Abstract
Recently it has been shown that long chain (C5 to C8) n-alkyl glucopyranosides completely inhibit ultrasound-induced cytolysis [1]. This protective effect has possible applications in HIFU (high intensity focused ultrasound) for tumor treatment, and in ultrasound assisted drug delivery and gene therapy. n-Alkyl glucopyranosides with hexyl (5mM), heptyl (3mM), octyl (2mM) n-alkyl chains protected 100% of HL-60 cells in-vitro from 1.057 MHz ultrasound induced cytolysis under a range of conditions that resulted in 35% to 100% cytolysis in the absence of glucopyranosides. However the hydrophilic methyl-β-D-glucopyranoside did not protect cells. The surface active n-alkyl glucopyranosides accumulate at the gas-liquid interface of cavitation bubbles. The OH radicals and H atoms formed in collapsing cavitation bubbles react by H-atom abstraction from either the n-alkyl chain or the glucose moiety of the n-alkyl glucopyranosides. Owing to the high concentration of the long chain surfactants at the gas-liquid interface of cavitation bubbles, the initially formed carbon radicals on the alkyl chains are transferred to the glucose moieties to yield radicals which react with oxygen leading to the formation of hydrogen peroxide. In this work we find that the sonochemically produced hydrogen peroxide yields from oxygen-saturated solutions of long chain (hexyl, octyl) n-alkyl glucopyranosides at 614 kHz and 1.057 MHz ultrasound increase with increasing n-alkyl glucopyranoside concentration but are independent of concentration for methyl-β-D-glucopyranoside. These results are consistent with the previously proposed mechanism of sonoprotection [1]. This sequence of events prevents sonodynamic cell killing by initiation of lipid peroxidation chain reactions in cellular membranes by peroxyl and/or alkoxyl radicals [2].
Keywords: n-alkyl glucopyranosides, protection mechanism, ultrasound-induced cell killing, HL-60 cells
1. Introduction
Recently Sostaric et al. [1] have shown that above certain concentrations, n-alkyl glucopyranosides completely inhibit ultrasound-induced cytolysis at 1.057 MHz. n-Alkyl glucopyranosides with hexyl (5mM), heptyl (3mM), octyl (2mM) n-alkyl chains protected 100% of HL-60 cells in-vitro from 1.057 MHz ultrasound induced cytolysis under a range of conditions that resulted in 35% to 100% cytolysis in the absence of glucopyranosides. However the hydrophilic methyl-β-D-glucopyranoside did not protect HL-60 cells.
The previously proposed mechanism [1] for sonoprotection of HL-60 cells is based on the quenching of organic peroxyl and alkoxyl radicals by the long chain n-alkyl glucopyranosides which accumulates at the gas-liquid interface of cavitation bubbles. Misik et al. have proposed a chemical mechanism for the increase in cytolysis from sonication with specific solutes (sonosensitizers) [2]. This mechanism involves a relatively small number of organic peroxyl radicals, which are formed from the sonosensitizers. These peroxyl radicals have long diffusion distances in a biological medium and thus initiate lipid peroxidation chain reactions in the cell membranes by attacking allylic hydrogens. Thus the sonoprotection of cells by n-alkyl glucopyranosides is achieved by quenching the peroxyl and alkoxyl radicals and preventing lipid peroxidation chain reactions from occurring. None of these n-alkyl glucopyranosides could prevent cytolysis of HL-60 cells by mechanical shear stress [1].
The initial chemical process involves OH radicals or H atoms formed in collapsing cavitation bubbles abstracting hydrogen atoms from either the glucose moiety (ring structure) or the n-alkyl chain of the glucopyranosides. If the abstraction of hydrogen atoms occurs on the glucose moiety, the rapid addition of oxygen to the carbon radicals of the glucose moiety leads to glucose peroxyl radicals. The fate of these glucose peroxyl radicals has been studied in detail by Schuchmann and von Sonntag [3] by extensive product analysis. Pulse radiolysis studies of D-glucose [4,5] have shown that the rates of HO2 elimination from D-glucose are in the order C-1 >> C-2, C-3, C-4 > C-6 >> C-5. The HO2 radicals largely react with one another or with O2−• to form H2O2 and oxygen.
| (1) |
If the abstraction of hydrogen atoms occurs on the n-alkyl chain to form n-alkyl carbon radicals, these have a high probability of encountering the weaker CH bonds of the glucose residues due to the high concentration of the surfactant n-alkyl glucopyranosides at the cavitation gas bubble - aqueous solution interface. In oxygen-saturated aqueous solutions, the resulting glucose radicals react with oxygen to form superoxide anion radicals leading to hydrogen peroxide [6].
The preferred localization of long chain n-alkyl glucopyranoside surfactants at the gas - aqueous interface of cavitation bubbles formed by 50 kHz ultrasound in aqueous solutions compared to non-volatile solutes possessing no surfactant properties was first demonstrated by Alegria et al. [7] by the detection of pyrolysis-derived methyl radicals in argon-saturated solutions at a 500 fold lower concentration than for the non-surfactant analogue methyl-β-D-glucopyranoside. Furthermore in studies of sonoluminescence from aqueous alcohol solutions, a good correlation was found between the decline in sonoluminescence intensity and the Gibbs Surface Excess [8] of the alcohol a the air - water interface of cavitation bubbles [9]. In addition Sostaric and Riesz [10] have reported that sonolysis of aqueous solutions of n-alkyl anionic surfactants with increasing n-alkyl chain lengths result in an increase in -•CH- radical yields due to the increase in their ability to equilibrate between the bulk solution and the gas - aqueous interface of the cavitation bubbles.
To elucidate the sonoprotective mechanism, we report a study of the chemical basis for sonoprotection of HL-60 cells by n-alkyl glucopyranosides exposed to ultrasound. Hydrogen peroxide yields of oxygen saturated aqueous solutions exposed to ultrasound at frequencies of 614 kHz and 1.057 MHz were determined. Consistent with the proposed mechanism of sonoprotection [1] which prevents the occurrence of lipid peroxidation chain reactions in cellular membranes, the surfactants n-hexyl and n-octyl glucopyranosides prevent the ultrasound-induced formation of peroxyl and alkoxyl radicals from the constituents of the biological medium and eventually leads to hydrogen peroxide formation. We observed the predicted increase of hydrogen peroxide yields with increasing concentration of n-hexyl and n-octyl glucopyranosides. Furthermore as predicted by this mechanism, an increase in the non-surfactant methyl glucopyranoside concentration was found to have no effect on the hydrogen peroxide yields.
2. Experimental
2.1 H2O2 Yield Determination
Hydrogen peroxide yields were measured by the method of Hochanadel as described by Alegria et al. [7]. This method involves the oxidation of iodide ion by H2O2 in neutral or slightly acidic solutions. The iodide reagent was prepared immediately before use and consisted of 1.25 ml of a solution containing 0.4 M KI, 0.05 M NaOH, and 1.6 × 10−4 M (NH4)6Mo7O24•4H2O; 1.25 ml of 0.1 M KHC8H4O4; and 0.5 ml of purified water (Milli-Q, 16 MΩ). This 3 ml of iodide reagent mixture was added to 2 ml of the sonicated sample and thoroughly mixed. The absorption of I3 − was measured at 352 nm with a diode array spectrophotometer (Hewlett-Packard) in a 1 cm cell. The H2O2 concentrations in μM were calculated by [H2O2] = (DS − DB) × 100 where DS and DB represent the absorbances of the sample and the blank, respectively. This classical method for determining H2O2 was calibrated with solutions of known H2O2 concentration [11].
2.2 Ultrasound conditions
Various concentrations of methyl- β-D-glucopyranoside (Sigma-Aldrich), hexyl (=98%, Fluka), and octyl n-alkyl glucopyranosides (=99%, Fluka) ranging from 0.1 to 6 mM were prepared with 2 ml of purified water (Milli-Q, 18 MΩ). These 2 ml of mixture was placed in 13 mm-diameter × 100 mm-length disposable borosilicate glass tubes (Kimble Glass Inc.) and saturated with oxygen for 5 minutes prior to sonication. The glass tube was then fixed at the center of a cylindrical thermostated sonication bath and maintained at 20°C by a circulating bath (model K20, Haake). The ultrasound system was composed of a power generator (model Cesar, L3 Communications, ELAC-Nautik GmbH) connected to a transducer unit operating at frequencies of 1.057 MHz (model USW 51-52) or 614 kHz (model USW 51-51) and is positioned at the bottom of the cylindrical circulating bath. The oxygen-saturated aqueous solutions (5 mM) were exposed to ultrasound for 5 minutes. Figure 1 and Figure 2 show the results of hydrogen peroxide yields (mean ± standard deviation) as a function of ultrasound sonication time for 614 kHz and 1.057 MHz, respectively. These figures show linear behavior for both frequencies up to 5 minutes.
Figure 1.

Effect of sonolysis time (minutes) and alkyl chain length (methyl -▽, hexyl -□, octyl -O) on hydrogen peroxide yields (mean ± standard deviation in μM) from oxygen-saturated aqueous solutions (5 mM) of n-alkyl glucopyranosides at 614 kHz.
Figure 2.

Effect of sonolysis time (minutes) and alkyl chain length (methyl -▽, hexyl -□, octyl -O) on hydrogen peroxide yields (mean ± standard deviation in μM) from oxygen-saturated aqueous solutions (5 mM) of n-alkyl glucopyranosides at 1.057 MHz.
A calibrated 1.0 mm-diameter PVDF needle hydrophone (model HPM01-1, Precision Acoustics Ltd) and a digital oscilloscope (model TDS5032B, Tektronix) were used to measure the pressure amplitude. The acoustical pressure amplitude inside of the glass tube fluctuates due to the complex acoustical field in our ultrasound exposure system. For suitable comparison of the acoustical pressure amplitude within the test tube, the pressure amplitudes were measured under the same conditions as during the hydrogen peroxide yield measurements. With the help of a micropositioner (Newport, model MS-500-XYZ), the needle hydrophone was placed at the bottom of the test tube and the peak-to-peak pressure amplitude was measured for 5 minutes of sonication within each setting of the power generator. The accumulated waveforms were averaged by the digital oscilloscope. Figure 3 shows the peak-to-peak pressure amplitude (mean ± standard deviation) averaged over 6 runs with respect to the setting in the power generator during 5 minutes of sonication at 614 kHz and 1.057 MHz. Note that due to the large fluctuates within the pressure measurements, we were unable to distinguish the pressure uniformity within the test tube or the attenuation of the test tube.
Figure 3.
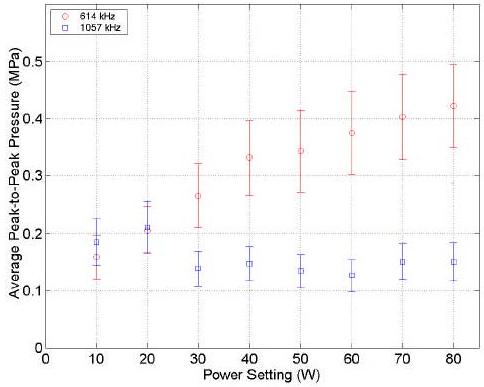
Peak-to-peak pressure amplitudes (mean ± standard deviation in MPa) averaged over 6 runs with respect to the setting in the power generator (W) during 5 minutes of sonication at 614 kHz (O), and 1.057 MHz (□).
3. Results
The effect of n-alkyl glucopyranosides on hydrogen peroxide formation in oxygen-saturated aqueous solutions exposed to ultrasound at 614 kHz and 1.057 MHz are shown in Figures 4 and 5 respectively. For hexyl and octyl glucopyranosides which accumulate at the gas/liquid interface of cavitation bubbles, the hydrogen peroxide yields exhibit an initial increase before saturation at constant values. For methyl glucopyranoside which does not accumulate at the gas/liquid interface of cavitation bubbles, the hydrogen peroxide yields remained constant at 64 μM and 11 μM for 614 kHz and 1.057 MHz, respectively.
Figure 4.

Hydrogen peroxide yields (mean ± standard deviation in μM) from oxygen-saturated aqueous solutions of n-alkyl glucopyranosides (methyl -▽, hexyl -□, octyl -O) after sonication (5 minutes, 20W) at 614 kHz.
Figure 5.

Hydrogen peroxide yields (mean ± standard deviation in μM) from oxygen-saturated aqueous solutions of n-alkyl glucopyranosides (methyl -▽, hexyl -□, octyl -O) after sonication (5 minutes, 20W) at 1.057 MHz.
4. Discussion
These results show that the hydrogen peroxide yields from oxygen saturated solutions of long chain (hexyl, octyl) n-alkyl glucopyranosides compared with methyl-β-D-glucopyranoside are consistent with the proposed mechanism for sonoprotection of cells discussed above. Although hydrogen peroxide above a certain concentration is toxic to cells, during the previously reported sonoprotection experiments [1], the cells are only exposed to ultrasound for 5 seconds in RPMI medium under air whereas the hydrogen peroxide measurement was performed for 5 minutes of sonication in water under oxygen. Results of hydrogen peroxide yields as a function of ultrasound sonication time shown in Figure 2 illustrate linear behavior for both frequencies up to 5 minutes. At 5 seconds of sonication at 1.057 MHz, the hydrogen peroxide concentration is approximately 0.5 μM. H2O2 can diffuse freely through cellular membranes and can activate many components of intracellular signaling cascades which influence cell survival [12]. Hachiya and Akashi have shown that for HL-60 cells, concentrations of more than 50 μM of hydrogen peroxide are needed for significant cell killing [13]. This indicates a very low percentage of cell death due to hydrogen peroxide at 0.5 μM. The critical factor in sonodynamic cell killing is the conversion of the OH radicals which react with organic molecules in biological media to form peroxyl radicals capable of attacking critical cellular sites due to their ability to diffuse significant distances and to initiate lipid peroxidation chain reactions in cellular membranes [2]. The presence of n-alkyl (≥ 5) glucopyranosides prevents this chain reaction from occurring and thus protects the cells.
It is interesting to note that at 614 kHz, the formation of H2O2 for hexyl and octyl glucopyranosides reach the same plateau level (∼ 100μM) for n-alkyl glucopyranoside above 2 mM as shown in Figure 4. However, at 1.057 MHz, the maximum H2O2 yield for hexyl glucopyranoside (∼ 47 μM) is larger than for octyl glucopyranoside (∼ 30 μM) as indicated in Figure 5. Changes in ultrasound frequency can result in detectable variations of the relative ability of surfactants to accumulate at the gas - aqueous interface of cavitation bubble [14] and hence affect the probability that OH radicals formed in cavitation bubbles will react with surfactant molecules at the gas/liquid interface or recombine in the bulk of the solution to form hydrogen peroxide. However, Birkin et al. [15] have shown that applying ultrasound frequencies between 20 - 160 kHz with a 1 kHz resolution in a cylindrical reactor, which is similar to our ultrasound setup, results in varying H2O2 yields, Fe+3 yields in the Fricke reaction, and the multibubble sonoluminescence emission intensity. They concluded that the frequency dependence of the system is the net result of the transducer frequencies, the reverberant sound field, the cavitation dynamics, and the chemistry. In addition, Sunartio et al. [16] have shown in a multibubble sonoluminescence study for aqueous solutions containing the anionic surfactant sodium dodecyl sulfate that the sonoluminescence emission intensity is strongly dependent on the applied acoustic power (or more specifically on the peak negative acoustical pressure). Since our ultrasound apparatus produces large fluctuations in acoustic pressure measurements, it is unclear whether the transducer frequency, the applied acoustic power, cavitation dynamics, or chemistry contribute a greater role to the hydrogen peroxide yields.
5. Conclusion
In summary, the results of measurements of the hydrogen peroxide yields from oxygen saturated solutions of long chains (hexyl, octyl) n-alkyl glucopyranosides compared with methyl-β-D-glucopyranoside are consistent with the previously proposed mechanism for sonoprotection of cells [1].
Acknowledgement
JYC acknowledges a Cancer Research Training Award Fellowship from the National Cancer Institute, NIH. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sostaric JZ, Miyoshi N, Riesz P, DeGraff WG, Mitchell JB. Free Radical Biology and Medicine. 2005;39:1539. doi: 10.1016/j.freeradbiomed.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Misik V, Riesz P. Ann. N.Y. Acad. Sci. 2000;899:335. doi: 10.1111/j.1749-6632.2000.tb06198.x. [DOI] [PubMed] [Google Scholar]
- 3.Schuchmann MN, von Sonntag C. J. Chem. Soc. Perkin Trans. 1977;II:1958. [Google Scholar]
- 4.Schuchmann MN, von Sonntag C. Z. Naturforsch. 1978;88b:321. [Google Scholar]
- 5.von Sonntag C. The Chemical Basis of Radiation Biology. Taylor & Francis, Inc.; Philadelphia: 1987. pp. 59–93. Ch 4. [Google Scholar]
- 6.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. Oxford Univ. Press; New York: 1999. [Google Scholar]
- 7.Alegria AE, Lion Y, Kondo T, Riesz P. J. Phys. Chem. 1989;93:4908. [Google Scholar]
- 8.Shaw DJ. Colloid and Surface Chemistry. 4th Edition Butterworth-Heinenmann; Oxford: 1992. p. 83. [Google Scholar]
- 9.Ashokkumar M, Hall R, Mulvaney P, Grieser F. J. Phys. Chem. B. 1997;101:10845. [Google Scholar]
- 10.Sostaric JZ, Riesz P. J. Am. Chem. Soc. 2001;123:11010. doi: 10.1021/ja010857b. [DOI] [PubMed] [Google Scholar]
- 11.Hochanadel CJ. J. Phys. Chem. 1952;56:587. [Google Scholar]
- 12.Boyd CS, Cadenas E. Biol. Chem. 2002;383:411. doi: 10.1515/BC.2002.045. [DOI] [PubMed] [Google Scholar]
- 13.Hachiya M, Akashi M. Radiation Research. 2005;163:271. doi: 10.1667/rr3306. [DOI] [PubMed] [Google Scholar]
- 14.Sostaric JZ, Riesz P. J. Phys. Chem. B. 2002;106:12537. [Google Scholar]
- 15.Birkin PR, Power JF, Vincotte AML, Leighton TG. Phys. Chem. Chem. Phys. 2003;5:4170. [Google Scholar]
- 16.Sunartio D, Ashokkumar M, Grieser F. J. Phys. Chem. B. 2005;109:20044. doi: 10.1021/jp052747n. [DOI] [PubMed] [Google Scholar]


