Abstract
Polycyclic aromatic hydrocarbons (PAH) such as benzo[a]pyrene (BaP) mainly induce lung cancer in humans, but induce liver cancer in fishes. The chemoprevention of cancers through inhibition of molecular events via phytochemicals is a potentially beneficial area of research, and has been carried out in human cell cultures in the past. Carcinogenesis initiation events are thought to occur in similar ways in fish and humans. Our study investigated the feasibility of using cultured rainbow trout CRL-2301 liver cells as a model for BaP-induced carcinogenesis and its prevention by dietary phytochemicals.
Treatment with 1 μM BaP resulted in extensive time-dependent covalent binding to cellular DNA and marked cytochrome P450 (CYP) 1A induction, for both about a 20-fold increase, which is similar to what has been observed in cultured human cells. A surprisingly high expression of epoxide hydrolase (EH) activity in these cells likely contributed substantially to the bioactivation of BaP. Two methoxylated flavones and the stilbene resveratrol were effective inhibitors of both the BaP-DNA binding and CYP 1A induction, in particular 5,7-dimethoxyflavone (5,7-DMF), supporting a role for these dietary compounds as cancer chemopreventive agents. Unlike in human liver or bronchial cells, the main mechanism of inhibition of BaP-induced CYP 1A activity in trout liver cells appears to be direct competition at the protein level. Different cellular responses in any particular model used can be expected and the effect of cell context on the biological responses to xenobiotics, including carcinogens as well as polyphenols, must be considered. The trout CRL-2301 cells' sensitivity to BaP treatment is a clear advantage when contemplating a model system for studies of PAH-induced carcinogenesis and cancer chemoprevention. However, extrapolation to human organs should be done cautiously.
Keywords: benzo[a]pyrene; trout; chemoprevention; flavonoids; CYP 1A; 5,7-dimethoxyflavone
1. Introduction
Benzo[a]pyrene (BaP) is a prototypical polycyclic aromatic hydrocarbon (PAH) formed by the incomplete combustion of many organic materials and is ubiquitously present in our environment. Various anatomical sites of cancer are related to ingestion and inhalation of PAHs [1] and survival rates for these types of cancer have not significantly improved over the past 10 years. The bioactivation of BaP, the enzymes involved and the subsequent binding to DNA has been well studied, including recent studies in both human liver [2] and lung [3] cells. The prevention of cancers through inhibition of such molecular events has become an important focus. Thus, chemoprevention via phytochemicals as a means of inhibiting chemically-induced carcinogenesis is a potentially beneficial area of research [4-6]. Recent studies have been carried out to assess the protective effects of bioavailable flavonoids on BaP-induced DNA binding in human cell cultures [2, 3, 7, 8].
Fish have been used for many years as experimental organisms across the disciplines, including ecotoxicological studies, risk assessment and chemical contamination studies [9-11]. Fundamental principles addressing biomedical questions are common for all vertebrates, and carcinogenesis initiation events are thought to occur in similar ways in fish and humans. In their diet and in their aquatic environment, fish and other aquatic organisms are exposed to xenobiotics, including contaminants such as BaP and other PAHs [11-14], but also flavonoids and other polyphenols [15-17]. Whereas BaP and BaP-like compounds mainly, but far from exclusively, induce lung cancer in humans, they mainly induce liver cancer in fishes [18].
Rainbow trout (Oncorhynchus mykiss) is one of the more commonly used fish species in aquaculture. Various cell lines derived from rainbow trout and other fishes have been used successfully as sensitive models [9-11] for many years. Our study investigated the feasibility of using a primary rainbow trout liver cell line (ATCC CRL-2301) [19] as a model for BaP-induced carcinogenesis and its prevention by dietary phytochemicals.
2. Materials & Methods
2.1. Chemicals
5,7-Dimethoxyflavone (5,7-DMF) and 3',4'-dimethoxyflavone (3',4'-DMF) were purchased from Indofine Chemical Co. (Somerville, NF). Resveratrol (RV), 7-ethoxyresorufin, resorufin, BaP, DL-dithiothreitol and bovine serum albumin were obtained from Sigma Chemical Co. (St. Louis, MO). Phenol:chloroform:isoamyl alcohol (25:24:1, v/v/v) was purchased from Amresco (Solon, OH). Generally-labeled [3H]-BaP (50 Ci/mmol, 99% radiochemical purity) was obtained through American Radiolabeled Chemicals (St. Louis, MO); BaP-trans-7,8-dihydrodiol(±) and BaP-7,8-epoxide(±) were bought from the NCI Chemical Carcinogen Repository at Midwest Research Institute (Kansas City, MO). All other chemicals were of analytical grade.
2.2. Cell Culture and Treatment
Normal primary rainbow trout hepatocytes (CRL-2301) obtained from American Type Culture Collection (Rockville, MD) were grown in cell growth medium (MEM) with 10% heat-inactivated fetal bovine serum, 0.1 mM non-essential amino acids, 2 mM L-glutamine, 1.32 g/L sodium chloride and 1% penicillin-streptomycin in uncoated flasks in a humidified atmosphere with 5% carbon dioxide at 18°C. At 80−90% confluency, the cells were treated with 1 μM BaP and/or 25 μM 5,7-DMF, 3',4'-DMF or RV. This polyphenol concentration was non-toxic to the trout liver cells, as determined visually (microscope) and by the cell number at the end of the experiments. Vehicle dimethyl sulfoxide (DMSO, ≤ 0.1% of final volume) was used as a control in all experiments. The cells were used at passages 5−15. While the basal levels of BaP-DNA binding and enzyme activity varied somewhat among different passages, the magnitude of effects remained the same.
2.3. Binding of Benzo[a]pyrene to DNA
DNA-binding was measured as previously described [2, 20] in cells grown in 6-well plates incubated with 1 μM [3H]-BaP for various times or with 1 μM [3H]-BaP +/−25 μM 5,7-DMF, 3',4'-DMF or RV for 48 h. The medium was changed every 24 h. The cells were washed with 0.9% saline and lifted off the plastic with lift buffer (10 mM Tris, 1 mM EDTA and 0.14 M NaCl) and pelleted. Cell pellets were subsequently lysed in swell buffer (100 mM HEPES, 10 mM KCl, 0.75 mM spermidine, 0.15 mM spermine, 0.1 mM EDTA and 0.1 mM EGTA) and 0.1 % Triton X-100, and then centrifuged to obtain crude nuclear pellets. Nuclei were purified by centrifugation through a 30% sucrose cushion. Nuclear protein and RNA were digested with proteinase K and RNAse, and the samples were extracted 3 times with phenol:chloroform:isoamyl alcohol. DNA was precipitated with sodium acetate and cold ethanol, and the pellet was washed with ethanol and dissolved in water. DNA quantity and purity were estimated spectrophotometrically at 260/280 nm. The amount of [3H]-BaP bound to DNA was quantified by liquid scintillation spectrometry.
2.4. Catalytic Activities
2.4.1. Cytochrome P450 (CYP) 1A/1B
Cells were treated with 1 μM BaP +/- 25 μM of the polyphenols in 6-well plates with fresh medium change every 24 h as applicable. Following treatment, cells were washed with fresh medium and incubated with 0.6 μM 7-ethoxyresorufin for 30 min [2, 3] in the presence of salicylamide to inhibit conjugation enzymes [21]. Ethoxyresorufin O-deethylation (EROD) was measured flurorometrically as resorufin formation in the cell culture medium with excitation/emission wavelengths of 530/590 nm [22]. The results were normalized for the amount of cellular protein in each well [23] with bovine serum albumin as standard. The reaction was linear over the 30-min time period used.
2.4.2. Epoxide Hydrolase (EH)
Cells were treated with 1 μM BaP in 6-well plates, as described in the respective figure legends, with fresh medium change every 24 h as applicable. Following treatment, the cells were washed with pH 7.4 Hanks' Balanced Salt Solution (HBSS) and intact cells were incubated with 20 μM BaP-7,8-epoxide in HBSS for 30 min in the dark to minimize autooxidation of the epoxide and the decomposition of the diol. Formation and release of BaP-7,8-dihydrodiol into the buffer was measured fluorometrically [24] with excitation/emission wavelengths of 360/420 nm and was linear over the 30-min time period used. A concentration curve using BaP-7,8-dihydrodiol was used as standard. The results were normalized for the amount of cellular protein in each well.
2.5. Western Analyses
Cells were treated with 1 μM BaP for 0.5, 2, 6, 24 or 48 h, or with 1 μM BaP +/- 25 μM 5,7-DMF, 3',4'-DMF, or RV for 48 h. After treatment, the cells were washed, scraped, resuspended in Tris/EDTA buffer with protease inhibitors and then sonicated on ice for 3 × 10 s to obtain whole cell lysate, and the protein content was determined. Proteins were denatured with DL-dithiothreitol and NuPAGE® LDS sample buffer at 70°C for 10 min, and then separated by electrophoresis on 10% NuPAGE® Novex Bis-Tris gels (Invitrogen, Carlsbad, CA) under reducing conditions, transferred to nitrocellulose membranes and blocked in 5% non-fat milk in 0.1% Tris-buffered saline/Tween 20 (TBST) for 3 h. The membranes were incubated overnight with rabbit anti-trout CYP 1A (1:500 dilution) (Cayman Chemical Co., Ann Arbor, MI) in 5% non-fat milk/TBST, washed three times with 0.1% TBST, incubated with goat anti-rabbit IgG peroxidase conjugate (1:1000 dilution) (BD Gentest, Woburn, MA), washed with 0.1% TBST, incubated with chemiluminescent substrate (KPL, Gaithersburg, MA) and exposed to ECL Hyperfilm (Amersham Biosciences, Piscataway, NJ). Baculovirus-expressed human CYP 1A1 supersomes, (0.3 μg protein corresponding to 30 fmol) were used as positive control.
2.7. Statistical Analyses
Results were expressed as means ± SEM of at least 3 experiments. Comparisons among means were made using two-tailed unpaired ANOVA (parametric or non-parametric) followed by Dunnett's or Dunn's Multiple Comparison Test (InStat, v. 2.00). The level of significance for all experiments was set at α = 0.05.
3. Results
3.1. BaP Bioactivation and DNA Binding
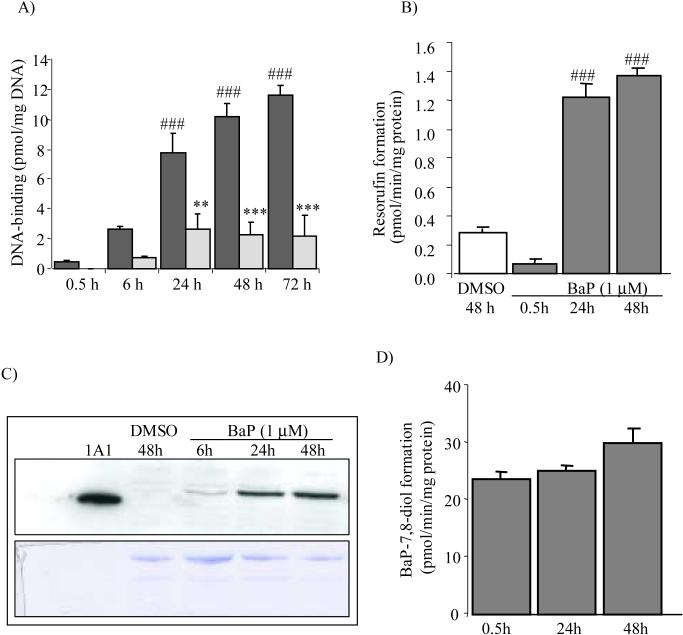
When the trout hepatic cells were exposed to a low concentration (1 μM) of tritium-labeled BaP, BaP was found to be covalently bound to cellular DNA and this binding increased with time (Fig. 1A, black bars). The greatest increase occurred from 0.5 to 24 h, a 20-fold increase. This clearly demonstrated the presence of the BaP-bioactivating enzymes in these trout hepatocytes.
Fig. 1.
Time-course of BaP-induced DNA-binding (dark bars) (A), CYP 1A catalytic activity (B), and CYP 1A protein expression (C), and EH catalytic activity (D) in trout cells. Values in A, B, and D represent means ± SEM (n = 3−6). Significantly different from 0.1% DMSO-treated controls, ###P<0.001. The Western blot (C) was repeated 3 times with recombinant human CYP 1A1 (0.3 μg protein, 30 fmol) as positive control and the coumassie-stained gel (lower panel) as the loading control. Also shown in (A) is the time-course of inhibition of BaP-DNA binding by 25 μM 5,7-DMF (light bars). Significantly different from respective BaP-treatment, **P<0.01; ***P<0.001.
When the EROD assay was used to measure the CYP 1A catalytic activity, the main BaP-bioactivating enzyme, exposure of the trout liver cells to 1 μM BaP resulted in a large induction, about 17-fold, at 24 h compared to 0.5 h (Fig. 1B). In support of these observations, BaP induced the CYP 1A protein expression, as determined by Western blotting (Fig. 1C), using the very sensitive polyclonal anti-trout CYP 1A antibody [25]. Whereas this protein was undetectable in the DMSO-treated cells, it was highly expressed after 24−48 h exposure to BaP. The epoxide hydrolase (EH) activity in trout cells, as measured by the formation of BaP-7,8-dihydrodiol from BaP-7,8-epoxide, was very high (Fig. 1D). However, there was no significant effect of BaP treatment of the cells on its activity.
3.2. Effects of Methoxylated Flavones and Resveratrol
Previous cellular studies with methoxylated flavones and resveratrol (RV) have used a 25 μM concentration, which appears to be nontoxic in various cell types [2, 7], including these trout liver cells. This is also a concentration that may be achieved in the oral cavity and intestine after dietary consumption of similar phytochemicals [26] and in rat [27] and fish [28] after in vivo exposure. When treating the trout cells simultaneously with 1 μM BaP and 25 μM 5,7-DMF there was an about 60−80% inhibition of the DNA binding compared to control at all time points (Fig. 1A, white bars). A comparison of the effects of 25 μM 3',4'-DMF, 5,7-DMF and RV during co-treatment of the cells with 1 μM BaP for 48 h is shown in Fig. 2A. All three compounds showed a significant (50−60%) inhibition of the DNA binding.
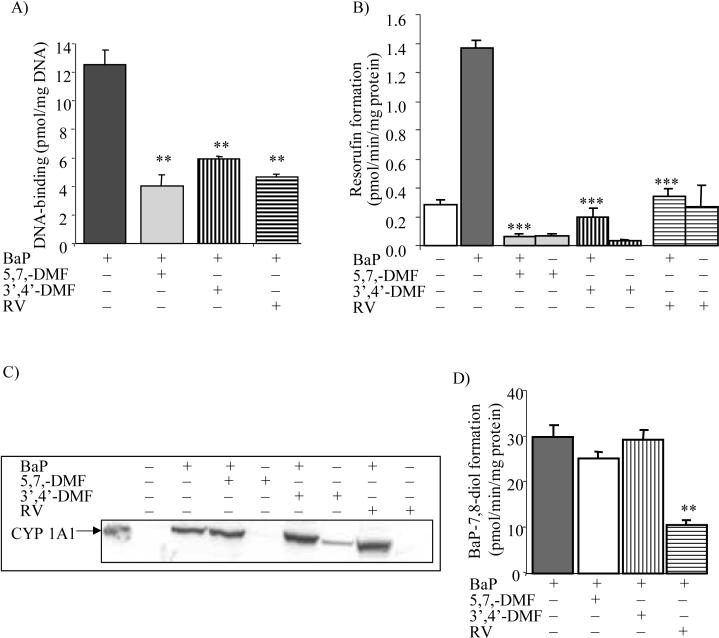
Fig. 2.
Effect of methoxylated flavonoids and resveratrol (RV) on BaP-induced DNA binding (A), CYP 1A catalytic activity (B), CYP 1A protein expression (C) and EH catalytic activity (D) in trout cells. Cells were treated with 1 μM BaP alone or with 25 μM 5,7-DMF, 3',4'-DMF, or RV alone or together with BaP for 48 h, as indicated in the figure. Controls were exposed to DMSO (<0.1%) for 48 h. Values in A, B and D represent means ± SEM (n = 3). Significantly different from BaP-treatment, **P<0.01; ***P<0.001.
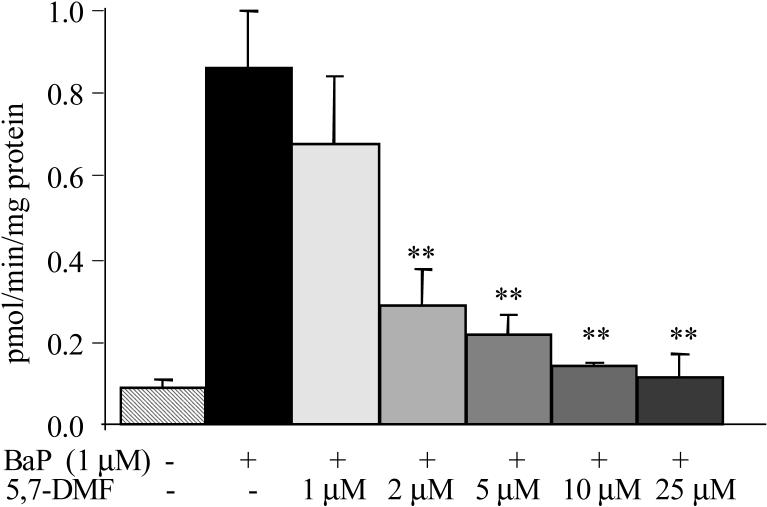
The three polyphenols, using 25 μM concentrations and 48 h BaP co-treatment, caused a potent inhibition of the BaP-induced EROD activity, ranging from about 75% for RV to >90% for 5,7-DMF (Fig. 2B). 5,7-DMF and 3',4'-DMF also inhibited basal (DMSO-treated) EROD activity. The seemingly potent inhibitory effect of 5,7-DMF on CYP 1A activity was investigated further in a concentration-effect study, Fig. 3. Significant inhibition occurred already with a 2 μM concentration, and an IC50 value of 1.3 μM was obtained.
Fig. 3.
Concentration-effect relationship of 5,7-DMF on inhibition of CYP1A-activity. Cells were treated with 1 μM BaP together with increasing concentrations of 5,7-DMF for 48 h. Inhibition of BaP-induced CYP 1A-activity was measured with the EROD-assay. Significantly different from BaP-treatment, **P<0.01.
To determine whether the potent inhibition of EROD activity by the three polyphenols was due to inhibition of transcription of the CYP 1A protein, we compared the protein expression by Western blot analysis (Fig. 2C) after identical treatments of the cells as for the EROD assay. None of the compounds had any inhibitory effect on BaP-induced CYP1A levels. 3',4'-DMF alone had a weakly stimulatory effect.
In cells treated with BaP and 25 μM of the polyphenols for 48 h, neither 5,7-DMF nor 3',4'-DMF had any effect on EH activity. In contrast, EH activity was inhibited by about 60% in RV-treated cells (Fig 2D).
4. Discussion
In the cultured primary trout hepatocytes treated with 1 μM BaP, extensive time-dependent covalent binding to cellular DNA occurred, which was secondary to marked CYP 1A induction, as measured by the catalytic EROD assay, as well as its protein expression. High EH expression in these cells likely contributed substantially to the bioactivation of BaP. The two methoxylated flavones 5,7-DMF and 3',4'-DMF and the stilbene resveratrol were all inhibitors of both the BaP-DNA binding and CYP 1A induction. The high bioavailability of 5,7-DMF [29] and its potent inhibition of CYP 1A activity, support a role for this dietary compound as a potential cancer chemopreventive agent.
In the trout hepatocytes studied, there were similarities with human hepatic as well as lung cells, but there were also distinct differences. In virtually identical experiments conducted in the human hepatoma Hep G2 cell line, the level of BaP-DNA binding was only about one third of that in the trout cells [2], in spite of the fact that the BaP-induced EROD activity was similar in the trout and human liver cells. As BaP-DNA binding is a reflection of both CYP 1A and EH activity, a potential reason for this difference may be the surprisingly high basal EH activity in the trout cells, exceeding that of the Hep G2 cells by about 10-fold. This high EH activity is even more remarkable when the low incubation temperature (18°C) is taken into account. Although the biological role of the very high EH activity in the trout cells is not known, it may serve a protective role in the detoxification of epoxides generated from both endogenous and exogenous compounds. However, for the PAHs, including BaP, it would be expected to lead to enhanced DNA binding. Whereas the EH activity in the trout cell was not affected significantly by BaP treatment, in the Hep G2 cells it is somewhat inducible [30], and in the human bronchial epithelial BEAS-2B cells it is quite low but highly inducible [3]. The EH assay used in our studies, measuring the conversion of BaP-7,8-epoxide to BaP-7,8-dihydrodiol, the precursor of the final BaP bioactivation step, although not commonly used, should be the most appropriate assay for this enzyme activity.
Whether EH is upregulated in our trout cells is not known. It would be important to investigate the expression of this enzyme in the trout liver in vivo. This would simultaneously address whether the trout may be a useful in vivo model of the human.
With respect to the time-course of induction by BaP, maximum binding of BaP to DNA in the trout cells occurred at 24 to 72 h after initiation of treatment, which appears to coincide with maximum induction of CYP 1A. This is very similar to our previous findings in the normal human bronchial epithelial BEAS-2B cells [3], but very different from the human hepatoma Hep G2 cells, where maximum induction occurred as early as after 6 h of exposure to BaP, but coincides with respective maxima for DNA-binding. Thus, the trout cells, representing normal fish liver cells, behaved similarly to the normal human bronchial cells with respect to BaP-induced CYP 1A activity and BaP-DNA binding, the latter having greater functional significance.
As in the human lung BEAS-2B cells, CYP 1A protein was undetectable in the trout cells at the basal state. However, after treatment with BaP for 48 h, both the trout and the BEAS-2B cells showed high expression of this protein. It is well known that many fish species express this protein even when living in uncontaminated water [31] thus, making them sensitive animal models for CYP 1A-mediated bioactivation of carcinogens.
The methoxylated flavones and resveratrol all inhibited the BaP-induced EROD activity potently, in particular 5,7-DMF. This has previously been reported also in the Hep G2 [2] and BEAS-2B [3] cells. The main mechanism of inhibition of BaP-induced CYP 1A (EROD) activity appears to be direct competition at the protein level by these dietary compounds. Thus, 5,7-DMF has been shown to inhibit human recombinant CYP 1A1 with an IC50 value as low as 0.8 μM [7]. The associated inhibition of BaP-DNA binding was not as potent in the trout cells, suggesting the presence of additional BaP bioactivating enzymes in this species, which were not inhibited by the polyphenols. The very high activity of EH may also blunt the inhibition of BaP-DNA binding. The mechanism associated with inhibition of CYP 1A protein also differed between trout cells and Hep G2 and BEAS-2B cells. Whereas these polyphenols appear to not only inhibit CYP 1A catalytic activity in the two human cell lines, they also inhibit its transcription and therefore protein expression [2, 3]. The latter effect was absent in trout cells at the concentrations used. Different cellular responses in any particular model used can be expected and the effect of cell context on the biological responses to xenobiotics, including carcinogens as well as polyphenols, must be considered [32].
The effects of the three polyphenols examined on BaP-induced cellular responses were very similar. However, in vivo, RV is expected to have very little utility as a cancer chemopreventive agent because of its extensive presystemic metabolism by the intestine and liver [33-35]. In contrast, the methoxylated flavones, in particular 5,7-DMF, have been shown to be metabolically stable [29, 36] with high oral bioavailability [27]. This was recently demonstrated in an in vivo fish study [28], where 5,7-DMF, unlike its methylated analogue chrysin, was detected in brain and especially in the liver in very high amounts after aqueous exposure. The high accumulation with very limited metabolism of 5,7-DMF in vivo makes this flavone a promising chemopreventive compound.
When contemplating the trout CRL-2301 cells as a model system for studies of PAH-induced carcinogenesis and cancer chemoprevention, their sensitivity to BaP treatment, as measured by the EROD assay, is a clear advantage. Thus, CYP 1A was highly inducible. Also, because of the very high basal EH activity in these cells, the binding of BaP to DNA was very high. Another advantage of these primary cells is that although they grow relatively slowly at 18°C, they are normal untransformed hepatocytes, which are commercially available. Compared with cancer cell lines, the responses of these normal cells are more likely to be reflective of fish in vivo. Due to the cell line's sensitivity, it has the potential of being an excellent model for screening potential carcinogens as well as xenobiotics such as flavonoids. Because of its similarities and differences to human hepatocarcinoma Hep G2 and human bronchial epithelial BEAS-2B cells, extrapolation to human organs should be done cautiously.
Acknowledgements
We would like to thank Kristina Walle for critical review of the manuscript. This study was supported by an EPA STAR fellowship (P.A. Tsuji) and in part by the National Institutes of Health grant GM55561. This project was performed in part using compounds provided by the National Cancer Institute's Chemical Carcinogen Reference Standards Repository operated under contract by Midwest Research Institute, NO. N02-CB-07008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services . Report on carcinogens. tenth edition U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program; 2002. [Google Scholar]
- 2.Wen X, Walle UK, Walle T. 5,7-Dimethoxyflavone downregulates CYP1A1 expression and benzo[a]pyrene-induced DNA binding in Hep G2 cells. Carcinogenesis. 2005;26:803–809. doi: 10.1093/carcin/bgi015. [DOI] [PubMed] [Google Scholar]
- 3.Tsuji PA, Walle T. Inhibition of benzo[a]pyrene-activating enzymes and DNA-binding in human bronchial epithelial BEAS-2B cells by methoxylated flavonoids. Carcinogenesis. 2006;27:1579–1585. doi: 10.1093/carcin/bgi358. [DOI] [PubMed] [Google Scholar]
- 4.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 5.Kim E, Hong WK, Khuri F. Chemoprevention of aerodigestive tract cancers. Annu. Rev. Med. 2002;53:223–243. doi: 10.1146/annurev.med.53.082901.104015. [DOI] [PubMed] [Google Scholar]
- 6.Lippman SM, Sudbø J, Hong WK. Oral cancer prevention and the evolution of molecular-targeted drug developmen. J. Clin. Oncol. 2005;23:346–356. doi: 10.1200/JCO.2005.09.128. [DOI] [PubMed] [Google Scholar]
- 7.Wen X, Walle T. Preferential induction of CYP1B1 by benzo[a]pyrene in human oral epithelial cells: impact on DNA adduct formation and prevention by polyphenols. Carcinogenesis. 2005;26:1774–1781. doi: 10.1093/carcin/bgi127. [DOI] [PubMed] [Google Scholar]
- 8.Wen X, Walle T. Cytochrome P450 1B1, a biomarker and chemopreventive target for benzo[a]pyrene-initiated human esophageal cancer. Cancer Lett. 2007;246:109–114. doi: 10.1016/j.canlet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Ahne W. Studies on the use of fish tissue cultures for toxicity tests in order to reduce and replace the fish tests. Zbl. Bakt. Hyg. 1985;180:480–504. [PubMed] [Google Scholar]
- 10.Babich H, Borenfreund E. Cultured fish cells for the ecotoxicity testing of aquatic pollutants. Toxicity Assessment: An International Quarterly. 1987;2:119–133. [Google Scholar]
- 11.Bailey GS, Williams DE, Hendricks JD. Fish models for environmental carcinogenesis: the rainbow trout. Environ. Health Persp. Suppl. 1996;104 doi: 10.1289/ehp.96104s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aas E, Beyer J, Jonsson G, Reichert WL, Andersen OK. Evidence of uptake, biotransformation and DNA binding of polyaromatic hydrocarbons in Atlantic cod and corkwing wrasses caught in the vicinity of an aluminium works. Mar. Env. Res. 2001;52:213–229. doi: 10.1016/s0141-1136(00)00269-5. [DOI] [PubMed] [Google Scholar]
- 13.Billiard SM, Hahn ME, Franks DG, Peterson RE, Bols NC, Hodson PV. Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs) Comp. Biochem. Physiol. B. 2002;133:55–68. doi: 10.1016/s1096-4959(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 14.James MO, Schell JD, Boyle SM, Altman AH, Cromer EA. Southern flounder hepatic and intestinal metabolism and DNA binding of benzo[a]pyrene (BaP) metabolites following dietary administration of low doses of BaP, BaP-7,8-dihydrodiol or a BaP metabolite mixture. Chem.-Biol. Interact. 1991;79:305–321. doi: 10.1016/0009-2797(91)90111-j. [DOI] [PubMed] [Google Scholar]
- 15.Iwashina T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000;113:287–299. [Google Scholar]
- 16.Kiparissis Y, Hughes R, Metcalfe C. Identification of the isoflavonoid genistein in bleached kraft mill effluent. Environ. Sci. Technol. 2001;35:2423–2427. doi: 10.1021/es001679+. [DOI] [PubMed] [Google Scholar]
- 17.Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seeweeds. Food Chem.Toxicol. 2006;44:1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Breinholt V, Hendricks J, Pereira C, Arbogast D, Bailey GS. Dietary chlorophyllin is a potent inhibitor of aflatoxin B1 hepatocarcinogenesis in rainbow trout. Cancer Res. 1995;55:57–62. [PubMed] [Google Scholar]
- 19.Ostrander GK, Blair JB, Stark BA, Marley GM, Bales WD, Veltri RW, Hinton DE, Okihiro M, Ortego S, Hawkins WE. Long-term primary culture of epithelial cells from Rainbow trout (Oncorhynchus mykiss) liver. In Vitro Cell. Develop. Biol. 1995;31:367–378. doi: 10.1007/BF02634286. [DOI] [PubMed] [Google Scholar]
- 20.Walle T, Vincent TS, Walle UK. Evidence of covalent binding of the dietary flavonoid quercetin to DNA and protein in human intestinal and hepatic cells. Biochem. Pharmacol. 2003;65:1603–1610. doi: 10.1016/s0006-2952(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 21.Ciolino HP, Daschner PJ, Wang TTY, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem. Pharmacol. 1998;56:197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 22.Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-, pentoxy- and benzyloxypheoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem. Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol agent. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Herrero ME, Castell JV. Fluorometric microassay to quantify microsomal epoxide hydrolase in 96-well plates. Anal. Biochem. 1995;230:154–158. doi: 10.1006/abio.1995.1450. [DOI] [PubMed] [Google Scholar]
- 25.Sarasquete C, Ortiz JB, Gisbert E. Immunohistochemical distribution of cytochrome P4501A in larvae and fingerlings of the Siberian sturgeon, Acipenser baeri. Histochem. J. 2001;33:101–110. doi: 10.1023/a:1017900314779. [DOI] [PubMed] [Google Scholar]
- 26.Walgren R, Walle U, Walle T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem. Pharmacol. 1998;55:1721–1727. doi: 10.1016/s0006-2952(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 27.Walle T, Ta N, Kawamori T, Wen X, Tsuji PA, Walle UK. Cancer chemopreventive properties of orally bioavailable flavonoids - methylated versus unmethylated flavones. Biochem. Pharmacol. 2007;79:1288–1296. doi: 10.1016/j.bcp.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji PA, Walle T. Accumulation and metabolism of the anticancer flavonoid 5,7-dimethoxyflavone compared to its unmethylated analog chrysin in the Atlantic killifish. Chem.-Biol. Interact. 2006;164:85–92. doi: 10.1016/j.cbi.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006;34 doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 30.Glatt HR, Wölfel T, Oesch F. Determination of epoxide hydrolase activity in whole cells (human lymphocytes) and activation of benzoflavones. Biochem. Biophys. Res. Commun. 1983;110:525–529. doi: 10.1016/0006-291x(83)91181-6. [DOI] [PubMed] [Google Scholar]
- 31.Monod G, Saucier D, Perdu-Durand E, Diallo M, Cravedi JP, Astic L. Catalytic and immunocytochemical detection of xenobiotic metabolizing enzymes in the olfactory organ of rainbow trout. Mar. Environ. Res. 1995;39:39–43. [Google Scholar]
- 32.Zhang S, Qin C, Safe S. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ. Health Perspect. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 34.Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 35.Walle T. Absorption and metabolism of flavonoids. Free Radic. Biol. Med. 2004;36:829–837. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Wen X, Walle T. Methylation protects dietary flavonoids from rapid hepatic metabolism. Xenobiotica. 2006;36:387–397. doi: 10.1080/00498250600630636. [DOI] [PubMed] [Google Scholar]