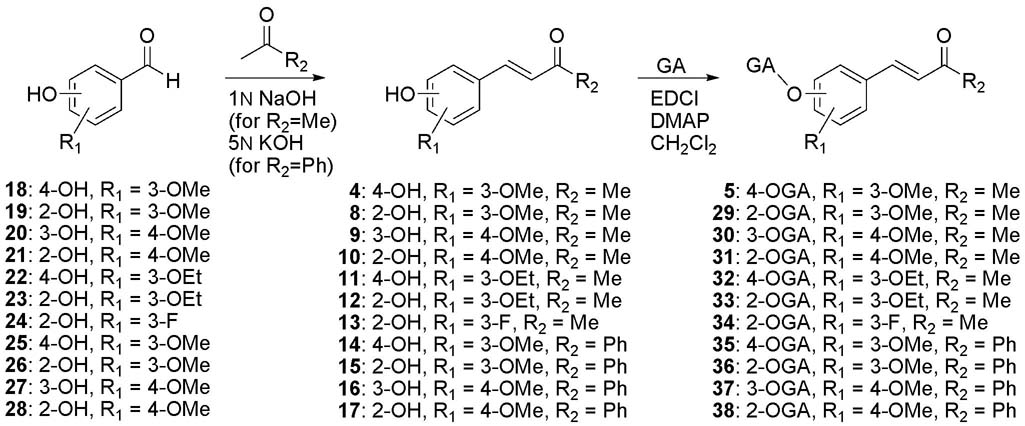
Conjugation of DZ with the terpene acids GA, OA, and UA was achieved by a well known esterification procedure using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) as condensation reagent in the presence of 4-dimethylaminopyridine (DMAP). Among the three initial conjugates, GA-DZ (5), OA-DZ (6), and UA-DZ (7), only compound 5 displayed significant cytotoxic activity as described later. This exciting finding prompted us to synthesize novel GA-DZ analogs 29–38 as shown in Scheme 1. DZ (4) and its analogs (8–17) were derived from the corresponding substituted benzaldehyde (18–28) with acetone or acetophenone via an aldol reaction. The conjugated compounds 29–38 were obtained from the resulting aldol products and GA using the same manner as described above.
Scheme 1.
Syntheses of GA-DZ conjugates 29–38.
Conjugates 5-7 were first evaluated against nasopharynx (KB) and multi-drug resistant KB sub-line expressing P-glycoprotein (KB-VIN) cancer cell lines as shown in Table 1. Interestingly, only GA-DZ (5) showed significant activity against these two cell lines with ED50 values of 1.7 and 2.8 μM, respectively, whereas its individual components (GA and DZ) were not active. OA-DZ (6) and UA-DZ (7) were also inactive. These results suggested that not only was conjugation important, but that a ketone on C11 and/or the position of carboxylic acid on C20 rather than C17 might be important factors for the activity. This exciting result prompted us to explore additional novel GA-DZ analogs 29–38.
Table 1.
Cytotoxicity screening of DZ-terpenoid conjugates
| Compound | ED50 (μM)a / Cell lineb | |
|---|---|---|
| KB | KB-VIN | |
| 5 (GA-DZ) | 1.7 | 2.8 |
| 6 (OA-DZ) | NAc | NA |
| 7 (UA-DZ) | 10.5 | NA |
| DZ | NA | NA |
| GA | NA | NA |
| OA | NA | NA |
| UA | 8.3 | 8.1 |
Cytotoxicity as ED50 values for each cell line, the concentration of compound that cause 50% reduction in absorbance at 562 nm relative to untreated cells using the sulforhodamine B assay. The average value is from two independent determinations and variation (SEM) was no greater than 10%.
Human epidermoid carcinoma of the nasopharynx (KB), multi-drug resistant KB sub-line expressing P-glycoprotein (KB-VIN)
Not active
All GA-DZ analogs 29–38 as well as GA-DZ (5) itself were tested against nine human tumor cell lines: KB, KB-VIN, lung (A549), ovarian (1A9), colon (HCT-8), breast (ZR-751), and prostate (PC-3, DU-145 and LN-Cap).10-13 Doxorubicin was used as a positive antitumor drug control, and GA and DZ were tested for comparison with the conjugates. The bioassay results are shown in Table 2.
Table 2.
Data for GA-DZ conjugates against human tumor cell replication
| Cmpd | ED50 (μM) / Cell linea | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| KB | KB-VIN | A549 | 1A9 | HCT-8 | ZR-751 | PC-3 | DU-145 | LN-Cap | |
| 5 | 1.6 | 2.5 | 2.0 | 0.9 | 1.7 | 2.8 | 1.4 | 3.1 | 0.6 |
| 29 | 0.8 | 2.8 | 2.2 | 0.8 | 1.9 | 3.0 | 1.1 | 3.6 | 2.8 |
| 30 | 0.9 | 1.9 | 2.8 | 1.6 | 2.0 | 1.9 | 2.8 | 9.9 | 6.5 |
| 31 | 6.2 | >15 | 15.5 | 5.9 | 2.6 | >15 | 7.4 | >15 | 1.9 |
| 32 | 1.8 | 1.7 | 1.7 | 1.1 | 2.7 | 5.2 | 3.3 | 5.8 | 1.1 |
| 33 | 2.9 | 13.2 | 3.0 | 1.8 | 4.9 | 8.8 | 3.5 | >15 | 6.8 |
| 34 | 3.0 | 8.7 | 3.2 | 1.3 | 2.2 | 2.7 | 1.6 | 2.7 | 4.4 |
| 35 | NA | NA | >14 | >14 | >14 | NA | >14 | >14 | >14 |
| 36 | 9.9 | NA | >14 | 13.3 | >14 | >14 | 14.1 | >14 | 14.1 |
| 37 | NA | NA | NA | >14 | >14 | NA | 14.1 | >14 | 14.1 |
| 38 | >14 | >14 | NA | NA | >14 | NA | >14 | 13.0 | >14 |
| GA | >21 | >21 | NA | >21 | 19.5 | NA | >21 | >21 | >21 |
| DZ | NA | NA | >52 | 33.9 | >52 | >52 | >52 | >52 | 51.0 |
| DOXb | 0.1 | 4.97 | 0.18 | 0.02 | 1.20 | 0.04 | 0.26 | 0.15 | 0.04 |
Human epidermoid carcinoma of the lung (A549), ovarian (1A9), colon (HCT-8), breast (ZR-751), prostate (PC-3, DU-145, LN-Cap).
Doxorubicin
These results indicate that, while conjugation between GA and DZ is necessary for significant cytotoxicity, the different substitution patterns on the DZ portion obviously affected the selectivity as well as activity against the nine tested cancer cell lines. Generally, conjugate 31, with a 2-GA ester and 4-OMe, was remarkably less potent than 5, 29, and 30, which showed significant cytotoxic effects against most of the cell lines with ED50 values less than 3.0 μM. This result suggested that an ortho relation between the methoxy and GA ester groups might be required for strong activity, possibly related to protein binding. Although both 29 and 31 bear the GA ester on the 2-position, 29 with 3-OMe showed broad spectrum cytotoxicity, while 31 with 4-OMe was selective against LN-Cap (EC50 = 1.9 μM) and HCT-8 (EC50 = 2.6 μM) cell lines. Regarding cell line selectivity, conjugate 5 with 4-GA ester and 3-OMe showed its highest potency (ED50 = 0.9 and 0.6 μM) against 1A9 and LN-Cap cells, respectively. Comparatively, conjugate 29 with 2-GA ester and 3-OMe displayed stronger activities against KB (EC50 = 0.8 μM), 1A9 (EC50 = 0.8 μM) and PC-3 (EC50 = 1.1 μM) than the remaining cell lines, and conjugate 30 with 3-GA ester and 4-OMe showed its strongest activity against KB cells with an ED50 value of 0.9 μM.
Replacing the methoxy (5, 29) with ethoxy (32, 33) caused decreased activity against all cell lines, with loss of activity against KB-VIN, A549, ZR-751, and DU-145 cells. To a lesser extent, a similar trend was found with a meta-fluoro group (compare 29 and 34). Replacing the methyl group at R2 with phenyl abolished the cytotoxic activity (see 35–38).
3. Conclusions
In summary, we found that conjugates between GA and DZ analogs showed significant cytotoxic activity, although the individual components, GA and DZ, and similar conjugates between DZ and similar terpenoids, OA and UA, did not. Novel GA-DZ analogs 5, 29, and 30, in which the methoxy and GA ester groups have an ortho relationship, showed significant cytotoxic activity against most of the tested cell lines. More interestingly, different DZ substitution patterns generated various cancer cell selectivities, with the highest potency against LN-Cap (ED50 = 0.6 μM) and 1A9 (ED50 = 0.9 μM) for 5, KB (ED50 = 0.8 μM) and 1A9 (ED50 = 0.8 μM) for 29, KB (ED50 = 0.9 μM) for 30. Thus, GA-DZ conjugates represent new hits for anticancer drug discovery and development.
4. Experimental
4.1. General
The proton nuclear magnetic resonance (1H-NMR) spectra were measured on a Varian Gemini 2000 (300 MHz) NMR spectrometer with TMS as the internal standard. All chemical shifts are reported in ppm. Mass spectra were obtained on a Hitachi M-4100H mass spectrometer. Analytical thin layer chromatography (TLC) was performed on Merck pre-coated aluminum silica gel sheets (Kieselgel 60 F 254). Column chromatography was performed on a CombiFlash Companion system using RediSep normal phase silica columns (ISCO, Inc., Lincoln, NE). All other chemicals were obtained from Aldrich, Inc. unless otherwise noted.
4.2. General Procedure for Aldol Reaction (4, 8–17)
The aldol intermediates were obtained by using the same procedure described by Elias and Rao.14 For 4 and 8–13, acetone with 1N NaOH was used, and for 14–17, acetophenone in MeOH with 5N KOH was used. Final compounds were purified with CombiFlash chromatography (EtOAc-hexane gradient).
4.3. General Procedure for Esterification (5, 29–38)
A CH2Cl2 solution of the same mol ratio of the corresponding terpenoid and DZ analog (4, 8–17) with a twofold mol ratio of EDCI and DMAP was stirred at room temperature under inert atmosphere overnight. The crude mixture was extracted with CH2Cl2, and the organic phase was washed with brine, dried over NaSO4, and concentrated in vacuo to obtain the product as a solid. The crude solid was purified with CombiFlash chromatography (EtOAc-hexane gradient).
4.3.1. DZ-GA conjugate (5)
1H NMR (300 MHz, CDCl3) δ 7.47 (d, 1H, J = 16.3 Hz, 1-H), 7.17-7.12 (m, 2H, 2′-H and 6′-H), 7.01 (d, 1H, J = 8.1 Hz, 5′-H), 6.60 (d, 1H, J = 16.3 Hz, 2-H), 5.70 (br s, 1H, 12″-H), 3.87 (s, 3H, OCH3), 3.22 (br s, 1H, 3β-H), 2.78 (br d, 1H, J = 13.5 Hz, 1″β-H), 2.44-2.38 (m, 1H, 1″α-H), 2.39 (s, 3H, 4-H), 2.35 (s, 1H, 9″α-H), 1.40 (s, 3H, CH3), 1.35 (s, 3H, CH3), 1.15 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.88 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.22 (C11″, C=O in GA), 198.18 (C-3, C=O in DZ), 174.33 [C-30″, GA-C(O)O-DZ], 169.14 (C-13″, -C=C- in GA), 151.57 (C-3′) 142.76 [-CH=CHC(O)Me in DZ], 141.74 (C-4′), 133.29 (C-1′), 128.55 [-CH=CHC(O)Me in DZ], 127.29 (=CH-), 123.21 (=CH-), 121.49 (=CH-), 111.25 (=CH-), 78.78 (C-3″, CH-OH in GA), 61.83 (C-9″, CH), 55.74 (-OCH3 in DZ), 54.97 (C-5″, CH), 48.10 (C-18″, CH), 45.36, 44.44 and 43.21 (C-8′, 14″ and 20″), 41.27 (C-19”, CH2), 39.12 (C-1″ or 21″, CH2), 37.46 (C-21″ or 1″, CH2), 37.08 (C-10″ or C-4″), 32.79 (C-7″, CH2), 31.87 (C-4″, or C10″), 31.21 (C-7″ or 21″, CH2), 28.54 (C-28″, CH3), 28.30 (C-24″, CH3), 28.11 (C-27″, CH3), 27.47 [C-1, -C(O)CH3], 27.31 (C-15″ or C-16″, CH2), 26.47 (C-16″ and C-15″, CH2×2), 23.40 (C-29″, CH3), 18.73 (CH3), 17.47 (C-6″, CH2), 16.35 (C-25″, CH3), 15.56 (C-23″, CH3); HR-SIMS m/z 645.4159 [M+H]+ (calcd for C41H57O6, 645.4152).
4.3.2. DZ-OA conjugate (6)
1H NMR (300 MHz, CDCl3): δ 7.46 (d, 1H, J = 16.3 Hz, 1-H), 7.16-7.10 (m, 2H, 2′-H and 6′-H), 6.97 (d, 1H, J = 8.1 Hz, 5′-H), 6.60 (d, 1H, J = 16.3 Hz, 2-H), 5.32 (br s, 1H, 12″-H), 3.83 (s, 3H, OCH3), 3.22 (br s, 1H, 3″α-H), 2.89 (m, 1H, 18″-H), 2.38 (s, 3H, 4-CH3), 1.19 (s, 3H, CH3), 1.00 (s, 3H, CH3), 0.94 (s, 3H, CH3), 0.92 (s, 3H, CH3), 0.90 (s, 3H, CH3), 0.85 (s, 3H, CH3), 0.79 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 198.23 (C-3, C=O in DZ), 175.48 (OA -C(O)O-DZ), 172.88 (C-13″, -C=C- in OA), 151.85 (C-3′), 143.20 [-CH=CHC(O)Me in DZ], 142.90 (C-4′), 142.20 (=CH-), 132.97 (C-1′), 127.09 [-CH=CHC(O)Me in DZ], 123.45 (=CH-), 123.04 (=CH-), 122.73 (=CH-), 111.20 (C′-2), 79.00 (C-3″, CH-OH in OA), 55.81 (-OCH3 in DZ), 55.23 (C-5″, CH), 48.35 (C-17″), 47.62 (C-9″, CH), 46.55 (C-19″, CH2), 41.80 (C-14″), 41.32 (C-18″, CH), 39.43 (C-8″), 38.75 (C-1″, CH2), 38.47 (C-4″). 37.00 (C-10″), 33.79 (CH2), 33.26 (CH3), 33.00 (CH2), 32.56 (CH2), 31.63 (CH2), 30.67 (C-20″), 28.11 (CH3), 27.59 (CH2), 27.45 [-C(O)CH3], 27.19 (CH2), 25.81 (CH3), 23.61 (CH3), 23.44 (CH2), 23.08 (C H2), 18.33 (CH2), 17.21 (CH3), 15.45 (CH3), 15.30 (CH3); MS m/z 631 [M+H]+; HR-SIMS m/z 669.4005 [M+K]+ (calcd for C41H58O5K, 669.3921).
4.3.3. DZ-UA conjugate (7)
1H NMR (300 MHz, CDCl3): δ 7.46 (d, 1H, J = 16.3 Hz, 1-H), 7.06 (dd, 1H, J = 8.1, 1.8 Hz, 6′-H), 7.09 (br s, 1H, 2′-H), 6.96 (d, 1H, J = 8.1 Hz, 5′-H), 6.60 (d, 1H, J = 16.3 Hz, 2-H), 5.31 (t, 1H, J = 3.9 Hz, 12″-H), 3.82 (s, 3H, OCH3), 3.21 (br s, 1H, 3″α-H), 2.35 (d, 1H, J = 11.1 Hz, 18-H), 2.38 (s, 3H, 4-CH3), 1.13 (s, 3H, CH3), 1.00 (s, 3H, CH3), 0.97 (d, 3H, J = 6.3 Hz, CH3), 0.94 (s, 3H, CH3), 0.89 (d, 3H, J = 4.5 Hz, CH3), 0.85 (s, 3H, CH3), 0.79 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 198.22 (C-3, C=O in DZ), 175.22 (C-30”, -C(O)O-DZ), 151.86 (C-3′or 4′), 142.89 [-CH=CHC(O)Me in DZ], 142.21 (C-4′ or 3′), 137.70 (=CH-), 132.97 (C-1′), 127.09 [-CH=CHC(O)Me in DZ], 126.02 (=CH-), 123.41 (=CH-), 121.52 (=CH-), 111.21 (C-2′), 79.03 (C-3″, CH-OH in UA), 55.80 (-OCH3 in DZ), 55.25 (C-5″, CH), 52.95 (C-18″ or 9″, CH), 48.78 (C-18″ or 9″, CH), 47.60 (C-17″), 42.26 (C-14″), 39.70 (C-8″), 39.19 (C-19″ or 20″, CH), 38.83 (C-19″ or 20″, CH), 38.76 (C-1″, CH2), 38.68 (C-4″), 37.00 (C-10″), 36.54 (CH2) 33.26 (CH3), 30.79 (CH2′), 28.15 (CH3), 27.46 [-C(O)CH3], 27.25 (CH2), 24.37 (CH2), 23.40 (CH2), 21.18 (CH3), 18.35 (CH2), 17.53 (CH3), 16.99 (CH3), 15.63 (CH3), 15.52 (CH3); HR-SIMS m/z 669.3967; [M+K]+ (calcd for C41H58O5K, 669.3921).MS m/z 631 [M+H]+.
4.3.4. DZ-GA conjugate (29)
1H NMR (300 MHz, CDCl3) δ 7.56 (d, 1H, J = 16.4 Hz, 1-H), 7.26-7.19 (m, 2H, 4′-H and 6′-H), 7.02-6.99 (m, 1H, 5′-H) 6.68 (d, 1H, J = 16.4 Hz, 2-H), 5.69 (s, 1H, 12″-H), 3.84 (s, 3H, OCH3), 3.23 (dd, 1H, J = 10.2, 6.3 Hz, 3″α-H), 2.77 (ddd, 1H, J = 13.7, 3.6, 3.6 Hz, 1″β-H), 2.53 (br-d, 1H, J = 13.7 Hz, 1″α-H) 2.35 (s, 1H, 9″α-H), 2.33 (s, 3H, 4-H), 1.45 (s, 3H, CH3), 1.41 (s, 3H, CH3), 1.15 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.16 (C11″, C=O in GA), 197.74 (C-3, C=O in DZ), 174.11 (C-30″, -C(O)O-DZ), 169.11 (C-13″, -C=C- in GA), 161.61 (C-3′or 2′), 138.96 (C-2′ or 3′), 136.23 [-CH=CHC(O)Me in DZ], 129.22 (=CH-), 128.63 (=CH-), 128.54 (C-1′), 126.60 (=CH-), 118.38 (=CH-), 113.79 (=CH-), 78.75 (C-3″, CH-OH in GA), 61.82 (C-9″, CH), 55.80 (-OCH3 in DZ), 54.95 (C-5″, CH), 47.77 (C-18″, CH), 45.37, 44.68 and 43.20 (C-8′, 14″ or 20″), 41.27 (C-19″, CH2), 39.12 (C-1″ or 22″, CH2), 37.08 (C-10″), 32.78 (C-7″ or 21″, CH2), 31.88 (C-4″), 31.30 (C-7″ or 21″, CH2), 28.62 (CH3), 28.54 (CH3), 28.09 (CH3), 27.50 [-C(O)CH3], 27.29 (CH2), 26.53 (CH2), 26.40 (C-16″ or -15″, CH2), 23.42 (C-29″, CH3), 18.71 (CH3), 17.49 (C-6”, CH2), 16.33 (C-25″, CH3), 15.56 (C-23″, CH3); HR-SIMS m/z 645.4160 [M+H]+ (calcd for C41H57O6, 645.4152).
4.3.5. DZ-GA conjugate (30)
1H NMR (300 MHz, CDCl3) δ 7.44 (d, 1H, J = 16.3 Hz, 1-H), 7.40 (dd, 1H, J = 8.5, 2.2 Hz, 6′-H), 7.20 (d, 1H, J = 2.2 Hz, 2′-H), 6.98 (d, 1H, J = 8.5 Hz, 5′-H) 6.59 (d, 1H, J = 16.3 Hz, 2-H), 5.70 (s, 1H, 12″-H), 3.87 (s, 3H, OCH3), 3.22 (m, 1H, 3″α-H), 2.78 (ddd, J = 13.5, 3.5, 3.5 Hz, 1″β-H), 2.42 (br-d, 1H, J = 13.5, 2.8 Hz, 1″α-H), 2.37 (s, 3H, 4-H), 2.35 (s, 1H, 9″α-H), 1.40 (s, 3H, CH3), 1.37 (s, 3H, CH3), 1.15 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.89 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.24 (C11″, C=O in GA), 198.21 (C-3, C=O in DZ), 174.42 (C-30″, -C(O)O-DZ), 169.19 (C-13″, -C=C- in GA), 155.12 (C-3′or 4′), 142.34 [-CH=CHC(O)Me in DZ], 140.08 (C-4′ or 3′), 128.52 (=CH-), 127.77 (C-1′), 127.48 (=CH-), 125.94 (=CH-), 121.99 (=CH-), 112.40 (=CH-), 78.76 (C-3″, CH-OH in GA), 61.83 (C-9″, CH), 55.86 (-OCH3 in DZ), 54.94 (C-5″, CH), 48.07 (C-18″, CH), 45.39, 44.42 and 43.20 (C-8’, 14″ or 20″), 41.25 (C-19″, CH2), 39.13 (C-1″ or 22″, CH2), 37.45 (C-10”), 37.08 (C-1″ or 22″, CH2), 32.78 (C-7″ or 21″, CH2), 31.88 (C-4″), 31.22 (C-7″ or 21″, CH2), 28.58 (CH3), 28.34 (CH3), 28.09 (CH3), 27.44 [-C(O)CH3], 27.30 (CH2), 26.53 (CH2), 26.44 (C-16″ or -15″, CH2), 23.40 (C-29″, CH3), 18.71 (CH3), 17.49 (C-6”, CH2), 16.36 (C-25”, CH3), 15.57 (C-23”, CH3); HR-SIMS m/z 645.4157 [M+H]+ (calcd for C41H57O6, 645.4152).
4.3.6. DZ-GA conjugate (31)
1H NMR (300 MHz, CDCl3) δ 7.63 (d, 1H, J = 8.8 Hz, 6’-H), 7.53 (d, 1H, J = 16.2 Hz, 1-H), 6.84 (dd, 1H, J = 8.8, 2.5 Hz, 5′-H) 6.56 (d, 1H, J = 2.5 Hz, 3′-H), 6.61 (d, 1H, J = 16.2 Hz, 2-H), 5.67 (s, 1H, 12″-H), 3.85 (s, 3H, OCH3), 3.23 (ddd, 1H, J = 9.9, 6.3, 6.3 Hz, 3″α-H), 2.78 (ddd, J = 13.7, 3.5, 3.5 Hz, 1″β-H), 2.30 (br-d, 1H, J = 13.7, 3.5 Hz, 1″α-H), 2.31 (s, 3H, 4-H), 2.35 (s, 1H, 9″α-H), 1.45 (s, 3H, CH3), 1.40 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.89 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 199.99 (C11″, C=O in GA), 197.63 (C-3, C=O in DZ), 174.63 (C-30″, -C(O)O-DZ), 168.40 (C-13″, -C=C- in GA), 162.22 (C-4′or 2′), 150.98 (C-4′ or 2′), 136.01 (=CH-), 128.80 (=CH-), 128.11 (=CH-), 126.48 (=CH-), 120.00 (C-1′), 112.48 (=CH-), 108.46 (=CH-), 78.76 (C-3″, CH-OH in GA), 61.84 (C-9″, CH), 55.65 (-OCH3 in DZ), 54.96 (C-5″, CH), 48.24 (C-18″, CH), 45.39, 44.63 and 43.22 (C-8′, 14″ or 20″), 41.04 (C-19″, CH2), 39.12 (C-1″ or 22″, CH2), 37.91 (C-1″ or 22″, CH2), 37.11 (C-10″), 32.79 (C-7″ or 21″, CH2), 31.98 (C-4″), 31.24 (C-7″ or 21″, CH2), 28.57 (CH3), 28.30 (CH3), 28.10 (CH3), 27.64 [-C(O)CH3], 27.31 (CH2), 26.46 (CH2), 26.39 (CH2), 23.46 (C-29″, CH3), 18.70 (CH3), 17.50 (C-6″, CH2), 16.34 (C-25″, CH3), 15.55 (C-23″, CH3); HR-SIMS m/z 645.4145 [M+H]+ (calcd for C41H57O6, 645.4152).
4.3.7. DZ-GA conjugate (32)
1H NMR (300 MHz, CDCl3) δ 7.47 (d, 1H, J = 16.2 Hz, 1-H), 7.13 (dd, 1H, J = 8.0, 1.9 Hz, 6′-H), 7.11 (d, 1H, J = 1.9 Hz, 2′-H), 6.98 (d, 1H, J = 8.0 Hz, 5′-H) 6.59 (d, 1H, J = 16.2 Hz, 2-H), 5.71 (s, 1H, 12-H), 4.10 (q, 2H, J = 6.9 Hz, OCH2CH3), 3.23 (m, 1H, 3″α-H), 2.78 (ddd, J = 13.5, 3.4, 3.4 Hz, 1″β-H), 2.35-2.40 (m, 1H, 1″α-H), 2.39 (s, 3H, 4-H), 2.35 (s, 1H, 9″α-H), 1.40 (t, 3H, J = 6.9 Hz, OCH2CH3), 1.40 (s, 3H, CH3), 1.37 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.87 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.14 (C11″, C=O in GA), 198.25 (C-3, C=O in DZ), 174.24 (C-30″, -C(O)O-DZ), 169.02 (C-13″, -C=C- in GA), 151.00 (C-4′or 3′), 142.88 [-CH=CHC(O)Me in DZ], 141.71 (C-4′ or 3′), 133.22 (C-1′), 128.63 (=CH-), 127.19 (=CH-), 123.67 (=CH-), 121.24 (=CH-), 111.96 (=CH-), 108.46 (=CH-), 78.75 (C-3″, CH-OH in GA), 64.23 (-OCH2CH3 in DZ), 61.82 (C-9″, CH), 54.94 (C-5″, CH), 48.11 (C-18″, CH), 45.39, 44.40 and 43.18 (C-8′, 14″ or 20″), 41.11 (C-19″, CH2), 39.12 (C-1″ or 22″, CH2), 37.62 (C-1″ or 22″, CH2), 37.09 (C-10″), 32.75 (C-7″ or 21″, CH2), 31.92 (C-4″), 31.14 (C-7″ or 21″, CH2), 28.49 (CH3), 28.38 (CH3), 28.08 (CH3), 27.45 [-C(O)CH3], 27.29 (CH2), 26.50 (CH2), 26.43 (CH2), 23.45 (CH3), 18.68 (CH3), 17.47 (C-6″, CH2), 16.33 (C-25″, CH3), 15.55 (C-23″, CH3), 14.85 (OCH2CH3 in DZ); HR-SIMS m/z 659.4315 [M+H]+ (calcd for C42H59O6, 659.4309).
4.3.8. DZ-GA conjugate (33)
1H NMR (300 MHz, CDCl3) δ 7.59 (d, 1H, J = 16.5 Hz, 1-H), 7.23-7.17 (m, 2H, 5′-H and 6′-H), 6.99 (dd, 1H, J = 6.8, 2.9 Hz, 4′-H), 6.68 (d, 1H, J = 16.5 Hz, 2-H), 5.70 (s, 1H, 12″-H), 4.10 (q, 2H, J = 6.9 Hz, OCH2CH3), 3.23 (m, 1H, 3″α-H), 2.78 (ddd, J = 9.9, 3.6, 3.6 Hz, 1″β-H), 2.49 (m, 1H, 1″α-H), 2.33 (s, 3H, 4-H), 2.35 (s, 1H, 9″α-H), 1.39 (t, 3H, J = 6.9 Hz, OCH2CH3), 1.46 (s, 3H, CH3), 1.41 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.23 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.91 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.07 (C11″, C=O in GA), 197.66 (C-3, C=O in DZ), 174.03 (C-30″, -C(O)O-DZ), 168.97 (C-13″, -C=C- in GA), 150.98 (C-2′or 3′), 138.96 (C-2′ or 3′), 136.35 (=CH-), 129.11 (=CH-), 128.78 (=CH-), 128.69 (=CH-), 126.56 (=CH-), 118.07 (=CH-), 114.38 (=CH-), 78.76 (C-3″, CH-OH in GA), 64.28 (-OCH2CH3 in DZ), 61.83 (C-9″, CH), 54.95 (C-5″, CH), 47.69 (C-18″, CH), 45.39, 44.64 and 43.17 (C-8′, 14″ or 20″), 41.07 (C-19″, CH2), 39.12 (C-1″ or 22″, CH2), 37.80 (C-1″ or 22″, CH2), 37.09 (C-10″), 32.75 (C-7″ or 21″, CH2), 31.96 (C-4″), 31.27 (C-7″ or 21″, CH2), 28.92 (CH3), 28.43 (CH3), 28.09 (CH3), 27.72 [-C(O)CH3], 27.30 (CH2), 26.50 (CH2), 26.36 (CH2), 23.54 (CH3), 18.70 (CH3), 17.49 (C-6″, CH2), 16.34 (C-25″, CH3), 15.56 (C-23″, CH3), 14.92 (OCH2CH3 in DZ); HR-SIMS m/z 659.4311 [M+H]+ (calcd for C42H59O6, 659.4309).
4.3.9. DZ-GA conjugate (34)
1H NMR (300 MHz, CDCl3) δ 7.53 (d, 1H, J = 16.5 Hz, 1-H), 7.45-7.17 (m, 3H, 4′, 5′, and 6’-H), 6.72 (d, 1H, J = 16.5 Hz, 2-H), 5.68 (s, 1H, 12″-H), 3.23 (m, 1H, 3″α-H), 2.78 (ddd, J = 9.6, 6.0, 6.0 Hz, 1″β-H), 2.38 (m, 1H, 1″α-H), 2.35 (s, 3H, 4-H), 2.35 (s, 1H, 9″α-H), 1.45 (s, 3H, CH3), 1.40 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.89 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.11 (C11″, C=O in GA), 197.36 (C-3, C=O in DZ), 173.75 (C-30″, -C(O)O-DZ), 168.61 (C-13”, -C=C- in GA), 156.26 (C-3′or 2′), 137.54 (C-3′ or 2′), 134.99 (=CH-), 129.97 (C-1′), 129.76 (=CH-), 128.76 (=CH-), 126.93 (=CH-), 122.29 (=CH-), 118.18 (=CH-), 78.77 (C-3″, CH-OH in GA), 61.83 (C-9″, CH), 54.96 (C-5″, CH), 47.98 (C-18″, CH), 45.38, 44.82 and 43.18 (C-8′, 14″ or 20″), 41.16 (C-19″, CH2), 39.13 (C-1″ or 22″, CH2), 37.57 (C-1″ or 22″, CH2), 37.10 (C-10″), 32.78 (C-7″ or 21″, CH2), 31.89 (C-4″), 31.29 (C-7″ or 21″, CH2), 28.42 (CH3), 28.38 (CH3), 28.10 (CH3), 27.79 [-C(O)CH3], 27.31 (CH2), 26.49 (CH2), 26.41 (CH2), 23.43 (CH3), 18.70 (CH3), 17.49 (C-6″, CH2), 16.34 (C-25″, CH3), 15.61 (C-23″, CH3); HR-SIMS m/z 633.3949 [M+H]+ (calcd for C40H54O5F5, 633.3953).
4.3.10. DZ-GA conjugate (35)
1H NMR (300 MHz, CDCl3) δ 8.03-7.49 (m, 5H, 2‴- 6‴-H) 7.77 (d, 1H, J = 15.8 Hz, 1-H), 7.46 (d, 1H, J = 15.8 Hz, 2-H), 7.26 (dd, 1H, J = 8.3 Hz, 6′-H), 7.21 (d, 1H, J = 1.9 Hz, 2′-H), 7.04 (d, 1H, J = 8.3 Hz, 5′-H), 5.71 (s, 1H, 12″-H), 3.90 (s, 3H, OCH3), 3.23 (dd, 1H, J = 10.0, 5.9 Hz, 3″α-H), 2.78 (ddd, 1H, J = 13.5, 3.2, 3.2 Hz, 1″β-H), 2.41 (br-d, 1H, J = 13.5 Hz, 1″α-H), 2.35 (s, 1H, 9″α-H), 1.40 (s, 3H, CH3), 1.37 (s, 3H, CH3), 1.16 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.89 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.48 (C11″ C=O in GA), 190.80 (C-3, C=O in DZ), 174.58 (C-30″, -C(O)O-DZ), 169.40 (C-13″, -C=C- in GA), 151.78 (C-4′or 3′), 144.51 [-CH=CHC(O)Ph in DZ], 141.95 (C-4′ or 3′), 138.43 (C-4), 133.99 (C-1′), 133.03 (=CH-), 128.87 (C×2 in Ph), 128.76 (C×2 in Ph), 123.44 (=CH-), 122.57 (=CH-), 121.62 (=CH-), 112.03 (=CH-), 79.01 (C-3″, CH-OH in GA), 62.07 (C-9”, CH), 56.03 (-OCH3 in DZ), 55.19 (C-5″, CH), 48.33 (C-18″, CH), 45.63, 44.68 and 43.45 (C-8′, 14″ or 20″), 41.52 (C-19”, CH2), 39.36 (C-1″ or 22″, CH2), 37.69 (C-1″ or 22″, CH2), 37.32 (C-10″), 33.02 (C-7″ or 21″, CH2), 32.12 (C-4″), 31.46 (C-7″ or 21″, CH2), 28.80 (CH3), 28.54 (CH3), 28.32 (CH3), 27.55 (CH2), 26.69 (CH2), 23.64 (CH2), 18.95 (CH3), 17.73 (C-6″, CH2), 16.60 (C-25″, CH3), 15.80 (C-23″, CH3); HR-SIMS m/z 707.4306 [M+H]+ (calcd for C46H59O6, 707.4309).
4.3.11. DZ-GA conjugate (36)
1H NMR (300 MHz, CDCl3) δ 8.00-7.46 (m, 5H, 2‴-6‴-H) 7.84 (d, 1H, J = 15.9 Hz, 1-H), 7.48 (d, 1H, J = 15.9 Hz, 2-H), 7.38 (dd, 1H, J = 8.1, 1.5 Hz, 6′-H), 7.25 (dd, 1H, J = 8.1, 8.1 Hz, 5′-H), 7.03 (dd, 1H, J = 8.1, 1.5 Hz, 4′-H), 5.69 (s, 1H, 12″-H), 3.85 (s, 3H, OCH3), 3.23 (m, 1H, 3″α-H), 2.77 (ddd, 1H, J = 13.5, 3.4, 3.4 Hz, 1″β-H), 2.77 (ddd, 1H, J = 13.5, 3.4, 3.4 Hz, 1″α-H), 2.34 (s, 1H, 9″α-H), 1.40 (s, 3H, CH3), 1.40 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.88 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.17 (C11″, C=O in GA), 190.24 (C-3, C=O in DZ), 174.24 (C-30″, -C(O)O-DZ), 151.74 (C-13″, -C=C- in GA), 139.27 (C-2′or 3′), 138.05 (C-2′ or 3′), 137.73 (=CH-), 132.74 (=CH-), 129.17 (C-4), 128.60 (C×2 in Ph), 128.50 (C×2 in Ph), 126.63 (=CH-), 124.47 (=CH-), 118.30 (=CH-), 113.81 (=CH-), 78.76 (C-3″, CH-OH in GA), 61.80 (C-9″, CH), 55.84 (-OCH3 in DZ), 54.95 (C-5″, CH), 47.79 (C-18″, CH), 45.37, 44.68 and 43.20 (C-8′, 14″ or 20″), 41.30 (C-19″, CH2), 39.12 (C-1″ or 22″, CH2), 37.47 (C-1″ or 22″, CH2), 37.08 (C-10″), 32.79 (C-7″ or 21″, CH2), 31.85 (C-4″), 31.31 (C-7″ or 21″, CH2), 28.55 (CH3), 28.51 (CH3), 28.09 (CH3), 27.30 (CH2), 26.55 (CH2), 26.42 (CH2), 23.39 (CH3), 18.71 (CH3), 17.50 (C-6″, CH2), 16.34 (C-25″, CH3), 15.56 (C-23″, CH3); HR-SIMS m/z 707.4315 [M+H]+ (calcd for C46H59O6, 707.4309).
4.3.12. DZ-GA conjugate (37)
1H NMR (300 MHz, CDCl3) δ 8.02-7.45 (m, 5H, 2‴-6‴-H) 7.74 (d, 1H, J = 15.7 Hz, 1-H), 7.39 (d, 1H, J = 15.7 Hz, 2-H), 7.49 (dd, 1H, J = 8.3 Hz, 6′-H), 7.31 (d, 1H, J = 1.9 Hz, 2′-H), 7.00 (d, 1H, J = 8.5 Hz, 5′-H), 5.72 (s, 1H, 12″-H), 3.89 (s, 3H, OCH3), 3.23 (m, 1H, 3″α-H), 2.78 (dm, 1H, J = 13.7 Hz, 1″β-H), 2.44 (dm, 1H, J = 13.7 Hz, 1″α-H), 2.35 (s, 1H, 9″α-H), 1.40 (s, 3H, CH3), 1.39 (s, 3H, CH3), 1.16 (s, 3H, CH3), 1.14 (s, 3H, CH3), 1.01 (s, 3H, CH3), 0.89 (s, 3H, CH3), 0.81 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.21 (C11″, C=O in GA), 190.53 (C-3, C=O in DZ), 174.45 (C-30″, -C(O)O-DZ), 169.19 (C-13″, -C=C- in GA), 153.17 (C-4′or 3′), 143.85 [-CH=CHC(O)-Ph in DZ], 140.10 (C-4′ or 3′), 138.33 (C-4), 132.61 (=CH-), 128.57 (C×2 in Ph), 128.47 (C×2 in Ph), 128.28 (=CH-), 128.15 (=CH-), 128.03 (C-1′), 121.93 (=CH-), 120.89 (=CH-), 112.38 (=CH-), 78.76 (C-3″, CH-OH in GA), 61.82 (C-9″, CH), 55.86 (-OCH3 in DZ), 54.94 (C-5″, CH), 48.00 (C-18″, CH), 45.39, 44.42 and 43.20 (C-8′, 14″ or 20″), 41.28 (C-19″, CH2), 39.12 (C-1″ or 22″, CH2), 37.47 (C-1″ or 22″, CH2), 37.08 (C-10″), 32.77 (C-7″ or 21″, CH2), 31.89 (C-4″), 31.25 (C-7″ or 21″, CH2), 28.58 (CH3), 28.39 (CH3), 28.09 (CH3), 27.30 (CH2), 26.53 (CH2), 26.45 (CH2), 23.41 (CH3), 18.71 (CH3), 17.49 (C-6″, CH2), 16.36 (C-25″, CH3), 15.57 (C-23″, CH3); HR-SIMS m/z 707.4305 [M+H]+ (calcd for C46H59O6, 707.4309).
4.3.13. DZ-GA conjugate (38)
1H NMR (300 MHz, CDCl3) δ 7.99-7.45 (m, 5H, Ar-H) 7.82 (d, 1H, J = 15.7 Hz, 1-H), 7.40 (d, 1H, J = 15.7 Hz, 2-H), 7.77 (dd, 1H, J = 8.5 Hz, 6′-H), 6.87 (dd, 1H, J = 8.8, 2.5 Hz, 5′-H), 6.59 (d, 1H, J = 2.5 Hz, 3′-H), 5.66 (s, 1H, 12″-H), 3.87 (s, 3H, OCH3), 3.22 (dd, 1H, J = 10.0, 6.2 Hz, 3″α-H), 2.76 (ddd, 1H, J = 13.5, 3.3, 3.3 Hz, 1″β-H), 2.29 (br-dd, 1H, J = 13.5, 4.6 Hz, 1″α-H), 2.34 (s, 1H, 9″α-H), 1.41 (s, 3H, CH3), 1.39 (s, 3H, CH3), 1.12 (s, 3H, CH3), 1.12 (s, 3H, CH3), 1.00 (s, 3H, CH3), 0.86 (s, 3H, CH3), 0.80 (s, 3H, CH3); 13C NMR (300 MHz, CDCl3) δ 200.02 (C11″, C=O in GA), 190.27 (C-3, C=O in DZ), 174.74 (C-30″, -C(O)O-DZ), 168.57 (C-13″, -C=C- in GA), 162.26 (C-4′or 2′), 151.29 (C-4′ or 2′), 138.33 (C-4), 137.61 (=CH-), 132.55 (=CH-), 128.73 (=CH-), 128.55 (C×2 in Ph), 128.42 (C×2 in Ph), 128.00 (=CH-), 121.61 (=CH-), 120.56 (C-1′), 112.53 (=CH-), 108.40 (=CH-), 78.77 (C-3″, CH-OH in GA), 61.80 (C-9″, CH), 55.68 (-OCH3 in DZ), 54.93 (C-5″, CH), 48.22 (C-18″, CH), 45.38, 44.632 and 43.20 (C-8′, 14″ or 20″), 41.04 (C-19″, CH2), 39.12 (C-1″ or 22″, CH2), 37.80 (C-1″ or 22″, CH2), 37.07 (C-10″), 32.78 (C-7″ or 21″, CH2), 31.95 (C-4″), 31.24 (C-7″ or 21″, CH2), 28.53 (CH3), 28.29 (CH3), 28.09 (CH3), 27.30 (CH2), 26.46 (CH2), 26.38 (CH2), 23.44 (CH3), 18.69 (CH3), 17.49 (C-6″, CH2), 16.35 (C-25″, CH3), 15.56 (C-23″, CH3); HR-SIMS m/z 707.4320 [M+H]+ (calcd for C46H59O6, 707.4309).
Figure 1.
Structures of GA, OA, UA, DZ, and their conjugates.
Acknowledgments
This investigation was supported in part by a grant from the National Cancer Institute (CA 17625) awarded to K. H. Lee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Finney RS, Tarnoky AL. J Pharm Pharmacol. 1960;12:49. doi: 10.1111/j.2042-7158.1960.tb12629.x. [DOI] [PubMed] [Google Scholar]
- 2.Pompei R, Flore O, Marccialis MA, Pani A, Loddo B. Nature. 1979;281:689. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka S, Uno C, Akimoto M, Tabata M, Honda C, Kamisako W. Planta Med. 1991;57:527. doi: 10.1055/s-2006-960199. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa Y, Mizoguchi Y, Kioka K, Kobayashi K, Tomekawa K, Morosawa S, Yamamoto S. Arerugi. 1989;38:365. [PubMed] [Google Scholar]
- 5.Okamoto H, Yoshida D, Mizusaki S. Cancer Lett. 1983;19:47. doi: 10.1016/0304-3835(83)90134-9. [DOI] [PubMed] [Google Scholar]
- 6.Liu J. J Ethnopharm. 1995;49:57.Liu J. J Ethnopharm. 2005;100:92. doi: 10.1016/j.jep.2005.05.024. and references therein.
- 7.Motohashi N, Yamagami C, Tokuda H, Konoshima T, Okuda Y, Okuda M, Mukainaka T, Nishino H, Saito Y. Cancer Lett. 1998;134:37. doi: 10.1016/s0304-3835(98)00239-0. [DOI] [PubMed] [Google Scholar]
- 8.Choi SK. Synthetic Multivalent Molecules. Wiley-Interscience; New York: 2004. [Google Scholar]
- 9.Bastow KF, Wang HK, Cheng YC, Lee KH. Bioorg Med Chem. 1997;5:1481. doi: 10.1016/s0968-0896(97)00102-8. [DOI] [PubMed] [Google Scholar]; Chang JY, Guo X, Chen HX, Jiang Z, Fu Q, Wang HK, Bastow KF, Zhu XK, Guan J, Lee KH, Cheng TC. Biochem Pharm. 2000;59:497. doi: 10.1016/s0006-2952(99)00363-9. [DOI] [PubMed] [Google Scholar]; Shi Q, Wang HK, Bastow KF, Tachibana Y, Chen K, Lee FY, Lee KH. Bioorg Med Chem. 2001;9:2999. doi: 10.1016/s0968-0896(01)00206-1. [DOI] [PubMed] [Google Scholar]; Ohtsu H, Nakanishi Y, Bastow KF, Lee FY, Lee KH. Bioorg Med Chem. 2003;11:1851. doi: 10.1016/s0968-0896(03)00040-3. [DOI] [PubMed] [Google Scholar]; Nakagawa-Goto K, Nakamura S, Bastow KF, Nyarko A, Peng CY, Lee YF, Lee FC, Lee KH. Bioorg Med Chem Lett. 2007;17(10):2894–2898. doi: 10.1016/j.bmcl.2007.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nakagawa-Goto K, Yamada K, Nakamura S, Chen TH, Bastow KF, Wang SC, Hung MC, Lee FY, Lee FC, Lee KH. Bioorg Med Chem Lett. doi: 10.1016/j.bmcl.2007.06.083. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The in vitro cytotoxicity assay was carried out according to procedures described in Rubinstein et al.11 Drug stock solutions were prepared in DMSO, and the final solvent concentration was <1% DMSO (v/v), a concentration without effect on cell replication. The human tumor cell line panel consisted of epidermoid carcinoma of the nasopharynx (KB), a multi-drug resistant KB sub-line expressing P-glycoprotein (KB-VIN), lung (A549), ovarian (1A9), colon (HCT-8), breast (ZR-751), and prostate (PC-3, DU-145, LN-Cap). The KB-VIN tumor cell line is cross-resistant to doxorubicin (Table 1). Detailed characterization of this cell is described elsewhere.10,11 Cells were cultured at 37 °C in RPMI-1640 with 100 μg/mL kanamycin and 10% (v/v) fetal bovine serum in a humidified atmosphere containing 5% CO2. Initial seeding densities varied among the cell lines to ensure a final absorbance of 1-2.5 A562 units. Drug exposure was for two days and the ED50 value, the drug concentration that reduced the absorbance by 50%, was interpolated from dose-response data. Each test was performed in duplicate, and absorbance readings varied no more than 5% between replicates.
- 11.Rubinstein LV, Shoemaker RH, Paull KD, Simo RM, Tosini S, Skehan P, Scudiero PA, Monks A, Boyd MR. J Natl Cancer Inst. 1990;82:1113. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson PJ, Fisher MH, Stephenson J, Li DH, Zhou BS, Cheng YC. Cancer Res. 1988;48:5956. [PubMed] [Google Scholar]
- 13.Beidler DR, Chang JY, Zhou BS, Cheng YC. Cancer Res. 1996;56:345. [PubMed] [Google Scholar]
- 14.Elias G, Rao MNA. Eur J Med Chem. 1988;23:379. [Google Scholar]