Summary
The extent to which areas in visual cerebral cortex differ in their ability to support perceptions has been the subject of considerable speculation. Experiments that have examined the activity of individual neurons have suggested that activity in later stages of visual cortex is more closely linked to perception than that in earlier stages [1-9]. In contrast, results from transcranial magnetic stimulation and lesion studies have been interpreted as showing that earlier stages are more closely coupled to perception [10-15]. We examined whether neuronal activity in early and later stages differs in its ability to support detectable signals by measuring behavioral thresholds for detecting electrical microstimulation in different cortical areas in two monkeys. By training the animals to perform a two-alternative temporal force-choice task, we obtained criterion-free thresholds from five visual areas: V1, V2, V3A, MT and inferotemporal cortex. Every site tested yielded a reliable threshold. Thresholds varied little within and between visual areas, rising gradually from early to later stages. We similarly found no systematic differences in the slopes of the psychometric detection functions from different areas. These results suggest that neuronal signals of similar magnitude evoked in any part of visual cortex can generate percepts.
Although it has long been known that visual cerebral cortex is subdivided into many distinct hierarchically-organized areas, it remains unclear whether certain regions of visual cortex are more tightly coupled to perception. The demonstration that neurons in later stages of visual cortex have more complicated response properties than those in early stages led to the suggestion that perceptions may depend on the activity of relatively few neurons in higher visual cortex [16, 17]. Recent measurements of the activity of individual neurons support the idea that later stages are more tightly associated with perception. For example, correlations between the responses of single cells and perceptual reports are stronger in later stages of cortex during binocular rivalry [see 1], during detection or discrimination of direction [2-5] or binocular disparity [6], and during viewing of anticorrelated random dot stereograms [7, 8]. Imaging studies in humans have similarly found that the changes in signals related to rivalry are weaker in V1 than in later stages of visual cortex [see 1, 9].
On the other hand, results from functional imaging, transcranial magnetic stimulation and lesion studies have been used to argue either that perception can depend specifically on neurons in the earliest levels of visual cortex [10-15], or else that neurons in all parts of visual cortex can contribute equally to perception [18, 19]. In still another formulation, it has been suggested that neuronal activity in the ventral stream of processing is more important for visual experience than activity in the dorsal stream [20].
To directly explore whether some cortical areas can more readily generate percepts than others, we have measured behavioral thresholds for detecting electrical microstimulation in a range of areas spanning all levels of visual cortex. It has long been known that electrical microstimulation of a site in or near the first stage of visual cortex, V1, can produce a specific, repeatable visual percept, called a phosphene [21-23, see 24]. Electrical stimulation of later stages of visual cortex can produce more elaborate visual or multimodal experiences [25]. If neuronal signals in some cortical areas are more tightly linked to perception, smaller changes in activity in those areas, relative to others, may suffice to create detectable percepts. Thus, thresholds for detecting electrical microstimulation are expected to be lower in those areas.
Microstimulation thresholds have been measured in monkey V1 [26, 27] and at a few sites in extrastriate visual cortex [28]. However, threshold measurements in previous experiments may have been substantially influenced by the criteria subjects used to determine whether they would report that they perceived something [29]. Internal response criteria could vary greatly for percepts arising from stimulation at different sites depending on whether they appear more or less natural, increasing the variance of thresholds within an area and producing systematic offsets between thresholds for different areas. We describe here the first measurements of cortical microstimulation thresholds using methods that essentially eliminate the effects of the subject's criterion and allow measurements from different brain regions to be compared directly.
Results
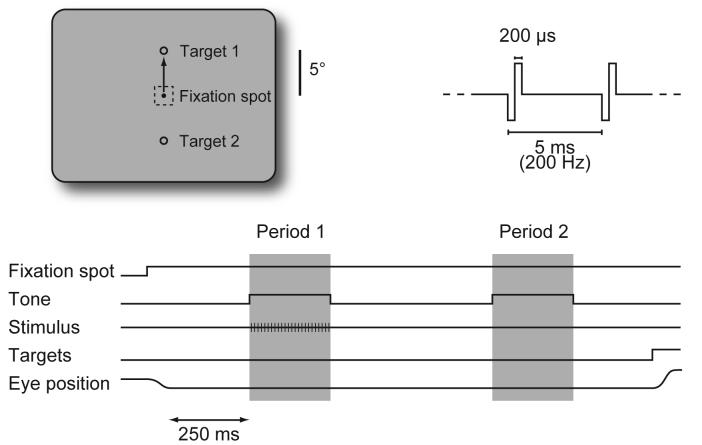
Two rhesus monkeys (Macaca mulatta) were trained to do a two-alternative temporal forced choice task (Figure 1). During each trial, the animal fixated on a small white spot centered on an unstructured gray background. While the animal fixated, two sequential 250 ms intervals were presented, each marked by a tone. A stimulus was delivered during one of the intervals, which was randomly selected for each trial. Shortly after the end of the second interval, two targets appeared, 5° above and below the fixation spot. The animal indicated which interval contained the stimulus by making a saccade directly to the appropriate target: up for interval one; down for interval two. Thus, the animals reported only the detection of the stimulus, not the apparent location or other properties of the percept.
Figure 1.
Two-alternative forced choice task. During fixation, a stimulus was delivered during one of two 250 ms time intervals that were marked by auditory tones and separated by 500 ms. Two-hundred and fifty milliseconds after the end of the second interval, two response targets appeared, and the animals indicated which interval contained the stimulus by making a direct saccade to the appropriate target (target 1 for period 1). The electrical stimuli were 250 ms trains of constant current pulses at 200 Hz, with the current randomly selected on each trial.
Each animal was initially trained with small, peripheral, low-contrast visual stimuli. During data collection, cortical microstimulation with a metal microelectrode replaced the visual stimulus. Behavioral detection thresholds were measured in V1, V2, and anterior inferotemporal cortex (IT) in both animals. Additionally, we measured thresholds in V3A and the middle temporal area (MT) in the second animal. For each visual area examined, both monkeys required a period of up to a few days of training during which microstimulation detection thresholds fell and stabilized. After thresholds had stabilized for an area, we attempted to sample uniformly from all layers of cortex, with successive stimulation sites typically separated by 250 μm (and by no less than 190 μm).
The animals detected microstimulation with low currents at every site tested in visual cortex, with each site yielding a well-formed psychometric function (Figure 2). Threshold for each site was taken as the current that yielded 82% correct detection (Experimental Procedures).
Figure 2.
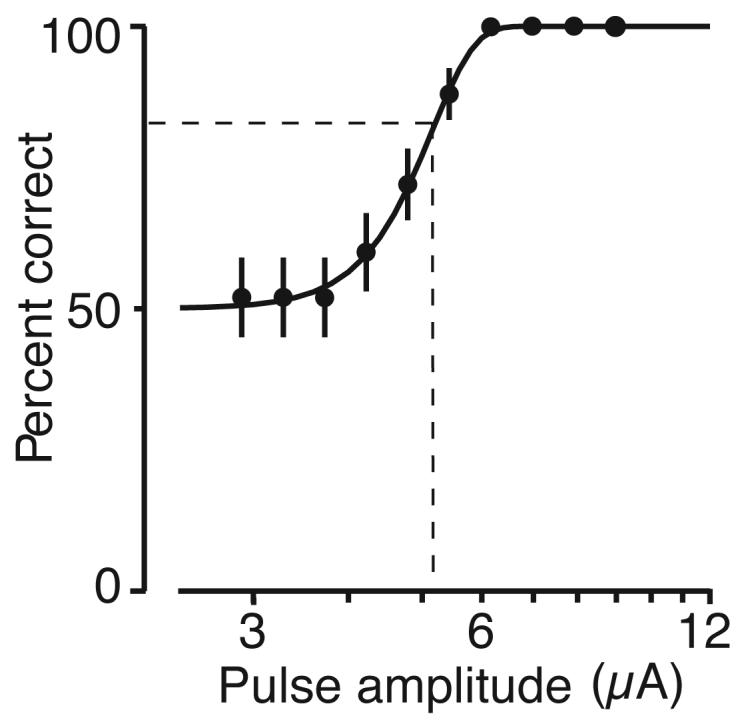
Representative psychometric function. Behavioral performance at a V1 site. Fifty repetitions of each of 10 currents were delivered in random order. The points show the average performance (±1 SE), and the curve is the best fitting psychometric function (see Methods). Threshold at each site was taken as the current corresponding to 82% correct performance on this curve (dashed lines).
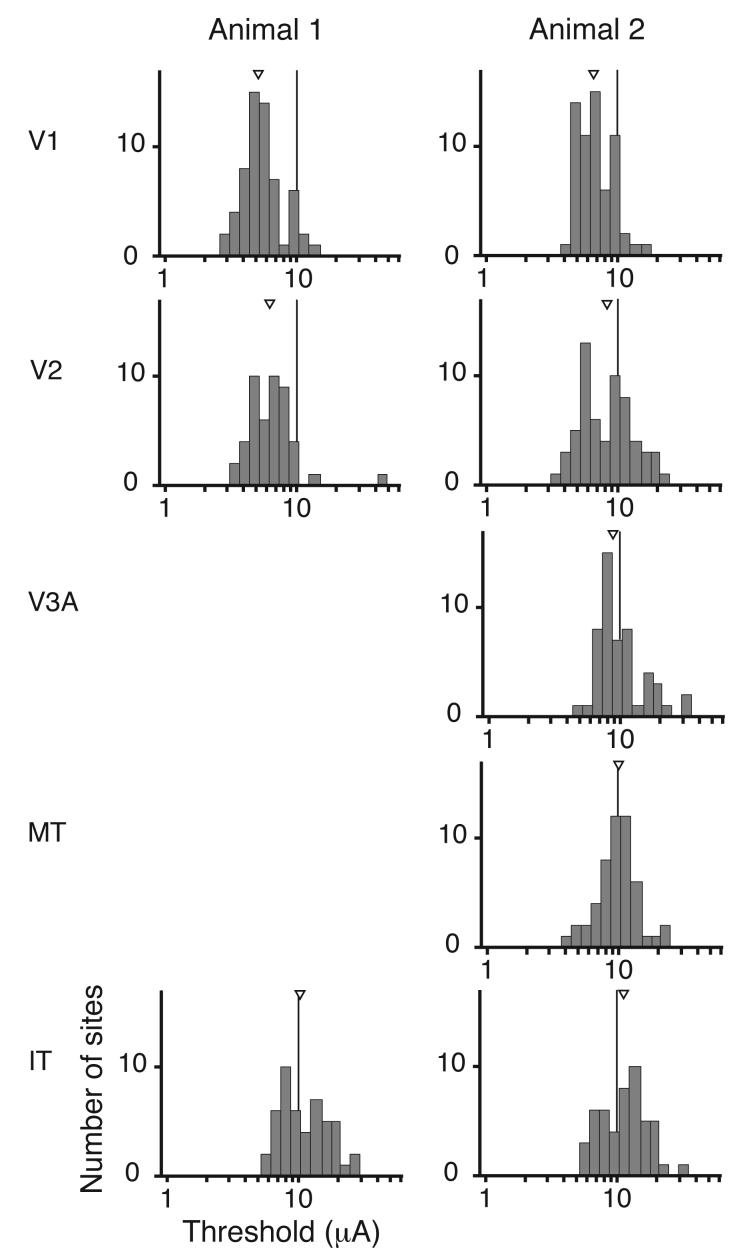
Threshold distributions for the different areas are plotted in Figure 3. Although we sampled from all layers, there was little variance in the distribution for each area. The average coefficient of variation for the threshold distributions in Figure 3 was 0.18. Moreover, thresholds were highly consistent for the two subjects. The median thresholds for V1 for the two animals were 5.2 and 6.6 μA (interquartile ranges 4.4-6.4 and 5.2-8.6 μA). Current pulses in this range (<10 μA) should directly excite neurons over no more than a 100 μm radius [27, 30], a small fraction of the thickness of macaque cortex. Although the anatomy of V2 is quite distinct from V1, thresholds in V2 differed only slightly (1.2 and 1.3 times greater those in V1 for the two animals). Thresholds for IT were somewhat higher (2.0 and 1.7 times those in V1 for the two animals). In the second animal we also measured thresholds in areas V3A and MT, which lie in the dorsal pathway. Thresholds in those areas were intermediate, consistent with their position in the hierarchy of cortical areas [31]. Overall, detection thresholds throughout visual cortex increased progressively from V1 to later stages. The differences between thresholds across areas were highly significant for both animals (p < 10−13 and p < 10−10; Kruskal-Wallis test). Nevertheless, the changes were moderate, with substantial overlap in the distributions for all areas and both animals reliably detecting 25 μA currents at virtually every site tested.
Figure 3.
Distributions of detection thresholds. Median thresholds are marked by a triangle. Medians and interquartile ranges for the two animals were (in microamps): Animal 1: V1, 5.2 (4.4-6.4), 6.6 (5.2-8.6); V2, 6.3 (4.9-7.6), 8.3 (5.8-11.5); V3A, 8.9 (7.7-12.2); MT, 10.1 (8.1-11.9); IT, 10.3 (8.2-15.5), 11.3 (7.5-15.2).
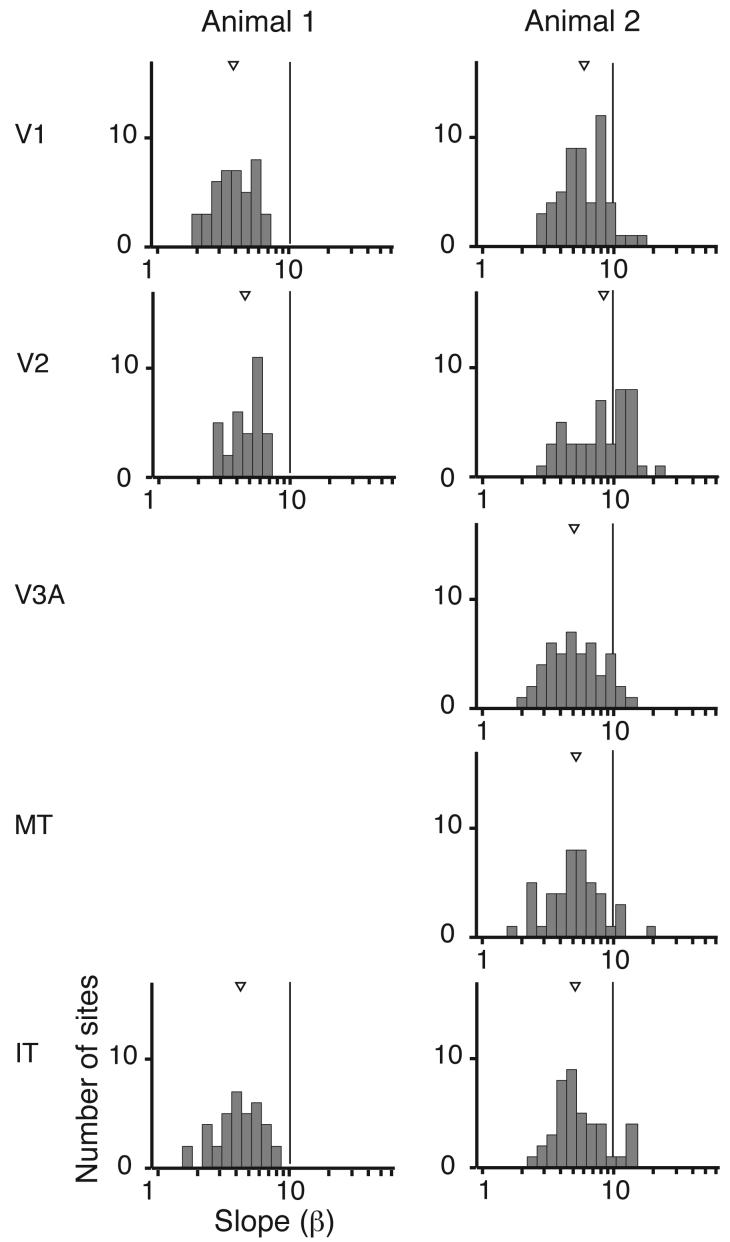
The psychometric detection function is characterized by a slope in addition to a threshold. Shallower slopes are associated with less sensitivity or greater noise and might be expected for stimulation sites that are further from a site of detection. Figure 4 illustrates the distribution of slopes for sites with dependable slope estimates (see Experimental Procedures). Only for Animal 2 was there a significant difference in slope across cortical areas (p<10−4; Kruskal-Wallis test), and neither animal had a significant correlation between slope and rank in the cortical hierarchy (p>0.32). Overall, the slopes of the psychometric functions did not suggest that particular cortical visual areas exercise special privilege in supporting percepts.
Figure 4.
Distributions of psychometric slopes. Summary slope histograms for all sites with reliable slope estimates (see Methods). Medians and interquartile ranges for the two animals were: V1, 4.5 (3.0-5.2), 6.0 (4.4-8.1); V2, 5.6 (4.9-7.6), 8.4 (5.2-11.5); V3A, 5.0 (3.6-6.6); MT, 5.2 (3.7-6.5); IT, 4.8 (3.3-5.7), 5.2 (4.0-7.0).
We tested for statistically significant correlations between threshold or slope and 1) depth in penetration (laminar differences), 2) time in data collection course for a given area (perceptual learning), and 3) time in day (learning or adaptation within a day). We also examined the relationship between threshold and slope, which are correlated when steeper slopes are achieved by suppressing responses to weak signals (“squelching” [see 32]). Seven tests were based on a correlation coefficient generated from a least squares fit of the data (p < 0.05 with Bonferroni correction). Across all the areas in both subjects this amounted to 56 tests, of which a few reached statistical significance. However, the significant correlations followed no obvious pattern: threshold versus time in data collection course for V1 in Animal 1, r =-0.60 (p<10−7) and threshold versus depth in penetration for IT in Animal 1, r = −0.53 (p<0.0001); threshold versus time in day for V3A for Animal 2, r = −0.48 (p<0.0003); threshold versus depth in penetration for V3A for Animal 2, r = −0.46 (p<0.0007). Both subjects had slightly higher thresholds in the superficial layers of V1 (sites <1.0 mm below the cortical surface), but the difference was small and significant only for one animal (Animal 1 27% higher, p = 0.18; Animal 2 18% higher, p=0.0001). For those areas in which the center of the multiunit receptive field was precisely mapped (V1, V2 and V3A), there was no correlation between receptive field eccentricity and the threshold or slope of the detection function.
Discussion
We have found that thresholds for detecting electrical microstimulation are similar across visual cerebral cortex, rising slowly and progressively in successive stages. There are several reasons these results must be interpreted with caution, however. First, while microstimulation makes it possible to measure the effects of changing the activity of neuronal elements in a single, identified cortical area, it does not create patterns of activity like those during normal vision. Although we modulated cortical activity arising from normal visual stimulation (viewing a fixation spot on an otherwise blank display), it remains possible that thresholds might have been different if it were possible to artificially activate spatial patterns of activity more closely resembling those created by visual stimuli. Second, it is possible that a close link between neuronal activity and perception in later stages was counterbalanced by unidentified factors that raised thresholds for detecting microstimulation in those areas. Finally, although thresholds were lower in earlier visual areas, it is possible that those thresholds were mediated by indirect, convergent activation of neurons in the highest levels of visual cortex. Conversely, it is possible that thresholds in later stages were mediated by indirect activation of neurons in V1 (which would explain why thresholds rose in later stages). However, obligatory activation of either the earliest or latest areas seems unlikely given the overlap in threshold and slope distributions found at opposite extremes of visual cortex. Additionally, a recent study using combined electrical microstimulation with functional imaging that found that most electrically-evoked activity in neocortex was limited to areas monosynaptically connected with the stimulation site (Logothetis, N., Sultan, F., Murayama, Y., Augath, M., Steudel, T., Oelterman, O, Microstimulation and fMRI in anesthetized and alert monkeys: conditions for transynaptic BOLD activation. Program No. 114.10. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience, 2006. Online).
While uncertainties remain about how microstimulation leads to perception, the overlapping threshold distributions for behavioral responses to microstimulation across different visual areas suggest that activation of a comparable number of neurons in any visual area suffices for generating a behaviorally detectable signal. They do not support the idea that later stages are more closely linked to perception, or that earlier stages have a distinctly privileged role. Similarly, the results from V3A and MT suggest that areas in dorsal pathway are not inferior to areas in the ventral pathway in their ability to support percepts. Our results are more consistent with the idea that neuronal signals anywhere in visual cortex have comparable capacity for contributing to perceptual decisions, and that performance on a particular visual task is based most directly on those neurons that provide the most reliable signals for that task, regardless of their position in the cortical visual hierarchy [19, 33].
The origin of the systematic changes in thresholds from V1 to IT remains unclear. Because we always tested in a sequence from V1 to IT, better performance in earlier stages cannot be due to the animals learning how to generalize to new percepts. Several anatomical differences mirror the progressive increases we found, including the spread of intrinsic connections [34, 35], the size of pyramidal cell bodies, and the extent and complexity of their dendritic fields [36]. On the other hand, the fact that the radically different architecture and thalamocortical inputs of V1 are not associated with markedly different thresholds suggests that thresholds are not greatly affected by details of cortical architectonics.
Further experiments will be needed to explain the disparity between the current results and previous studies that suggested that later stages in visual cortex are more closely linked to perception. One possibility is that previous studies involved visual functions that are specifically mediated by those regions of cortex. For example, studies of rivalry may have found closer correlations between neuronal activity and perceptual reports in later visual cortex because later stages play a special role in rivalry. Because lesions restricted to higher stages in the cortical hierarchy do not affect performance on simple discrimination tasks [37, 38], it is possible that tasks involving simple visual attributes or those involving precise spatial localization, such as hyperacuity, might reveal stronger correlations between behavior and neuronal activity in intermediate or early areas.
The detection thresholds we found in V1 are comparable to those found in an earlier study of detection of microstimulation in monkey V1 [26], and with thresholds for microstimulation with chronically implanted microelectrodes near V1 in a human subject [39]. We found less variance in the thresholds of different stimulation sites, probably owing to the use of a forced-choice design. Other studies in monkeys [30] and humans [40] have described higher thresholds for generating a behavioral response in the superficial layers of V1, as we describe here. In contrast, DeYoe and his colleagues [26] reported that the lowest detection thresholds in monkey V1 are found in the superficial layers. This discrepancy may arise from differences in behavioral tasks or stimulation protocols.
The current results are relevant to experiments that explore how microstimulation affects the perception of visual stimuli [e.g., 41, 42, 43]. These studies seek to perturb perception without the animal knowing when the electrical stimulus was delivered, but currents used are often higher than those that were reliably detectable here. In our task, microstimulation was detected while viewing a blank display, and it is possible that higher currents would be needed to detect microstimulation in conjunction with visual stimuli [but see 22]. Nevertheless, these other microstimulation studies must be carefully interpreted, as the subjects were rewarded for responses that could be influenced by an induced percept, either visual or otherwise [44]. Our results raise questions about interpretations that specifically argue against the detection of microstimulation during particular tasks [45].
Finally, we note that much of the work on developing neural prostheses has focused on stimulation of primary sensory and motor areas. The relatively uniform detection thresholds found in visual cortex and similarly low detection thresholds described in somatosensory and prefrontal cortex [46] raise the possibility that most regions of neocortex might be exploited for evoking percepts.
Experimental Procedures
All experiments conformed to protocols approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Electrical Stimulation
The electrical stimulus was a train of constant current 200 μs biphasic pulses delivered at 200 Hz for 250 ms through a Pt/Ir electrode (∼0.2-1.5MΩ at 1 kHz). At each cortical stimulation site, 50 repetitions of 6-10 current levels spanning behavioral threshold were tested in a randomly interleaved order. All currents are given as the pulse amplitude (not peak-to-peak). In both animals we collected data first from V1 and then sampled areas in hierarchical order [31], ending with IT. We typically stimulated at many regularly-spaced sites along one electrode penetration each day. In Animal 1, we found that thresholds began to rise over the course of 13 penetrations in a small region of V1. We excluded those data from analysis and limited sampling density for the data reported here.
Identifying Visual Areas
V1, V2, MT, and IT were targeted using sulcal landmarks in structural MR images and stereotaxic locations [47]. Their locations were confirmed using response properties and receptive field locations and sizes. Recordings were made from the general area of V3A on the anterior bank of the lunate sulcus, but because it lacks response properties or a topographic organization that can distinguish it from V3 [48], it is possible that some putative V3A sites may have been in V3. Because most penetrations were in a region that included receptive fields in the superior contralateral quadrant, we strongly suspect that all the sites were in V3A.
Psychometric Functions
Neither animal had an obvious response bias. During data collection interval 1 was selected 52% of the time by Animal 1 and 47% of the time by Animal 2. Behavioral responses on the two-alternative forced choice detection task was fit to the cumulative Weibull function [49]:
The parameter α is the current yielding to 82% correct responses and was taken as the threshold. The parameter β determines the steepness (slope) of the function. Sites were excluded from the analysis of slopes fewer than two sampled currents fell between 55 and 95% correct on the best fitting function, because those cases tended to have artificially high slopes.
Acknowledgements
We thank William Bosking, Joonyeol Lee, Xinmiao Peng, and Daniel Yoshor for their insights and suggestions during all stages of this project. We also thank Michael Beauchamp for comments on the manuscript and Vivian Imamura, Dennis Murray and Tori Williford for technical assistance. Supported by NIH R01EY05911. J.H.R.M. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 2.Williams ZM, Elfar JC, Eskandar EN, Toth LJ, Assad JA. Parietal activity and the perceived direction of ambiguous apparent motion. Nat Neurosci. 2003;6:616–623. doi: 10.1038/nn1055. [DOI] [PubMed] [Google Scholar]
- 3.Cook EP, Maunsell JH. Attentional modulation of behavioral performance and neuronal responses in middle temporal and ventral intraparietal areas of macaque monkey. J Neurosci. 2002;22:1994–2004. doi: 10.1523/JNEUROSCI.22-05-01994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J Neurosci. 1994;14:4109–4124. doi: 10.1523/JNEUROSCI.14-07-04109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 6.Nienborg H, Cumming BG. Macaque V2 neurons, but not V1 neurons, show choice-related activity. J Neurosci. 2006;26:9567–9578. doi: 10.1523/JNEUROSCI.2256-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen P, Vogels R, Liu Y, Orban GA. At least at the level of inferior temporal cortex, the stereo correspondence problem is solved. Neuron. 2003;37:693–701. doi: 10.1016/s0896-6273(03)00023-0. [DOI] [PubMed] [Google Scholar]
- 8.Cumming BG, Parker AJ. Responses of primary visual cortical neurons to binocular disparity without depth perception. Nature. 1997;389:280–283. doi: 10.1038/38487. [DOI] [PubMed] [Google Scholar]
- 9.Tong F, Nakayama K, Vaughan JT, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21:753–759. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- 10.Tong F, Engel SA. Interocular rivalry revealed in the human cortical blind-spot representation. Nature. 2001;411:195–199. doi: 10.1038/35075583. [DOI] [PubMed] [Google Scholar]
- 11.Juan CH, Campana G, Walsh V. Cortical interactions in vision and awareness: hierarchies in reverse. Prog Brain Res. 2004;144:117–130. doi: 10.1016/S0079-6123(03)14408-1. [DOI] [PubMed] [Google Scholar]
- 12.Tong F. Primary visual cortex and visual awareness. Nat Rev Neurosci. 2003;4:219–229. doi: 10.1038/nrn1055. [DOI] [PubMed] [Google Scholar]
- 13.Bullier J. Feedback connections and conscious vision. Trends Cogn Sci. 2001;5:369–370. doi: 10.1016/s1364-6613(00)01730-7. [DOI] [PubMed] [Google Scholar]
- 14.Silvanto J, Lavie N, Walsh V. Double dissociation of V1 and V5/MT activity in visual awareness. Cereb Cortex. 2005;15:1736–1741. doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- 15.Silvanto J, Cowey A, Lavie N, Walsh V. Striate cortex (V1) activity gates awareness of motion. Nat Neurosci. 2005;8:143–144. doi: 10.1038/nn1379. [DOI] [PubMed] [Google Scholar]
- 16.Barlow HB. Single units and sensation: a neuron doctrine for perceptual psychology? Perception. 1972;1:371–394. doi: 10.1068/p010371. [DOI] [PubMed] [Google Scholar]
- 17.Barlow HB. The twelfth Bartlett memorial lecture: the role of single neurons in the psychology of perception. Q J Exp Psychol A. 1985;37:121–145. doi: 10.1080/14640748508400927. [DOI] [PubMed] [Google Scholar]
- 18.Pollen DA. On the neural correlates of visual perception. Cereb Cortex. 1999;9:4–19. doi: 10.1093/cercor/9.1.4. [DOI] [PubMed] [Google Scholar]
- 19.Zeki S, Bartels A. Toward a theory of visual consciousness. Conscious Cogn. 1999;8:225–259. doi: 10.1006/ccog.1999.0390. [DOI] [PubMed] [Google Scholar]
- 20.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 21.Foerster O. The Cerebral Cortex in Man. Lancet. 1931:309–312. [Google Scholar]
- 22.Bartlett JR, DeYoe EA, Doty RW, Lee BB, Lewine JD, Negrao N, Overman WH., Jr. Psychophysics of electrical stimulation of striate cortex in macaques. J Neurophysiol. 2005;94:3430–3442. doi: 10.1152/jn.00406.2005. [DOI] [PubMed] [Google Scholar]
- 23.Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol. 1968;196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tehovnik EJ, Slocum WM, Carvey CE, Schiller PH. Phosphene induction and the generation of saccadic eye movements by striate cortex. J Neurophysiol. 2005;93:1–19. doi: 10.1152/jn.00736.2004. [DOI] [PubMed] [Google Scholar]
- 25.Penfield W. Some Mechanisms Of Consciousness Discovered During Electrical Stimulation Of The Brain. Proc Natl Acad Sci U S A. 1958;44:51–66. doi: 10.1073/pnas.44.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeYoe EA, Lewine JD, Doty RW. Laminar variation in threshold for detection of electrical excitation of striate cortex by macaques. J Neurophysiol. 2005;94:3443–3450. doi: 10.1152/jn.00407.2005. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett JR, Doty RW. An exploration of the ability of macaques to detect microstimulation of striate cortex. Acta Neurobiol Exp (Wars) 1980;40:713–727. [PubMed] [Google Scholar]
- 28.Doty RW. Conditioned Reflexes Elicited By Electrical Stimulation Of The Brain In Macaques. J Neurophysiol. 1965;28:623–640. doi: 10.1152/jn.1965.28.4.623. [DOI] [PubMed] [Google Scholar]
- 29.Green DM, Swets JA. Signal Detection Theory and Psychophysics. 1966 [Google Scholar]
- 30.Tehovnik EJ, Slocum WM, Schiller PH. Differential effects of laminar stimulation of V1 cortex on target selection by macaque monkeys. Eur J Neurosci. 2002;16:751–760. doi: 10.1046/j.1460-9568.2002.02123.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- 32.Pelli DG, Farell B, Moore DC. The remarkable inefficiency of word recognition. Nature. 2003;423:752–756. doi: 10.1038/nature01516. [DOI] [PubMed] [Google Scholar]
- 33.Maunsell JH, Cook EP. The role of attention in visual processing. Philos Trans R Soc Lond B Biol Sci. 2002;357:1063–1072. doi: 10.1098/rstb.2002.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amir Y, Harel M, Malach R. Cortical hierarchy reflected in the organization of intrinsic connections in macaque monkey visual cortex. J Comp Neurol. 1993;334:19–46. doi: 10.1002/cne.903340103. [DOI] [PubMed] [Google Scholar]
- 35.Yoshioka T, Levitt JB, Lund JS. Intrinsic lattice connections of macaque monkey visual cortical area V4. J Neurosci. 1992;12:2785–2802. doi: 10.1523/JNEUROSCI.12-07-02785.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elston GN, Rosa MG. Morphological variation of layer III pyramidal neurones in the occipitotemporal pathway of the macaque monkey visual cortex. Cereb Cortex. 1998;8:278–294. doi: 10.1093/cercor/8.3.278. [DOI] [PubMed] [Google Scholar]
- 37.Vogels R, Saunders RC, Orban GA. Effects of inferior temporal lesions on two types of orientation discrimination in the macaque monkey. Eur J Neurosci. 1997;9:229–245. doi: 10.1111/j.1460-9568.1997.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 38.De Weerd P, Desimone R, Ungerleider LG. Impairments in spatial generalization of visual skills after V4 and TEO lesions in macaques (Macaca mulatta) Behav Neurosci. 2003;117:1441–1447. doi: 10.1037/0735-7044.117.6.1441. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt EM, Bak MJ, Hambrecht FT, Kufta CV, O'Rourke DK, Vallabhanath P. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain. 1996;119(Pt 2):507–522. doi: 10.1093/brain/119.2.507. [DOI] [PubMed] [Google Scholar]
- 40.Bak M, Girvin JP, Hambrecht FT, Kufta CV, Loeb GE, Schmidt EM. Visual sensations produced by intracortical microstimulation of the human occipital cortex. Med Biol Eng Comput. 1990;28:257–259. doi: 10.1007/BF02442682. [DOI] [PubMed] [Google Scholar]
- 41.Britten KH, van Wezel RJ. Electrical microstimulation of cortical area MST biases heading perception in monkeys. Nat Neurosci. 1998;1:59–63. doi: 10.1038/259. [DOI] [PubMed] [Google Scholar]
- 42.Ditterich J, Mazurek ME, Shadlen MN. Microstimulation of visual cortex affects the speed of perceptual decisions. Nat Neurosci. 2003;6:891–898. doi: 10.1038/nn1094. [DOI] [PubMed] [Google Scholar]
- 43.Nichols MJ, Newsome WT. Middle temporal visual area microstimulation influences veridical judgments of motion direction. J Neurosci. 2002;22:9530–9540. doi: 10.1523/JNEUROSCI.22-21-09530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- 46.de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 47.Van Essen DC. Surface-based approaches to spatial localization and registration in primate cerebral cortex. Neuroimage. 2004;23(Suppl 1):S97–107. doi: 10.1016/j.neuroimage.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 48.Zeki SM. The third visual complex of rhesus monkey prestriate cortex. J Physiol. 1978;277:245–272. doi: 10.1113/jphysiol.1978.sp012271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quick RF., Jr. A vector-magnitude model of contrast detection. Kybernetik. 1974;16:65–67. doi: 10.1007/BF00271628. [DOI] [PubMed] [Google Scholar]