Abstract
During hibernation animals oscillate from near ambient (Ta) to euthermic body temperatures (Tb). As animals arouse, the rate of rewarming (RRW) might be expected to simply increase as a function of time. We monitored the Tb of golden-mantled ground squirrels (Spermophilus lateralis) housed at 4, 8, 12, and 16° C during natural arousals. The maximum RRW, the time required to reach a maximum RRW, and the relative time index all demonstrated negative relationships with Ta. The Tb corresponding to maximal RRW demonstrated a positive relationship with Ta. Squirrels reached maximal RRW when they had generated 30 to 40% of the heat required to reach a euthermic Tb. These data suggest that arousal is more constrained than expected and that both time and temperature influence the RRW.
Keywords: ground squirrel, torpor, arousal
1. Introduction
For many mammals, the onset of winter presents formidable challenges in the form of cold temperatures and scarce food supply. Hibernation provides a means of evading many of these challenges and is a strategy employed throughout the mammalian kingdom (see reviews by van Breukelen and Martin, 2002a; Carey et al., 2003). Hibernators experience tremendous energetic savings as a result of severely reduced metabolic rates. Torpid metabolic rates may be as low as ∼ 1% of euthermic rates in ground squirrels (reviewed in Wang and Lee, 2000). The effects of this drastically reduced energy supply can be seen in the depression of many physiologically significant processes during torpor. Transcription, translation, mitosis, and mitochondrial respiration are all depressed during torpor (Carey et al., 2003; van Breukelen and Martin, 2001; 2002a and b and references therein).
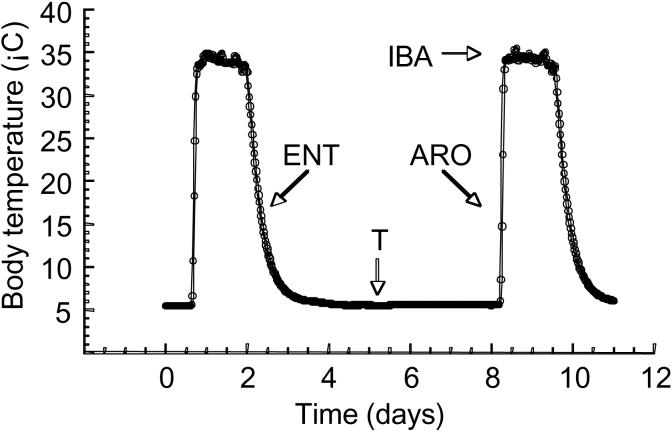
However, hibernators are not always torpid. Across a hibernation season there are dynamic fluctuations in body temperature (Tb) and physiological activity as they oscillate from near ambient (Ta) to euthermic body temperature (Fig. 1). The duration of torpor phases can change with species, Ta, and progression through the hibernation season (e.g. Buck & Barnes, 2000; French, 1982; Carey et al., 2003). As ground squirrels enter into torpor, Tb falls slowly and normally stabilizes within ∼ 1° C of Ta. When challenged with temperatures below freezing, animals actively thermoregulate to maintain Tb near 0° C (Buck and Barnes, 2000). The length of a typical torpor bout for golden-mantled ground squirrels (Spermophilus lateralis) housed at 4° C is ∼ 8 days (personal observation). Torpor bouts are regularly interrupted by arousals (interbout arousal, IBA) wherein animals rapidly rewarm to euthermic levels (Tb is ∼ 36° C). Upon completion of the interbout arousal, animals spontaneously reenter another torpor bout.
Fig. 1.
Body temperature (Tb) in a hibernating ground squirrel. Tb was measured every 30 minutes for an animal housed at 4° C. During entrance (ENT) into torpor, Tb slowly decreases from euthermic (∼ 36° C) to near ambient temperature (Ta). During the approximately week long period of torpor (T), Tb is maintained within 1° C of Ta. Animals rapidly rewarm to euthermic Tb during arousal (ARO). Euthermic Tb is maintained during the interbout arousal (IBA).
During arousal, animals may increase Tb by > 30° C within a period of 2 to 3 hours, which represents a substantial quantity of heat generation. Given the general relationship between biological reactions and temperature, a simple expectation might be that as animals arouse from torpor and rewarm to euthermic Tb, an increasing number of biochemical processes will be restored. Biological reactions release heat, and their rates are usually temperature dependant (Withers, 1992). Thus, the rate of rewarming (RRW) might be expected to simply increase as a function of time during the arousal process. To better understand heat gains during arousal, golden-mantled ground squirrels were implanted with temperature sensitive radio telemeters and housed at 4, 8, 12, and 16° C. Since torpid Tb is generally within 1° C of Ta, the various housing temperatures allow for modulation of torpid Tb. All squirrels were allowed to proceed through natural undisturbed arousals. Tb was recorded; relationships between Ta, RRW, time to reach maximal RRW, and Tb at maximal RRW were investigated.
2. Materials and Methods
2.1 Animals
Golden-mantled ground squirrels, Spermophilus lateralis, were live-trapped from the Southern California/Southern Nevada area. Prior to the beginning of the hibernation season, temperature sensitive radio telemeters (model VM-FH disc; Mini Mitter, Sun River, OR) were surgically implanted in the abdominal cavity. Animals were housed in an environmental chamber at 4, 8, 12, and 16° C. Body temperature was recorded every minute. The University of Nevada, Las Vegas Institutional Animal Care and Use Committee approved all procedures.
2.2 A relative definition of arousal
It has been noted that the time required to complete an arousal changes with Ta (i.e. it takes longer to rewarm from 4° C to ∼ 36° C than from 16° C to ∼ 36° C; e.g. French, 1982). In order to compare arousal dynamics across varying Ta, the initiation and termination of an arousal must be clearly defined. Tb was recorded every minute. The first derivative (FD) and its associated standard deviation (SD) for temperature as a function of time were calculated. The onset of arousal was defined as the point where the instantaneous rate of rewarming (RRW) exceeded the FD ± 3 SD (threshold rate; TR) for a 10-minute period of consistent Tb. The first Tb measurement with a FD > TR was defined as the onset of arousal. The onset of arousal was typically associated with a rate of rewarming of 0.02° C · min−1. The termination of arousal was defined as the first point at which the instantaneous rate was < TR for the following 5 min.
2.3 Calculations
For each Ta, arousals from 5 animals were examined. A mean value was used in analysis of rewarming rates. Mean maximum rate of rewarming was calculated for each animal from the 5 highest RRW values for a given arousal. The period of time between the onset of arousal and the single highest RRW value was used for analysis of the time required to attain maximum rewarming rate. The relative time index is the time to reach maximum RRW divided by the arousal duration. The Tb at which maximum RRW occurs was investigated; the 5 highest RRW values within a single arousal were identified, and the 5 corresponding Tb values were used to calculate a mean Tb. Mean Tb was also used to calculate the temperature gain index wherein it was divided by the difference in Tb between the initiation and termination of arousal.
2.4 Statistical Analyses
When appropriate, data were subjected to regression analyses. Statistical significance was indicated when p < 0.05.
3. Results
3.1 The dynamics of a typical torpor bout
The body temperature during a typical torpor bout for golden-mantled ground squirrels is depicted in Fig. 1. Changes in body temperature during arousal from torpor for an animal housed at 4, 8, 12, and 16° C were analyzed (Fig. 2a). A pattern of a relatively slow increase in Tb, followed by a faster increase in Tb, and finally an additional period characterized by a slow increase in Tb was noted for all animals at all ambient temperatures. The onset of arousal was typically marked by instantaneous rates of rewarming of 0.02° C · min−1 (Fig. 2b). During early arousal, the RRW progressively increases and may be as high as 1.4° C · min−1. Interestingly, the RRW begins to decrease during mid arousal despite as much as an additional 25° C yet to be gained in Tb.
Fig. 2.
Body temperature as a function of time during arousals from one individual. Panel A) Body temperature was measured every minute for a squirrel housed at 4, 8, 12, and 16° C. Panel B) Instantaneous rate changes as demonstrated by plotting the first derivative as a function of time across the same range of ambient temperatures.
3.2 Maximum rewarming rate
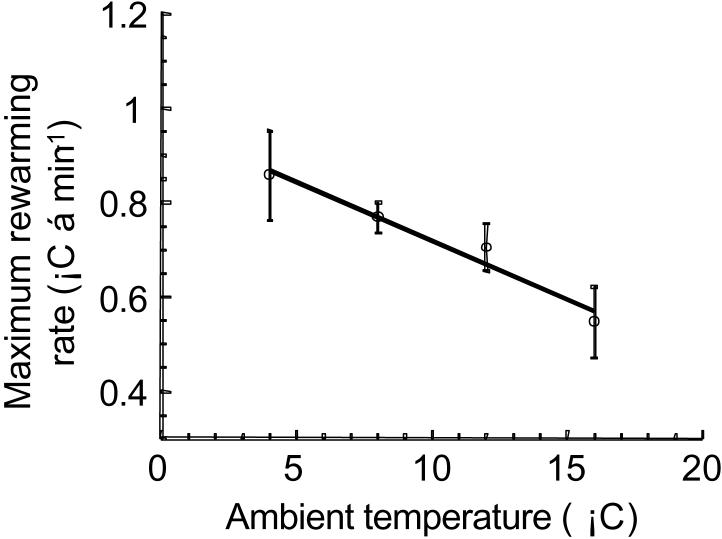
The maximum rate of rewarming was determined. A negative relationship between mean maximum RRW and Ta was found (Fig. 3). Mean maximum RRW decreased ∼ 35% from 0.86 ± 0.09° C · min−1 for squirrels housed at 4° C to 0.55 ± 0.08° C · min−1 for squirrels maintained at 16° C.
Fig. 3.
Effect of ambient temperature on maximum rewarming rate. Symbols represent means ± SE, n = 5. There is a significant effect of Ta on maximum RRW, p < 0.05, r2 = 0.98.
3.3 Time to attain maximum rewarming rate
Data were analyzed to determine whether there is a defined period of time required to reach maximum RRW e.g. is the RRW a simple function of time with no effect of Ta? There is a negative relationship between the time required to attain maximum RRW and Ta (Fig. 4). Squirrels housed at 4° C took ∼ 3.5 fold more time to reach a maximum RRW.
Fig. 4.
Effect of ambient temperature on time to reach maximum rewarming rate. Symbols represent means ± SE, n = 5. There is a significant effect of Ta on time to attain maximum RRW, p < 0.05, r2 = 0.98.
3.4 Relative time index
Hibernators arouse more rapidly when housed at elevated ambient temperatures (French, 1982). Thus, the waxing and waning pattern observed for RRW may be dependant on a relative rather than absolute amount of time. For example, it is plausible that maximum RRW is reached when 40% of the time required to complete arousal has passed. To investigate this possibility, the relative time index was examined. Even when accounting for differences in arousal duration, animals housed at 16° C reached maximal rates approximately twice as fast as those housed at 4° C (Fig. 5).
Fig. 5.
Effect of ambient temperature on relative time index. Relative time index was calculated as the fraction of elapsed arousal duration required to reach maximum RRW; i.e. 40 min to maximum RRW/100 min total arousal duration. Symbols represent means ± SE, n = 5. There is a significant effect of Ta on relative time index, p < 0.05, r2 = 0.96.
3.5 Body temperature associated with maximum rewarming rate
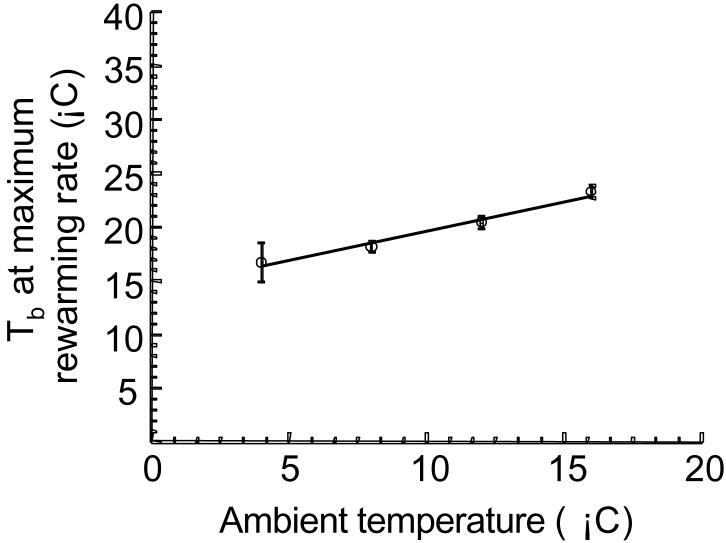
Since we found that the RRW is not a simple function of time (Figs. 4-5), we investigated the role of Tb in determining RRW. Does maximum RRW occur at a specific Tb? There is no specific temperature for maximum RRW (Fig. 6). Rather, the mean Tb for the occurrence of maximal RRW at Ta of 16° C is ∼ 6.5° C higher than for Ta of 4° C.
Fig. 6.
Effect of ambient temperature on the body temperature at which the maximum rewarming rate occurred. Symbols represent means ± SE, n = 5. There is a significant effect of Ta on the Tb at which the maximum RRW occurred, p < 0.05, r2 = 0.99.
3.6 Temperature gain index
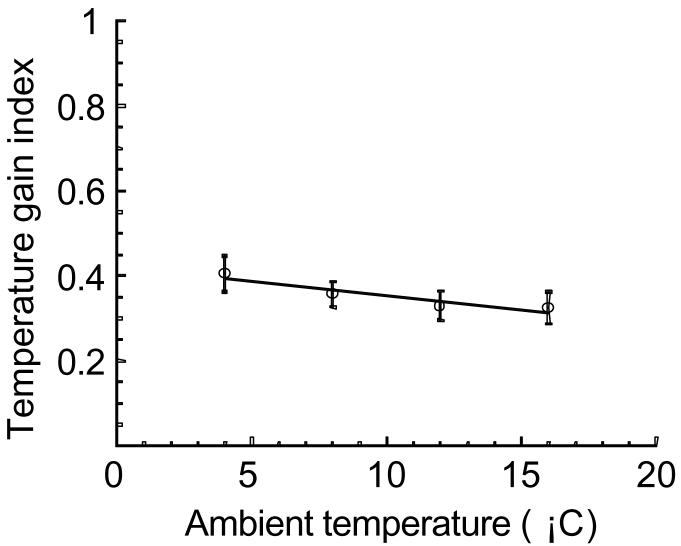
The heat required to transition from a torpid Tb of 16° C to ∼ 36° C is much less than from 4° C to ∼ 36° C. It is plausible that maximum rewarming rates are reached when an animal has generated enough heat to reach some fraction of the required temperature gain. For all ambient temperatures, squirrels reached maximum rewarming rates when they had generated 30 to 40% of the heat required to reach euthermic body temperatures (Fig. 7).
Fig. 7.
Effect of ambient temperature on the temperature gain index. Temperature gain index is defined as the fraction of temperature gain achieved when animals reach maximal rewarming rates; i.e. an animal housed at 4° C must gain ∼ 32° C to reach euthermic Tb. If a maximum RRW occurs at a Tb of 14° C, the animal has gained 10° C thus the temperature gain index is 0.31 (10° C/32° C). Symbols represent means ± SE, n = 5. Ta has no effect on the temperature gain index, p > 0.05, r2 = 0.94.
4. Discussion
The maximum RRW, the time required to reach a maximum RRW, and the relative time index all demonstrated negative relationships with ambient temperature (Ta). As torpid body temperature (Tb) increases, animals reach their maximal rewarming rates in less time e.g. 20.2 ± 0.73 min for animals housed at 16° C compared to 70.0 ± 8.5 min for animals housed at 4° C (Fig. 4). When changes in arousal duration are considered, animals at 16° C still reach a maximum RRW ∼ 2 fold faster than those at 4° C (Fig. 5). Although animals housed at warmer temperatures reach a maximum RRW faster, the rates themselves are reduced when compared to animals housed at cooler temperatures e.g. maximum RRW was 0.546 ± 0.075° C · min−1 for 16° C and 0.857 ± 0.095° C · min−1 for 4° C (Fig. 3). The Tb corresponding to maximal rewarming rates demonstrated a positive relationship with Ta (Fig. 6). Squirrels housed at 16° C reached maximal rewarming rates at a Tb of 23.3 ± 0.58° C while those housed at 4° C were at a Tb of 16.7 ± 1.8° C. Interestingly, one parameter was unchanged by Ta, the temperature gain index. For all ambient temperatures, squirrels reached maximal rewarming rates when they had generated 30 to 40% of the heat required to reach a euthermic Tb (Fig. 7).
A simplistic explanation of heat production at the subcellular level is based on the release of heat from inefficient biological reactions (Withers, 1992). When one couples this inefficiency with the notion that most enzymatic rates are temperature dependent, a simple expectation might be that as Tb increases and biochemical processes are restored during arousal from torpor the RRW would increase as a function of time. In a review of standard metabolic rate, Rolfe and Brown (1997) compiled data for major oxygen consuming reactions in six rat tissues. Protein synthesis generally accounts for 20 to 30% but may account for as much as 74% of total oxygen consumption depending on tissue type. Initiation of translation is acutely depressed at Tb = 18° C during entrance into hibernation and is fully recoupled with elongation as animals arouse at Tb = 18° C (van Breukelen and Martin, 2001). Thus, it appears that there is a critical temperature for the bulk of initiation at 18° C. Given the role of protein synthesis in generating metabolic heat, one might assume that at Tb = 18° C, there would be a significant input of heat as initiation of translation was restored. The data do not support a significant heat input at 18° C (Fig. 6). One explanation may be that this heat input is obfuscated by heat generation at other sites.
A consideration of major sites of heat production in the whole organism reveals localized thermogenesis. During arousal from hibernation, the primary sources of heat generation are skeletal muscle (SM) and brown adipose tissue (BAT; e.g. Carey et al., 2003; Fons et al., 1997; Genin et al. 2003 and references therein). For a typical arousal, initial increases in Tb appear to be due to heat production in BAT with SM beginning to contribute heat midway through the arousal process (Fons et al., 1997). One plausible explanation for our data may be that the relative contributions or timing of SM and BAT thermogenesis is shifted depending on Ta. The relative contribution of SM activity during arousal following transfer from 4° C to 22° C in Etruscan shrews was examined; only after Tb > 17° C did SM activity occur (Fons et al., 1997). In two species of ground squirrels, the onset of SM thermogenesis may vary from when Tb > 12° C (housed at 5° C) to Tb > 15° C (housed at 2° C; taken from Tøien et al., 2001; Phillips and Heath, 2004). We found that the body temperature at which maximal RRW was achieved was correlated with Ta (Fig. 6). Future efforts will directly ascertain the effects of Ta on the onset of shivering thermogenesis in hibernators.
Body temperature is not just a function of heat gain but may also be influenced by heat loss. Models for animals to rewarm as fast as possible have been addressed in the literature; one of the consequences of rewarming slowly is increased heat loss to the environment and thus a greater overall cost of rewarming to the animal (c.f. Stone and Purvis, 1992). Changes in regional blood flow dramatically influence both heat gain and loss. Regional blood flow is regulated during arousal from torpor. Blood flow to the hind foot is markedly reduced during early arousal in hibernators (Osborne et al., 2005). Blood flow posterior to the diaphragm is restricted in arousing thirteen-lined ground squirrels until the thoracic temperature is ∼ 25° C (Bullard and Funkhouser, 1962). These data are consistent with data from the big brown bat where anterior organs receive greater fractions of cardiac output during early arousal (Rauch and Beatty, 1975). The restoration of blood flow to the periphery and skin may also have large effects on the level of heat loss to the environment. In larger mammals like marmots, redistribution of blood flow to peripheral circulation has been shown to slow rates of rewarming (Phillips and Heath, 2004). We are not aware of any studies to date that have examined the effects of Ta on regional blood flow regulation. If the regulation of regional blood flow were dependent on Ta, temporal and temperature effects consistent with our findings might be expected.
The sigmoidal nature of the Tb increase during arousal has been noted before (c.f. Stone and Purvis, 1992). This sigmoidal pattern is characteristic of issues with substrate delivery and utilization. Animals that are arousing from warmer ambient temperatures have lower maximal RRW (Fig. 2B, Fig. 3). One explanation may be that tissue metabolism cannot be supported by available supply mechanisms e.g. since much of thermogenesis occurs in SM and BAT, perhaps there is insufficient blood flow to these regions to support additional thermogenesis at warmer Tb. Interestingly although maximum RRW is much higher for squirrels housed at lower ambient temperatures, the time required for an animal to rewarm when Ta is 16° C is equal to the amount of time required for an animal housed at 4° C to complete the final 20° C (i.e. from 16° C to 36° C; p > 0.05; data not shown). Thus, it appears there are constraints on the maximum RRW achievable at warmer Tb.
Biological reaction rates are temperature dependent and many reactions release heat. Therefore as Tb increases, an increasing number of biochemical processes should contribute additional heat. Using this model, RRW might be expected to simply increase as a function of time until a euthermic set point is approached. While this assertion is sensible, the data support a more complex regulation. A more likely model for heat production during arousal integrates systemic physiology with the subcellular biochemical processes that underlie thermogenesis. Contributions of regional blood flow, substrate delivery and utilization, and unequal contributions of heat from different tissues must be reconciled in the context of both heat gain and heat loss. Our data indicate the dynamics of rewarming are affected by both time and temperature. Maximum RRW and the time to reach maximum RRW decrease with increasing Ta. The Tb associated with maximum RRW increases with increasing Ta. Thus, there does not appear to be a simple relationship for heat gain during arousal. Instead, it appears that the process of arousal is more constrained than we initially expected. All animals reached their maximum rewarming rates when they had generated 30 to 40% of the heat required to reach a euthermic Tb. Future efforts will be directed towards further elucidating the interactions among the systemic and subcellular events that modulate thermogenesis.
Acknowledgements
We would like to acknowledge the assistance of the various members of the laboratory with animal care and housing. This study was supported by grants from the National Science Foundation (IOB 0448396) and the National Institutes of Health (2 P20 RR016464).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol. 2000;279:R255–262. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- Bullard RW, Funkhouser GE. Estimated regional blood flow by rubidium 86 distribution during arousal from hibernation. Am J Physiol. 1962;203:266–270. doi: 10.1152/ajplegacy.1962.203.2.266. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Fons R, Sender S, Peters T, Jurgens KD. Rates of rewarming, heart and respiratory rates and their significance for oxygen transport during arousal from torpor in the smallest mammal, the Etruscan shrew Suncus etruscus. J Exp Biol. 1997;200:1451–1458. doi: 10.1242/jeb.200.10.1451. [DOI] [PubMed] [Google Scholar]
- French AR. Effects of temperature on the duration of arousal episodes during hibernation. J Appl Physiol. 1982;52:216–220. doi: 10.1152/jappl.1982.52.1.216. [DOI] [PubMed] [Google Scholar]
- Genin F, Nibbelink M, Galand M, Perret M, Ambid L. Brown fat and nonshivering thermogenesis in the gray mouse lemur (Microcebus murinus). Am J Physiol Regul Integr Comp Physiol. 2003;284:R811–818. doi: 10.1152/ajpregu.00525.2002. [DOI] [PubMed] [Google Scholar]
- Osborne PG, Sato J, Shuke N, Hashimoto M. Sympathetic alpha-adrenergic regulation of blood flow and volume in hamsters arousing from hibernation. Am J Physiol Regul Integr Comp Physiol. 2005;289:R554–R562. doi: 10.1152/ajpregu.00004.2005. [DOI] [PubMed] [Google Scholar]
- Phillips PK, Heath JE. Comparison of surface temperature in 13-lined ground squirrel (Spermophilus tridecimlineatus) and yellow-bellied marmot (Marmota flaviventris) during arousal from hibernation. Comp Biochem Physiol A Mol Integr Physiol. 2004;138:451–457. doi: 10.1016/j.cbpb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Rauch JC, Beatty DD. Comparison of regional blood distribution in Eptesicus fuscus (big brown bat) during torpor (summer), hibernation (winter), and arousal. Can J Zool. 1975;53:207–214. doi: 10.1139/z75-025. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Stone GN, Purvis A. Warm-up rates during arousal from torpor in heterothermic mammals: physiological correlates and a comparison with heterothermic insects. J Comp Physiol [B] 1992;162:284–295. doi: 10.1007/BF00357536. [DOI] [PubMed] [Google Scholar]
- Tøien Ø, Drew KL, Chao ML, Rice ME. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2001;281:R572–583. doi: 10.1152/ajpregu.2001.281.2.R572. [DOI] [PubMed] [Google Scholar]
- van Breukelen F, Martin SL. Translational initiation is uncoupled from elongation at 18 degrees C during mammalian hibernation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1374–1379. doi: 10.1152/ajpregu.2001.281.5.R1374. [DOI] [PubMed] [Google Scholar]
- van Breukelen F, Martin SL. Invited review: molecular adaptations in mammalian hibernators: unique adaptations or generalized responses? J Appl Physiol. 2002a;92:2640–2647. doi: 10.1152/japplphysiol.01007.2001. [DOI] [PubMed] [Google Scholar]
- van Breukelen F, Martin SL. Reversible depression of transcription during hibernation. J Comp Physiol [B] 2002b;172:355–361. doi: 10.1007/s00360-002-0256-1. [DOI] [PubMed] [Google Scholar]
- Wang LCH, Lee TF. Perspectives on metabolic suppression during mammalian hibernation and daily torpor. In: Heldmaier G, Klingenspor M, editors. Life in the Cold. Springer-Verlag; Berlin: 2000. pp. 152–158. [Google Scholar]
- Withers PC. Comparative Animal Physiology. Saunders College Publishing; Fort Worth: 1992. [Google Scholar]