Abstract
Endothelialization of expanded polytetrafluoroethylene (ePTFE) has the potential to improve long-term patency for small-diameter vascular grafts. Successful endothelialization requires ePTFE surface modification to permit cell attachment to this otherwise non-adhesive substrate. We report here on a peptide fluorosurfactant polymer (FSP) biomimetic construct that promotes endothelial cell (EC)-selective attachment, growth, shear stability, and function on ePTFE. The peptide FSP consists of a flexible poly(vinyl amine) backbone with EC-selective peptide ligands for specific cell adhesion and pendant fluorocarbon branches for stable anchorage to underlying ePTFE. The EC-selective peptide (primary sequence- Cys-Arg-Arg-Glu-Thr-Ala-Trp-Ala-Cys, CRRETAWAC) has demonstrated high binding affinity for the α5β1 integrin found on ECs. Here, we demonstrate low affinity of CRRETAWAC for platelets and platelet integrins, thus providing it with EC-selectivity. This EC-selectivity could potentially facilitate rapid in vivo endothelialization and healing without thrombosis for small-diameter ePTFE vascular grafts.
Introduction
There is a pressing clinical need for suitable small-diameter vascular prostheses to bypass diseased coronary and peripheral arteries. Materials, such as expanded polytetrafluoroethylene (ePTFE), that are successful as large-diameter vascular prostheses have proven inadequate when used in small-diameter applications because of thrombosis and occlusion [1, 2]. The ideal blood interface is a confluent layer of healthy endothelial cells (ECs). Pre-implantation endothelialization of ePTFE has demonstrated limited clinical improvement in long-term patency for small-diameter grafts [3, 4]. The challenge of tissue engineering the ideal blood interface is that the same matrix proteins (e.g. fibronectin, FN) or FN-derived peptides that bind ECs also will bind platelets and initiate thrombosis. We have previously reported a peptide fluorosurfactant polymer (FSP) modification of ePTFE that facilitates EC adhesion, growth and function [5]. However, the RGD-containing peptide sequence used for cell binding on our previous construct has roughly equivalent affinity for EC and platelet integrins [6-8]. In this study, we hypothesized that platelet binding could be reduced while maintaining EC adhesion, growth, shear stability, and hemostatic function if an alternative, EC integrin-selective peptide was employed in our FSP biomimetic construct.
The cyclic peptide sequence Cys-Arg-Arg-Glu-Thr-Ala-Trp-Ala-Cys (CRRETAWAC; Fig. 1) has been shown to bind with high specificity and affinity to the α5β1 integrin (IC50 for α5β1 integrin binding is 0.01 μM, but >1000 μM for αvβ3 integrin) [9]; this has been attributed to RRE motif interaction with the β1 subunit (similar to RGD interaction) [10] and Trp interaction with a Trp residue in the α5 subunit [11-13]. High affinity binding of CRRETAWAC to α5β1 integrin confers EC selectivity due to the relative prominence of α5β1 integrin on ECs compared with platelets [14].
Figure 1.

Reaction scheme for synthesis of fluorosurfactant polymer with CRRETAWAC peptide. The RRE motif is believed to interact with β1 integrin subunit [10]; the Trp residue is believed to interact with Trp157 within the α5 integrin subunit [11]. Peptide FSP consists of poly(vinyl amine) backbone with free amines (x=7), glutaraldehyde linked cyclic GSSSCRRETAWAC cell-binding peptide (y=2), and substrate adsorbing perfluorocarbon pendant branches (z=1).
In this report, we examine the binding affinity of cyclic CRRETAWAC for platelet receptors, specifically the αIIbβ3 integrin. The synthesis and characterization of CRRETAWAC FSP (Fig. 1) is detailed. We also investigate endothelial cell adhesion, growth, shear stability, and function on CRRETAWAC FSP-modified fluorocarbon substrates. Finally, we demonstrate limited platelet adhesion on CRRETAWAC FSP, establishing its potential to promote rapid endothelialization without thrombosis for small-diameter vascular grafts.
Materials and Methods
Peptide fluorosurfactant polymer synthesis and characterization
Peptide synthesis, cyclization, and purification
The cell adhesive peptide was synthesized using an Applied Biosystems (model 433A) solid-phase peptide synthesizer, utilizing 9-fluorenylmethoxycarbonyl (Fmoc) methodology, common solvents, packing resin and capped amino acids. This peptide is a 13 amino acid molecule having the following sequence: CRRETAWACSSSG (Fig. 1, or negative control scrambled peptide CATAERWRCSSSG). Poly(vinyl amine) (PVAm, Mn=11,000) was synthesized as described [15] with molecular weight characterized by GPC using a cationic column and a laser scattering detector [16]. The hydrophilic SSSG spacer present at the N-terminus of the peptide was included to allow for elevation of the functional CRRETAWAC peptide sequence from the PVAm backbone. Formation of disulfide bonds between sulfhydryl group of cysteine residues was achieved by a solution oxidation method [17]. The linear peptide (150 mg), cleaved and deprotected, was dissolved in 300 ml of 0.01% aqueous acetic acid, and the pH was adjusted to 7.5-8.0 with 8 M aqueous ammonium hydroxide. The solution was titrated at room temperature (RT) with 0.01 M K3Fe(CN)6 until the yellow color was maintained for 10 min. The conversion of sulfhydryl groups to disulfide bonds was confirmed by a negative Ellman's test [18]. Ion-exchange resin (3.7 g of AG3X4, acetate form) was added, and stirring was continued for 30 min. The suspension was then filtered to remove resin, and the filtrate was lyophilized, resuspended in water, and lyophilized for two additional cycles. The peptide was then purified by preparatory scale high performance liquid chromatography (HPLC) to yield 46 mg (30 % yield) and characterized for composition by mass-spectroscopy (single peak 1410.98, expected 1411).
Synthesis of peptide fluorosurfactant polymer
To facilitate peptide attachment to amino groups of PVAm, cyclized and purified peptide was first reacted with glutaraldehyde (5x excess) using the standard Schiff base reaction in the presence of a reducing agent, NaCNBH3, in aqueous solution. Ellman's test for sulfhydryl groups on the modified peptide was negative. The aldehyde-terminated peptide (Pep-ald) was purified from excess glutaraldehyde by dialysis against water, filtered to remove a small amount of aggregates, and lyophilized. Reactive CRRETAWAC peptide (Pep-ald, 27 mg, 0.02 mmol) was attached to PVAm (1.4 mg, 0.033 mmol) using the standard Schiff base reaction described above. The conjugate was purified by extensive dialysis against water, filtered, and lyophilized (24 mg). Ellman's test for sulfhydryl groups on this product was also negative. IR: 3305 cm−1(ν (OH), 2926-2968 cm−1(ν (CH) of CH2 and CH, 1650 cm−1(amide-I) and 1547 cm−1 (amide-II). No residual aldehyde peak (at 9.7 ppm) was present in the 1H-NMR spectrum.
To attach perfluorocarbon pendant groups to PVAm polymer chain, the N-(perfluoroundecanoyloxy) succinimide [5] (3.64 mg, 0.0056 mmol in 1 ml methanol) was added to PVAm-Pep (20 mg, 0.0112 mmol) dissolved in 2 ml DMSO and stirred for 5 h at RT. The initial molar ratio of fluorocarbon to peptide was 1:2. The final product was purified by extensive dialysis against water and lyophilized (10.6 mg). IR (KBr, cm−1) 3305 (ν (OH), 2926-2968 (ν (CH) of CH2 and CH), 1650 (amide-I) and 1547 (amide-II), 1224 and 1154 (-CF2-). XPS atomic composition data on polyethylene: %O 17.0; %N 13.3; %F 8.9.
UV spectroscopy
UV absorption of peptide and PVAm-peptide conjugate in aqueous solution was measured by UV-VIS scanning spectrophotometer (UV-2101PC, Shimadzu) at 200-400 nm range, with sampling interval 0.2 and slit width 1.0. At least 5 dilutions were made using Millipore water as a diluent. Maximum absorbance (at 280 nm) was plotted against sample concentration. The molar absorption coefficient was calculated using the Beer-Lambert equation (ε=A/c).
Solid-phase integrin binding competition
αIIbβ3 integrin (5 μg/ml, Enzyme Research Laboratories) in 50 mM Tris-HCl, pH 7.4 with 150 mM NaCl, 1 mM MgCl2, and 1 mM CaCl2 (Buffer A) was adsorbed overnight at 4°C onto 96 well high-binding plates (Fisher). Wells were then blocked for 1 h with 50 mM Tris-HCl, pH 7.4, 150 mM NaCl containing 0.2% (w/v) bovine serum albumin (BSA, Sigma). Wells were then rinsed three times with Buffer A which contained 0.04% Tween 20. Fibrinogen (FG, 10 μg/ml, Enzyme Research Laboratories) mixed with various concentrations of GRGDSP or CRRETAWAC in Buffer A was incubated and allowed to bind to immobilized integrin in wells for 2 h at RT. Wells were then rinsed three times with Buffer A. Bound FG was detected by incubating wells with 1:500 primary sheep anti-human FG affinity purified peroxidase conjugated antibody (Enzyme Research Laboratories) in 50 mM Tris-HCl, pH 7.4, 150 mM NaCl (1xTBS) containing 4% (v/v) fetal bovine serum (FBS, Sigma) for 45 min at RT. Wells were then rinsed three times with Buffer A. To develop the wells, 3,3′,5,5′-Tetramethylbenzidine (TMB, Calbiochem) substrate was added, and the absorbance was monitored at 650 nm. Normalized FG binding was determined by correlating absorbance from wells with peptide-FG competition to absorbance values obtained from wells with varying concentrations of FG and no peptide. IC50 values for peptide inhibition of FG binding to immobilized αIIbβ3 integrin were determined by software fitting (Origin 7.5, OriginLab Corporation) of 60 data points for each peptide (at least 11 concentrations/peptide with at least 4 replicates for each concentration) from 3 independent experiments to a logistic nonlinear regression model. The coefficients of determination (R2) for the CRRETAWAC and GRGDSP regression fits were 0.92 and 0.83, respectively.
Platelet aggregometry
The effect of peptides on platelet aggregation was assessed using a Bio/Data PAP-4 aggregometer according to a standardized protocol [19]. Platelet-rich plasma (PRP; 231-324×103 platelets/μL) and platelet-poor plasma (PPP; 15-23 × 103 platelets/μL) were obtained from two healthy consenting donors in compliance with IRB approved protocol by methods described previously [20]. PPP was used to obtain baseline data for the aggregometer before each run. Samples were prepared by adding 450 μL of PRP, 25 μL of 200 μM adenosine-5'-diphosphate (ADP) solution (Bio/Data Corporation, final [ADP] of platelet suspension was 10 μM), and 25 μL of various concentrations of CRRETAWAC or GRGDSP peptide to an aggregometer cuvette. Platelet aggregation was monitored for 5 min with stirring, and the maximum percent platelet aggregation for each sample was recorded. All data were compared with the maximum platelet aggregation value from a platelet suspension of PRP with 10 μM ADP to calculate the % platelet aggregation inhibition. Inhibitory concentrations for GRGDSP and CRRETAWAC at which 50% of platelet aggregation was inhibited (IC50) were determined by software fitting (Origin 7.5) of at least 15 data points for each peptide (at least 5 concentrations/peptide with at least 3 replicates for each concentration) from 2 independent experiments to a logistic nonlinear regression model. The coefficients of determination (R2) for the CRRETAWAC and GRGDSP regression fits were 0.84 and 0.83, respectively.
Surface preparation and modification
The peptide FSP was dissolved in water (2 mg/ml) and adsorbed on fluorosilane self-assembled monolayers (FSAM, prepared as described [5]) and ePTFE (wall thickness 0.005″, internodal distance 20–30 μm, ZEUS) for 24 h. The samples were removed from the solution and dried. Surfaces were sterilized at RT by a 5 h exposure to ethylene oxide followed by a 24 h outgassing period.
Peptide FSP stability on solid PTFE was tested under water for up to 20 weeks. Upon removal, samples were rinsed 3 times with pure water and dried at RT for 24 h. The degree of peptide FSP modification and stability were determined by water contact angle measurements and XPS analysis.
Cell culture
Human pulmonary artery endothelial cells (HPAECs, Cambrex) obtained at passage 3 were grown to 80–90% confluence from a 1:3 split ratio in 25 cm2 polystyrene tissue culture flasks (Costar) coated with human fibronectin (FN, Sigma, 1 μg/cm2) in complete growth medium (CGM) and trypsinized using trypsin/EDTA (BioWhittaker). Culture medium was made by supplementing basal media (MCDB 131, Sigma) with 0.015% (w/v) EC growth factor supplement (ECGS, Core Facilities Laboratory, Cell Biology Department, Cleveland Clinic Foundation), 0.009% heparin (w/v, heparin activity, 16.3 U/ml; isolated from porcine mucosa, Sigma) and 10% (v/v) FBS. ECs for experiments were used at passages 6–8.
Peptide and integrin specificity
To determine the CRRETAWAC-dependence of EC interaction with the peptide FSP, ECs were incubated with either soluble CRRETAWAC or CATAERWRC (1 mM), or no peptide in Hank's Buffered Salt Solution with 0.1% BSA, 25 mM HEPES, 1 mM MgCl2, and 1 mM CaCl2 at 37°C for 30 min. Peptide-incubated ECs were then seeded at an estimated density of 50,000 cells/cm2 onto CRRETAWAC FSP coated FSAM surfaces (n=6, 2 surfaces/pre-incubation condition). After seeding for 30 min under controlled conditions (37°C, 5% CO2), the EC seeding solution was aspirated and CellTiter assay (Promega) was employed to determine relative cell viability. EC attachment as reported is normalized by attachment to CRRETAWAC FSP by ECs not incubated with peptide.
To establish α5β1 integrin specificity of CRRETAWAC peptide, integrin function blocking antibodies were employed to disrupt EC attachment to CRRETAWAC FSP. For this, ECs were incubated with no integrin antibody, α5β1 integrin blocking antibodies (MAB1969 at 30 μl/ml and AB1950 at 10 μl/ml, both antibodies from Chemicon), or αvβ3 integrin blocking antibody (MAB1976Z at 30 μg/ml, Chemicon) in Hank's Buffered Salt Solution with 0.1% BSA, 25 mM HEPES, 1 mM MgCl2, and 1 mM CaCl2 at 37°C for 30 min. Cells were then seeded on FN-coated glass (n=10, at least 3 surfaces/pre-incubation condition) or CRRETAWAC FSP coated FSAM (n=10, at least 3 surfaces/pre-incubation condition). EC attachment was assessed by phase contrast imaging and manual cell counting for at least 26 fields/surface.
Adhesion and growth studies
Confluent ECs were trypsinized and resuspended in serum-free Opti-Mem I (Invitrogen) supplemented with 1 mM CaCl2 for seeding at a subconfluent density (via hemocytometer count) of 15,000 cells/cm2. Experimental surfaces included CRRETAWAC FSP modified FSAM (n=4/time point) and ePTFE (n=3 at 3, 48, and 96 h time points). FN coated glass surfaces (1 μg/cm2, n=at least 8/time point) were used as the positive control and unmodified ePTFE (n=3 for 3, 48, and 96 h time points, no surfaces at 19 and 72 h) surfaces served as negative controls; these surfaces were rendered aseptic by immersion in ethanol. ECs were permitted to attach for 2 h under controlled conditions (37°C, 5% CO2). After 2 h, the Opti-Mem I was aspirated and replaced with CGM. Culture media was changed every 2 d. The cell population was assessed 3 h, 19 h, 48 h, 72 h and 96 h after seeding by DAPI (4',6-diamidino-2-phenylindole, dihydrochloride, Invitrogen) staining of cell nuclei and by phase contrast imaging and manual cell counting. For the DAPI stain and count, ECs were fixed with 4% (w/v) paraformaldehyde (PFA, Sigma) in phosphate-buffered saline (PBS, Gibco), permeabilized with 0.1% (v/v) Triton-X 100 in TBS, and stained with DAPI (1 μM), a double-stranded DNA binding dye. For enumeration of fluorescently labeled EC nuclei, 33 epifluorescent images along 8 axes at 4 radial distances for each surface were acquired using a Nikon Diaphot 200 microscope with a 10x objective. Cells were counted automatically (ImageJ, NIH) in a 0.4 mm2 region of interest and the average cell population was determined. Phase contrast imaging and manual cell counting on surfaces proceeded in a similar fashion, but with 12 fields/surface analyzed for cell population estimation (4 axes at 3 radial distances).
Attachment efficiency was calculated as the ratio of attached cell density to seeded cell density. Attached cell density was calculated 3 h after initial seeding; seeding density was determined by a hemocytometer count of the seeding solution. Doubling time, a reflection of cell growth rate during the logarithmic growth phase, was estimated for CRRETAWAC FSP and FN from nonlinear regression (Origin 7.5) of EC population data to the following sigmoidal Boltzmann equation: , where A1 is the initial cell population, A2 is the maximum cell population, t0 is the time at which cell population is halfway between the initial and maximum cell population, and k is a growth coefficient with units of h−1 [21]. Residual analysis for the nonlinear regression revealed good model fit. The model coefficients of determination (R2) for CRRETAWAC FSP and FN were 0.946 and 0.623, respectively. The growth coefficient (k) was used to obtain the doubling time parameter during the exponential phase of growth using this equation: [21]. Significant differences in the growth coefficients were investigated using a two sample t-test with the error and degrees of freedom for each growth coefficient estimated by the model fit.
Shear stability testing
A rotating disk system was used as previously described [22] to investigate shear stability of adherent ECs on CRRETAWAC FSP. Shear stability on CRRETAWAC FSP modified FSAM (n=4) was compared with EC shear stability on RGD FSP modified FSAM (n=4, peptide ligand sequence- GSSSGRGDSPA) and on FN coated glass (n=4, 1 μg/cm2). For these experiments, ECs were seeded at a confluent density (65,000 cells/cm2) and cultured on surfaces for 18-22 h to allow for EC attachment and spreading. EC-seeded surfaces were then subject to shear stress (τ) in CGM according to the following equation: τ = 0.8*η*r*ω1.5*ν−0.5 where η = dynamic viscosity, r = radial distance, ω = angular velocity, and ν = kinematic viscosity. The EC population on the different surfaces was quantified using a DAPI nuclear stain and count (described above) after 4 h of applied shear stress ranging from 0-57.4 dynes/cm2. Cell retention was calculated by comparing the EC population at radial distances after shear to the analogous locations on the same surface before shear, as determined by phase contrast imaging and manual counting.
Cell alignment was determined by tracing cell cytoskeleton outlines revealed by Alexafluor 488 phalloidin staining of actin stress fibers (1:20 dilution in TBS for 20 min, Invitrogen). A software package (ImageJ v1.34, NIH) was used to determine the angle of the long axis of traced cells for at least 80 cells/surface/shear condition for 4 replicates of each surface type. This angle was compared with the direction of flow and cells found to be within ± 20° were considered aligned.
Hemostatic function studies
Adherent cell hemostatic function was investigated with two enzyme immunoassay kits that measure cellular production of 6-keto prostaglandin F1α (6-keto PGF1α, Cayman Chemical) or tissue plasminogen activator (tPA, American Diagnostica Inc.). To collect cell culture media samples for assay, HPAECs (p. 7, Cambrex) were seeded on CRRETAWAC FSP coated ePTFE (n=6/assay), FN (1 μg/cm2) coated glass (n= at least 6/assay), and RGD FSP coated ePTFE (n=6/assay) at a confluent density (40,000 cells/cm2) in serum-free Opti-mem I supplemented with 1 mM CaCl2. After 2 h, the seeding solution was aspirated and replaced with CGM for 22 h. After 6-22 h, conditioned CGM was assayed according to the manufacture's protocol for both enzyme immunoassay kits. PGF1α or tPA concentrations were converted to production rates by normalizing by time and cell population. Reported production rates were normalized by the production rate for ECs adherent to FN for a particular experimental run.
To confirm EC phenotype, adherent cells were incubated with 10 μg/ml acetylated low density lipoprotein, labeled with 1,1'-dioctadecyl–3,3,3',3'-tetramethyl-indocarbocyanine perchlorate (DiI AcLDL; Biomedical Technologies) in CGM for 4 h at 37°C. Cells were then fixed and counterstained with DAPI as described above. DiI AcLDL uptake was visualized with a Nikon Diaphot 200 epifluorescent inverted microscope using a 40x objective and the appropriate excitation/emission filters.
Platelet adhesion to surfaces
Venous blood was drawn from healthy, aspirin-refraining adult donors in compliance with IRB approved protocols. Blood was drawn into 3.2% sodium citrate anticoagulant (Sigma) at a 9:1 ratio. Blood was centrifuged at 200g for 15 min. The top layer was then removed and centrifuged at 2000g for 10 min to pellet platelets. The supernatant was then discarded and the platelet pellet resuspended in a solution of 1% BSA in PBS to a final concentration of approximately 50,000 platelets/μl (measured with an AcTDiff Coulter Counter).
All surfaces (n=3 for CRRETAWAC and RGD FSP coated FSAM, n=6 for FN coated glass) were passivated with 2% BSA in PBS for 30 min prior to platelet suspension exposure. Immediately before surface incubation, CaCl2 and MgCl2 were added to the platelet suspension to a final concentration of 2 mM and 1 mM, respectively. The platelet suspension was incubated on experimental surfaces for 30 min at 37°C. Surfaces were then rinsed four times with PBS, fixed in a solution of 4% PFA in PBS for 20 min, and stained with FITC-labeled anti-CD41a monoclonal antibody (anti-GPIIb, BD Biosciences) at 1:100 dilution in PBS with 1% FBS. Adherent platelets were visualized with a Nikon Diaphot 200 epifluorescent inverted microscope using a 40x objective with the appropriate excitation/emission filters. The % surface area coverage was determined by applying a threshold for light objects to 14-33 fields/surface using software (ImageJ v1.34, NIH).
Statistical analysis
Residual analysis for nonlinear logistic regression of normalized FG binding and % inhibition of platelet aggregation revealed good model fit. A two-sample t-test was used to compare IC50 model parameters using the degrees of freedom from the model as the sample size. ANOVA with Tukey's comparison of means (α=0.05, Minitab 14) was used to assess differences in groups for the following data sets: platelet surface coverage, integrin and peptide specificity of attachment, attachment and growth, shear stability, alignment with shear, tPA production, and 6-keto PGF1α production. Residual analysis ensured data satisfied ANOVA assumptions.
Results and Discussion
Solid-phase αIIbβ3 integrin binding competition and platelet aggregometry
As a first step in examining EC over platelet selectivity, the extent of CRRETAWAC interaction with platelets and platelet integrins was explored. CRRETAWAC affinity for the predominant platelet integrin, αIIbβ3, was investigated using a solid-phase integrin binding assay. As expected, increasing concentration of GRGDSP resulted in decreased bound FG due to competitive binding to the adsorbed αIIbβ3 integrin (Fig. 2a). A decrease in bound FG was also observed with high concentrations of CRRETAWAC, although much higher peptide concentrations were required to out compete FG for binding the adsorbed αIIbβ3 integrin (Fig. 2a). IC50 values for inhibition of FG binding were determined from non-linear regression analysis. The IC50 for CRRETAWAC was 3,054 ± 90 μM; the IC50 for GRGDSP was significantly lower (p<0.001) at 52 ± 6 μM. These results confirm that CRRETAWAC has much lower affinity for the platelet αIIbβ3 integrin than RGD-containing peptides.
Figure 2.
Platelet interaction with CRRETAWAC. A) Inhibition of fibrinogen binding to immobilized αIIbβ3 platelet integrin by GRGDSP ( ) or CRRETAWAC (
) or CRRETAWAC ( ) with nonlinear logistic regression fits. Higher IC50 for CRRETAWAC indicates lower affinity for αIIbβ3 integrin. B) Inhibition of platelet aggregation by GRGDSP (
) with nonlinear logistic regression fits. Higher IC50 for CRRETAWAC indicates lower affinity for αIIbβ3 integrin. B) Inhibition of platelet aggregation by GRGDSP ( ) or CRRETAWAC (
) or CRRETAWAC ( ) with nonlinear logistic regression fits. Higher IC50 for CRRETAWAC indicates lower affinity for platelet receptors involved in aggregation.
) with nonlinear logistic regression fits. Higher IC50 for CRRETAWAC indicates lower affinity for platelet receptors involved in aggregation.
Inhibition of platelet aggregation by soluble peptide was also used to examine the degree of platelet-CRRETAWAC interaction. When soluble peptide out competes FG for binding to platelet receptors (principally, the αIIbβ3 integrin [23]), platelet aggregation is inhibited. Both GRGDSP and CRRETAWAC were capable of inhibiting platelet aggregation; however, CRRETAWAC required a much higher concentration to produce the same inhibitory effect as GRGDSP (Fig. 2b). Platelet aggregation was inhibited by CRRETAWAC peptide with an IC50 = 2,700 ± 390 μM; this was significantly higher (p<0.001) than IC50 = 280 ± 40 μM for GRGDSP peptide. Both the peptide-FG competition for adsorbed integrin and platelet aggregation inhibition experiments indicate low specific CRRETAWAC-platelet interaction.
Peptide fluorosurfactant polymer synthesis and characterization
To modify the surface of fluorocarbon substrates, like ePTFE, with the EC-selective peptide, we used a peptide fluorocarbon surfactant polymer (FSP) as previously described [5] (Fig. 1). The main concern in the first stages of synthesis was the possibility of disulfide bond reductive cleavage in cyclic CRRETAWAC peptide upon attachment of glutaraldehyde. Ellman's test for sulfhydryl groups on the modified peptide was negative, confirming the retention of disulfide bonds.
The NMR spectrum of aldehyde-modified CRRETAWAC-SSSG peptide in d6-DMSO showed the characteristic proton peak from the aldehyde group (9.7 ppm); the disappearance of this peak for the PVAm-peptide conjugate confirmed successful peptide incorporation. Quantitative analysis of this intermediate product was performed by UV spectroscopy. The tryptophan residue in CRRETAWAC peptide absorbs strongly at 280 nm. The amount of 280 nm absorbance was found to be directly proportional to CRRETAWAC-SSSG peptide concentration (R2=0.9998) with a molar absorption coefficient of 4062.9 cm−1M−1. The composition of the PVAm-peptide conjugate was determined using the molar absorption coefficient: for every 15 amino groups on the PVAm backbone, there were 3 attached peptides.
IR spectroscopy confirmed successful incorporation of fluorocarbon branches on the PVAm backbone for the next step of the reaction: the final product contained 1224 cm−1 and 1154 cm−1 (-CF2-) bands which were not observed for the corresponding precursor. XPS atomic composition analysis of the peptide fluorocarbon surfactant polymer on polyethylene indicated the peptide:fluorocarbon ratio to be 2:1. This ratio, combined with the result from UV analysis of the PVAm-peptide precursor, gives the peptide fluorocarbon surfactant polymer composition: on average, for every 10 amino groups on the PVAm backbone, there are 2 peptide ligands and 1 fluorocarbon pendant branch.
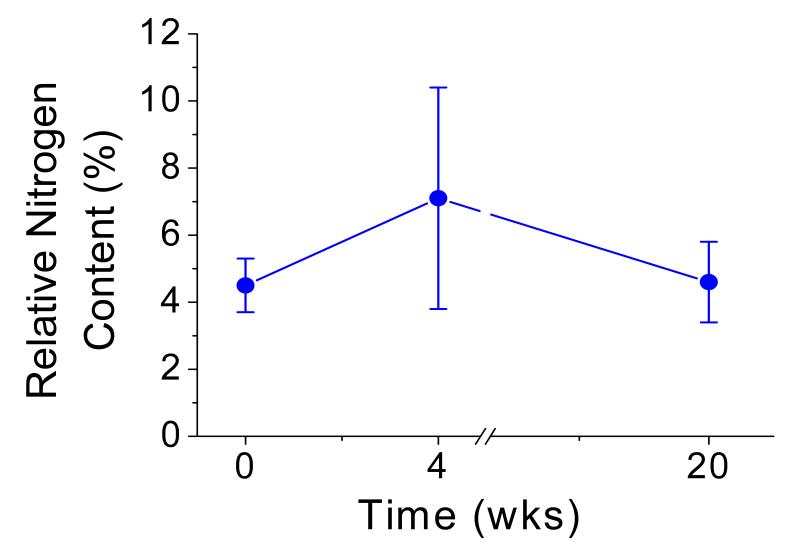
Water contact angle measurement demonstrated significant reduction in surface tension for ePTFE and FSAM on glass after surface modification with CRRETAWAC FSP (Table 1). The peptide fluorocarbon surfactant polymer adsorbs irreversibly on fluorocarbon substrates and remains stable under aqueous conditions for at least 20 weeks, as evidenced by low water contact angles (Table 1) and constant surface nitrogen content determined by XPS (Fig. 3). The CRRETAWAC peptide density on the surface was calculated to be 0.24-0.41 peptides/nm2, which is comparable to previously determined RGD FSP peptide density [5].
Table 1.
Advancing (θa) and receding (θr) water contact angles for unmodified and CRRETAWAC fluorosurfactant polymer (FSP) modified fluorocarbon substrates. Water contact angles for CRRETAWAC FSP modified surfaces subjected to aqueous conditions for 4 and 20 weeks are also given.
| Substrate | Unmodified | CRRETAWAC FSP | CRRETAWAC FSP 4 wks |
CRRETAWAC FSP 20 wks |
||||
|---|---|---|---|---|---|---|---|---|
| θa1 | θr2 | θa1 | θr2 | θa1 | θr2 | θa1 | θr2 | |
| FSAM | 105±2° | 95±2° | 65±7° | 7±2° | 55±7° | 15±4° | ||
| PTFE | 110±1° | 105±1° | 60±6° | 8±2° | 61±1° | 12±4° | 47±4° | 10±6° |
| ePTFE | 124±1° | 120±2° | 68±8° | 12±4° | 63±4° | 17±1° | ||
Highest advancing water contact angle ± s.d. from 3 measurements.
Lowest receding water contact angle ± s.d. from 3 measurements.
Figure 3.
Stability of CRRETAWAC FSP on PTFE in aqueous conditions. Change in relative nitrogen content (%N  ) after exposure to static aqueous conditions for 4 and 20 weeks. Bars represent standard deviation for 3 measurements for each data point.
) after exposure to static aqueous conditions for 4 and 20 weeks. Bars represent standard deviation for 3 measurements for each data point.
Peptide and integrin specificity of EC attachment
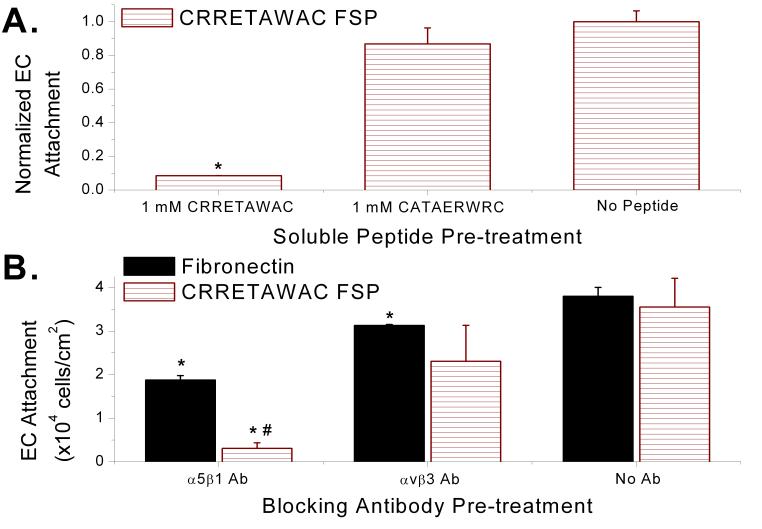
Peptide dependence for EC interaction with the FSP was confirmed when soluble CRRETAWAC, but not CATAERWRC, disrupted EC attachment to CRRETAWAC FSP (Fig. 4a). Pre-incubating ECs with soluble CRRETAWAC (1 mM) reduced cell attachment to CRRETAWAC FSP by over 90% (p=0.002) compared with ECs not incubated with any peptide before seeding on the same surface. Soluble CRRETAWAC most likely exerted its inhibitory effect by binding and occupying cell surface receptors ordinarily involved in binding immobilized CRRETAWAC for cell-surface attachment. Soluble CRRETAWAC inhibition of EC attachment to CRRETAWAC FSP indicates that the peptide presented on the surface by our biomimetic construct is specifically responsible for cell adhesion.
Figure 4.
Endothelial cell (EC) specificity of attachment to CRRETAWAC fluorosurfactant polymer (FSP). A) CRRETAWAC dependence of EC attachment to CRRETAWAC FSP. ECs pre-incubated with soluble CRRETAWAC showed reduced attachment to CRRETAWAC FSP compared with cells pre-incubated with CATAERWRC or no peptide. * Significantly different (p<0.05) cell attachment compared with no peptide pre-treatment. B) α5β1 integrin specificity of EC attachment to CRRETAWAC FSP. Cells pre-incubated with α5β1 integrin blocking antibody (Ab) demonstrated significantly reduced attachment to CRRETAWAC FSP, indicating that the α5β1 integrin is principally responsible for cell attachment. * Significantly different (p<0.05) cell attachment compared with no Ab pre-treatment for same surface type. # Significantly different (p<0.05) cell attachment compared with same Ab pre-treatment for FN surface. Bars represent standard deviation for at least 3 replicates per data point.
We also demonstrated the α5β1 integrin specificity of the CRRETAWAC ligand on the FSP. ECs that had been pre-incubated with α5β1 integrin blocking antibodies before seeding on CRRETAWAC FSP demonstrated over 90% reduction in attachment to CRRETAWAC FSP compared with cells not incubated with blocking antibody (p=0.001, Fig. 4b). The decrease in cell attachment after incubation with α5β1 integrin blocking antibodies was much more pronounced (p<0.001) for CRRETAWAC FSP than FN. This is most likely due to the contribution of other non-α5β1 integrins (α4β1 and αvβ3) in FN adhesion; these other integrins would be expected to play a substantially reduced role in adhesion to CRRETAWAC FSP. There was no statistical difference in EC attachment on CRRETAWAC FSP for cells incubated with αvβ3 integrin blocking antibody compared with cells not incubated with any antibody. This confirms the α5β1 integrin specificity of the CRRETAWAC ligand on the FSP. The 51% reduction (p<0.001 for difference) in attachment to FN for ECs incubated with α5β1 integrin blocking antibodies compared with ECs not incubated with blocking antibody confirms the abundant presence and importance of α5β1 integrin for EC attachment to native extracellular matrix protein. The specificity of the CRRETAWAC ligand for the α5β1 integrin underlies the endothelial cell selectivity due to the prominent role of the α5β1 integrin for EC binding and the relative scarcity of the α5β1 integrin on platelets (present at less than 1,000/platelet [14]).
EC attachment and growth
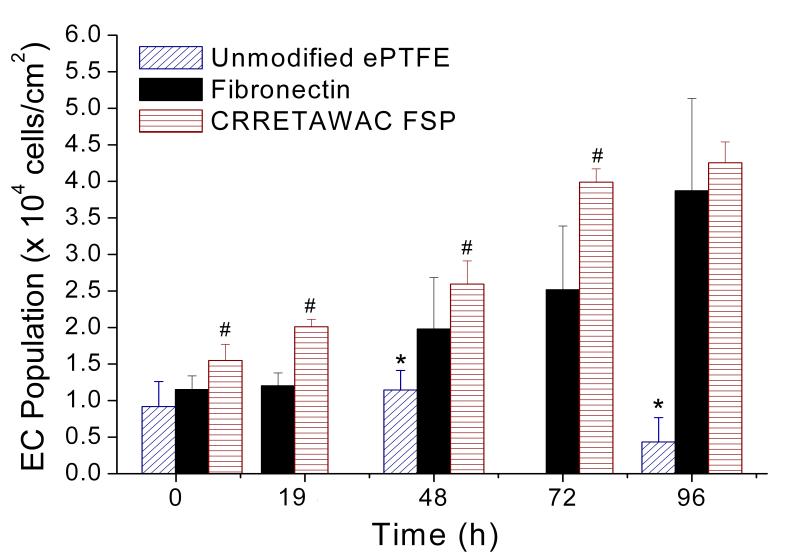
ECs seeded on CRRETAWAC FSP-modified ePTFE and FSAM demonstrated high attachment efficiency and growth (Fig. 5). Attachment efficiency was higher for ECs on CRRETAWAC FSP than for FN (103 vs. 74%, p=0.001 for difference). A significant (p<0.001) increase in EC population was observed on CRRETAWAC FSP and FN, but not on unmodified ePTFE. ECs seeded on CRRETAWAC FSP reached confluence the quickest with a shorter doubling time (7.5 h) compared to FN (10.3 h, p<0.001 for difference). Final confluent cell population was comparable on CRRETAWAC FSP and FN at 96 h. Unmodified ePTFE had significantly lower (p<0.001) EC population than CRRETAWAC FSP for all time points and significantly lower (p<0.001) population than FN from 48 h onward.
Figure 5.
Endothelial cell (EC) attachment and growth on unmodified ePTFE, fibronectin (FN), and CRRETAWAC fluorosurfactant polymer (FSP) modified ePTFE and FSAM. Cell attachment and growth on CRRETAWAC FSP was higher than on FN and substantially greater than on unmodified ePTFE. # Significantly different (p<0.05) cell population compared with FN and ePTFE surfaces at the same time point. * Significantly different (p<0.05) cell population compared with FN and CRRETAWAC FSP surfaces at the same time point. Bars represent standard deviation for at least 3 replicates per data point.
Comparing EC adhesion and growth on CRRETAWAC FSP and RGD FSP [5] relative to FN illustrates that CRRETAWAC FSP supported EC attachment and growth as well as RGD FSP. ECs attached more efficiently to CRRETAWAC FSP relative to FN than on RGD FSP (Table 2). The EC growth rate on both CRRETAWAC and RGD FSPs was significantly greater than for FN (shorter doubling times provide peptide FSP : FN ratio < 1, Table 2). Also, both CRRETAWAC and RGD FSPs supported a confluent population that was equal to or greater than on FN (Table 2). These results demonstrate similar high attachment efficiency, rapid proliferation, and high confluent density on both CRRETAWAC FSP and RGD FSP.
Table 2.
EC attachment efficiency, doubling time, and confluent density comparisons between CRRETAWAC FSP and RGD FSP. The value of each parameter from peptide FSP is normalized by the FN value from the same experiment ± normalized standard deviation. RGD FSP data is from Larsen et al. [5].
| Attachment Efficiency |
Doubling Time |
Confluent Density |
|
|---|---|---|---|
| CRRETAWAC FSP : FN | 1.4 ± 0.2 | 0.7 ± 0.2 | 1.1 ± 0.1 |
| RGD FSP : FN | 0.8 ± 0.3 | 0.5 ± 0.2 | 1.7 ± 0.7 |
Efficient attachment and rapid growth of ECs on CRRETAWAC FSP indicate sufficient peptide ligand density and affinity to support survival and proliferation. Compositional data along with molecular modeling gives an estimated peptide density of 0.04-0.07 nmol/cm2 for CRRETAWAC FSP. This is at least two orders of magnitude greater than the estimated monolayer density of FN (0.0003-0.001 nmol/cm2) [24-26]. High attachment peptide density has been shown to enhance cell spreading, survival, focal contact formation, and proliferation [27-30]. The high cell-binding ligand density on the CRRETAWAC FSP leads to more rapid EC attachment and spreading compared with FN, giving this surface a higher attachment efficiency. This higher initial cell population advantage on CRRETAWAC FSP was maintained through the exponential growth phase due to CRRETAWAC FSP's ability to support a faster growth rate (shorter doubling time). The EC population on both FN and CRRETAWAC FSP reached a similar confluent density and experienced contact inhibition during the plateau phase. The similarity in EC adhesion and growth on CRRETAWAC and RGD FSP may be explained by similar peptide density on both constructs (0.11-0.19 nmol/cm2 for RGD FSP [5]). Despite the known integrin specificity and affinity differences for these peptides [8, 9], the combination of ligand affinity and number were sufficient for both to support high attachment efficiency, rapid proliferation, and high confluent density. These results indicate feasibility of rapid EC expansion to confluence for a subconfluently seeded construct. This has particular application to a one-stage seeding clinical scenario where patient ECs are harvested and seeded at a subconfluent density on a CRRETAWAC FSP modified ePTFE graft at the time of surgery.
EC shear stability
The shear stability of ECs attached to CRRETAWAC and RGD FSP coated FSAM surfaces was assessed using a rotating disk system described elsewhere [22]. ECs were subjected to 4 h of shear stress in CGM ranging from 0-57.4 dynes/cm2 on confluently seeded peptide FSP or FN coated surfaces. ECs on CRRETAWAC FSP modified FSAM displayed shear stability with no significant cell loss after 4 h of 47.8 dynes/cm2 applied shear stress (Fig. 6a). ECs on RGD FSP and FN coated surfaces displayed similar shear stability. Shear stability of ECs on the CRRETAWAC FSP at the shear ranges examined indicates feasibility of application for our biomimetic construct at small-diameter arterial shear stresses.
Figure 6.

Endothelial cell (EC) shear stability and morphology on CRRETAWAC fluorosurfactant polymer (FSP). A) EC shear stability on FN (△), CRRETAWAC FSP ( ), and RGD FSP (
), and RGD FSP ( ). ECs remained stably adherent to CRRETAWAC FSP after 4 h of 47.8 dynes/cm2 applied shear stress. * Significant difference in cell population (p<0.05) compared with 9.6 dynes/cm2 shear stress for same surface. B) Alignment of ECs on FN, CRRETAWAC FSP, and RGD FSP after 4 h of applied shear stress. ECs reoriented and aligned with shear upon application of minimal shear stress for all surfaces. * Significantly different (p<0.05) % of aligned cells compared with 9.6 and 38.2 dynes/cm2 shear stress for the same surface type. Bars represent standard deviation for 4 replicates per data point. C) Actin cytoskeleton staining for ECs on FN displaying alignment with shear after 4 h at 28.7 dynes/cm2. Scale bars for images are 100 μm. Arrows indicates direction of applied shear stress. D) Actin cytoskeleton staining for ECs on CRRETAWAC FSP displaying alignment with shear after 4 h at 28.7 dynes/cm2. E) Actin cytoskeleton staining for ECs on RGD FSP displaying alignment with shear after 4 h at 28.7 dynes/cm2.
). ECs remained stably adherent to CRRETAWAC FSP after 4 h of 47.8 dynes/cm2 applied shear stress. * Significant difference in cell population (p<0.05) compared with 9.6 dynes/cm2 shear stress for same surface. B) Alignment of ECs on FN, CRRETAWAC FSP, and RGD FSP after 4 h of applied shear stress. ECs reoriented and aligned with shear upon application of minimal shear stress for all surfaces. * Significantly different (p<0.05) % of aligned cells compared with 9.6 and 38.2 dynes/cm2 shear stress for the same surface type. Bars represent standard deviation for 4 replicates per data point. C) Actin cytoskeleton staining for ECs on FN displaying alignment with shear after 4 h at 28.7 dynes/cm2. Scale bars for images are 100 μm. Arrows indicates direction of applied shear stress. D) Actin cytoskeleton staining for ECs on CRRETAWAC FSP displaying alignment with shear after 4 h at 28.7 dynes/cm2. E) Actin cytoskeleton staining for ECs on RGD FSP displaying alignment with shear after 4 h at 28.7 dynes/cm2.
ECs on all surfaces were able to achieve significant alignment (p<0.05) with minimal applied shear stress; 36-43% of ECs aligned within ± 20° of flow direction upon 9.6 dynes/cm2 of applied shear (Fig. 6b-e). EC alignment remained relatively constant over the applied shear stress range examined with no statistical differences in alignment between 9.6 and 38.2 dynes/cm2 for all surfaces. Taken together, the shear stability and alignment data indicate that the combination of ligand affinity and number for the CRRETAWAC FSP is sufficient to permit EC cytoskeleton reorganization and results in stable cell anchorage at arterial shear stresses.
EC hemostatic function
In order for ECs to provide maximum biocompatibility benefit for graft material, they must not only be stably adherent, but also provide anti-thrombotic function. Production of prostacyclin, a potent inhibitor of platelet aggregation and important hemostatic mediator [31], was assessed for ECs adherent to CRRETAWAC and RGD FSP and FN by quantifying 6-keto prostaglandin F1α (PGF1α), a stable prostacyclin hydrolysis product, using an enzyme immunoassay (EIA). ECs on CRRETAWAC FSP demonstrated 6-keto PGF1α production rates comparable to ECs on RGD FSP and FN surfaces (Fig. 7a). Actual 6-keto PGF1α production rates for ECs on CRRETAWAC FSP, RGD FSP, and FN ranged from 7.9×10−5 to 2.5×10−3 pg/cell/h, 1.5×10−4 to 8.7×10−4 pg/cell/h, and 5.6×10−5 to 5.5×10−4 pg/cell/h, respectively.
Figure 7.
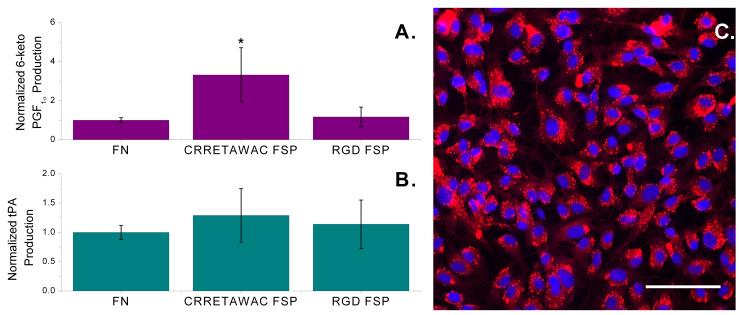
Endothelial cell (EC) hemostatic function on fibronectin (FN) and peptide fluorosurfactant polymer (FSP) surfaces. A) Prostacyclin production by ECs on FN, CRRETAWAC FSP, and RGD FSP as measured by a surrogate breakdown product, 6-keto prostaglandin F1α (PGF1α). Cellular production values (pg/cell/h) were normalized to the production rate from ECs on FN for a particular experimental run. * Significantly different (p<0.05) normalized production compared with ECs on FN and RGD FSP surfaces. Bars represent standard deviation for at least 6 replicates per data point. B) Equivalent tissue plasminogen activator (tPA) production by ECs on FN, CRRETAWAC FSP, and RGD FSP. Cellular production values (ng/cell/h) were normalized to the production rate from ECs on FN for a particular experimental run. Bars represent standard deviation for at least 6 replicates per data point. C) Uptake of fluorescently labeled acetylated low density lipoprotein (red) by ECs (nuclei are blue) adherent to CRRETAWAC FSP modified FSAM. Scale bar is 100 μm.
ECs also play an important hemostatic role in regulating fibrinolysis. Tissue plasminogen activator (tPA), produced and secreted by ECs, activates plasminogen to plasmin, which in turn degrades fibrin resulting in thrombolysis [31]. tPA production rates for ECs on CRRETAWAC and RGD FSP and FN were assessed using an EIA. ECs on CRRETAWAC FSP demonstrated tPA production rates comparable to ECs on RGD FSP and FN (Fig. 7b). Actual tPA production rates for ECs on CRRETAWAC FSP, RGD FSP, and FN ranged from 1.2×10−6 to 3.5×10−5 ng/cell/h, 2.7×10−6 to 2.4×10−5 ng/cell/h, and 2.0×10−6 to 2.6×10−5 ng/cell/h, respectively. Production of prostacyclin and tPA, along with uptake of acetylated low-density lipoprotein (Fig. 7c), an EC-specific function [32], indicates that CRRETAWAC FSP is capable of promoting an anti-thrombotic EC phenotype.
Platelet adhesion
A suspension of washed platelets was incubated with FN, RGD, and CRRETAWAC FSP surfaces to further establish the EC-selectivity of the CRRETAWAC ligand (Fig. 8a). CRRETAWAC FSP demonstrated significantly less (p<0.01) platelet surface coverage (11%, Fig. 8b) than either FN (33%, Fig. 8c) or RGD FSP (46%, Fig. 8d). Differences in platelet surface coverage may be reasonably explained by the differential affinity of presented ligands for platelet integrins. Future studies using a porcine carotid artery interposition model are planned to assess if low in vitro platelet binding translates into low platelet adhesion in vivo for the CRRETAWAC FSP.
Figure 8.
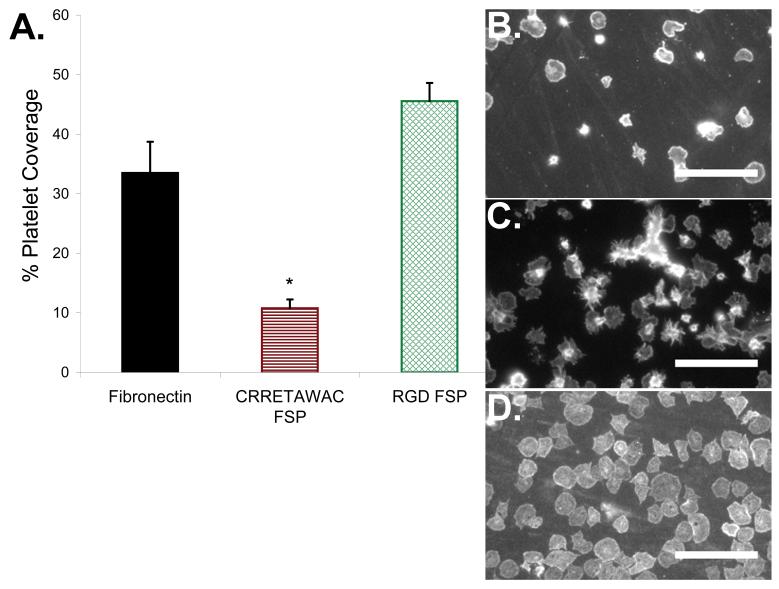
Platelet interaction with CRRETAWAC fluorosurfactant polymer (FSP) surface. A) Platelet surface coverage for FN, CRRETAWAC FSP, and RGD FSP. * Significantly different (p<0.05) platelet surface coverage for CRRETWAC FSP compared with FN and RGD FSP. Bars represent standard deviation for at least 3 replicates per data point. B) FITC-anti CD41a labeled platelets adherent to CRRETAWAC FSP modified FSAM. Scale bars for all images are 30 μm. C) FITC-anti CD41a labeled platelets adherent to fibronectin (FN) coated glass. D) FITC-anti CD41a labeled platelets adherent to RGD FSP modified FSAM.
Conclusion
The challenge of tissue engineering an EC interface on vascular graft material is that many surface modifications that bind ECs also will bind platelets and initiate thrombosis. Here, we provide data demonstrating that cyclic CRRETAWAC peptide has much lower affinity for platelet receptors, including the αIIbβ3 integrin, than an RGD-containing peptide. A FSP incorporating cyclic CRRETAWAC has been synthesized and shown to be a simple, quantitative, and effective surface modification of ePTFE. ECs adhere specifically to the CRRETAWAC FSP in an α5β1 integrin-dependent fashion. CRRETAWAC FSP is capable of supporting high efficiency EC attachment and proliferation. ECs remain stably adherent to CRRETAWAC FSP for at least 4 h at 47.8 dynes/cm2 and are capable of cytoskeletal reorganization and alignment under physiologically relevant shear stress conditions. ECs adherent to CRRETAWAC FSP are capable of providing anti-thrombotic function by producing prostacyclin and fibrinolytic function by producing tissue plasminogen activator. Specific platelet adhesion to CRRETAWAC FSP is limited compared with RGD FSP and fibronectin. EC-selective FSP modification of ePTFE could significantly reduce the time required to ready a cell-seeded vascular graft for implantation. Ultimately, this technology has potential to promote rapid in vivo endothelialization and healing without thrombosis and occlusion for small-diameter vascular grafts.
Acknowledgements
The authors gratefully acknowledge the financial support provided by NIH Grant 5R01EB002067 and the facilities provided by the Center for Cardiovascular Biomaterials. Graduate training support was provided for C.C.L. from NIH Grant 5T32GM007250 and an American Heart Association predoctoral fellowship. We thank Aryavarta M.S. Kumar and Eric H. Anderson for assistance in generating graphical models. We thank Jeffrey A. Beamish for helpful discussions. We thank Charles B. Becker for cell counting.
Footnotes
Competing Interests Statement
The authors declare that they have no competing financial interests.
References
- 1.Faries PL, Logerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000 Dec;32(6):1080–1090. doi: 10.1067/mva.2000.111279. [DOI] [PubMed] [Google Scholar]
- 2.Sayers RD, Raptis S, Berce M, Miller JH. Long-term results of femorotibial bypass with vein or polytetrafluoroethylene. Br J Surg. 1998 Jul;85(7):934–938. doi: 10.1046/j.1365-2168.1998.00765.x. [DOI] [PubMed] [Google Scholar]
- 3.Laube HR, Duwe J, Rutsch W, Konertz W. Clinical experience with autologous endothelial cell-seeded polytetrafluoroethylene coronary artery bypass grafts. J Thorac Cardiovasc Surg. 2000 Jul;120(1):134–141. doi: 10.1067/mtc.2000.106327. [DOI] [PubMed] [Google Scholar]
- 4.Meinhart JG, Deutsch M, Fischlein T, Howanietz N, Froschl A, Zilla P. Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann Thorac Surg. 2001 May;71(5 Suppl):S327–331. doi: 10.1016/s0003-4975(01)02555-3. [DOI] [PubMed] [Google Scholar]
- 5.Larsen CC, Kligman F, Kottke-Marchant K, Marchant RE. The effect of RGD fluorosurfactant polymer modification of ePTFE on endothelial cell adhesion, growth, and function. Biomaterials. 2006 Oct;27(28):4846–4855. doi: 10.1016/j.biomaterials.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechantsreiter MA, Planker E, Matha B, Lohof E, Holzemann G, Jonczyk A, et al. N-Methylated cyclic RGD peptides as highly active and selective alpha(V)beta(3) integrin antagonists. J Med Chem. 1999 Aug 12;42(16):3033–3040. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 7.Haubner R, Schmitt W, Holzemann G, Goodman SL, Jonczyk A, Kessler H. Cyclic RGD peptides containing beta-turn mimetics. Journal of the American Chemical Society. 1996;118(34):7881–7891. [Google Scholar]
- 8.Pfaff M, Tangemann K, Muller B, Gurrath M, Muller G, Kessler H, et al. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem. 1994 Aug 12;269(32):20233–20238. [PubMed] [Google Scholar]
- 9.Koivunen E, Wang B, Ruoslahti E. Isolation of a highly specific ligand for the alpha 5 beta 1 integrin from a phage display library. J Cell Biol. 1994 Feb;124(3):373–380. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 11.Humphries JD, Askari JA, Zhang XP, Takada Y, Humphries MJ, Mould AP. Molecular basis of ligand recognition by integrin alpha5beta 1. II. Specificity of arg-gly-Asp binding is determined by Trp157 of the alpha subunit. J Biol Chem. 2000 Jul 7;275(27):20337–20345. doi: 10.1074/jbc.M000568200. [DOI] [PubMed] [Google Scholar]
- 12.Mould AP, Askari JA, Humphries MJ. Molecular basis of ligand recognition by integrin alpha 5 beta 1. I. Specificity of ligand binding is determined by amino acid sequences in the second and third NH2-terminal repeats of the alpha subunit. J Biol Chem. 2000 Jul 7;275(27):20324–20336. doi: 10.1074/jbc.M000572200. [DOI] [PubMed] [Google Scholar]
- 13.Mould AP, Burrows L, Humphries MJ. Identification of amino acid residues that form part of the ligand-binding pocket of integrin alpha5 beta1. J Biol Chem. 1998 Oct 2;273(40):25664–25672. doi: 10.1074/jbc.273.40.25664. [DOI] [PubMed] [Google Scholar]
- 14.Tomiyama Y, Shiraga M, Shattil SJ. Platelet membrane proteins as adhesion receptors. In: Gresele P, editor. Platelets in thrombotic and non-thrombotic disorders : pathophysiology, pharmacology, and therapeutics. Cambridge University Press; Cambridge; New York: 2002. pp. 80–92. [Google Scholar]
- 15.Qiu YX, Zhang TH, Ruegsegger M, Marchant RE. Novel nonionic oligosaccharide surfactant polymers derived from poly(vinylamine) with pendant dextran and hexanoyl groups. Macromolecules. 1998 Jan;31(1):165–171. [Google Scholar]
- 16.Zhu J, Gosen C, Marchant RE. Synthesis and characterization of Poly (vinyl amine)-based amphiphilic comb-like dextran glycopolymers by a two step method. J Poly Sci A. 2006;44:192–199. [Google Scholar]
- 17.Darlak K, Wiegandt Long D, Czerwinski A, Darlak M, Valenzuela F, Spatola AF, et al. Facile preparation of disulfide-bridged peptides using the polymer-supported oxidant CLEAR-OX. J Pept Res. 2004 Mar;63(3):303–312. doi: 10.1111/j.1399-3011.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 18.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 19.Lestini BJ, Sagnella SM, Xu Z, Shive MS, Richter NJ, Jayaseharan J, et al. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J Control Release. 2002 Jan 17;78(13):235–247. doi: 10.1016/s0168-3659(01)00505-3. [DOI] [PubMed] [Google Scholar]
- 20.Gupta AS, Huang G, Lestini BJ, Sagnella S, Kottke-Marchant K, Marchant RE. RGD-modified liposomes targeted to activated platelets as a potential vascular drug delivery system. Thromb Haemost. 2005 Jan;93(1):106–114. doi: 10.1160/TH04-06-0340. [DOI] [PubMed] [Google Scholar]
- 21.Mundermann L, Erasmus Y, Lane B, Coen E, Prusinkiewicz P. Quantitative modeling of Arabidopsis development. Plant Physiol. 2005 Oct;139(2):960–968. doi: 10.1104/pp.105.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagnella S, Kligman F, Marchant RE, Kottke-Marchant K. Biomimetic surfactant polymers designed for shear-stable endothelialization on biomaterials. J Biomed Mater Res A. 2003 Dec 1;67(3):689–701. doi: 10.1002/jbm.a.10035. [DOI] [PubMed] [Google Scholar]
- 23.Plow EF, Shattil SJ. Integrin aIIbb3 and platelet aggregation. In: Coleman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors. Hemostasis and Thrombosis. 4th ed Lippincott Williams & Wilkins; 2001. pp. 479–491. [Google Scholar]
- 24.Pompe T, Kobe F, Salchert K, Jorgensen B, Oswald J, Werner C. Fibronectin anchorage to polymer substrates controls the initial phase of endothelial cell adhesion. J Biomed Mater Res A. 2003 Nov 1;67(2):647–657. doi: 10.1002/jbm.a.10130. [DOI] [PubMed] [Google Scholar]
- 25.Kowalczynska HM, Nowak-Wyrzykowska M, Dobkowski J, Kolos R, Kaminski J, Makowska-Cynka A, et al. Adsorption characteristics of human plasma fibronectin in relationship to cell adhesion. J Biomed Mater Res. 2002 Aug;61(2):260–269. doi: 10.1002/jbm.10151. [DOI] [PubMed] [Google Scholar]
- 26.Bhat VD, Truskey GA, Reichert WM. Fibronectin and avidin-biotin as a heterogeneous ligand system for enhanced endothelial cell adhesion. J Biomed Mater Res. 1998 Sep 5;41(3):377–385. doi: 10.1002/(sici)1097-4636(19980905)41:3<377::aid-jbm6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Dee KC, Andersen TT, Bizios R. Cell function on substrates containing immobilized bioactive peptides. Mater Res Soc Symp Proc. 1994;331:115–119. [Google Scholar]
- 28.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003 Nov;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 29.Lin YS, Wang SS, Chung TW, Wang YH, Chiou SH, Hsu JJ, et al. Growth of endothelial cells on different concentrations of Gly-Arg-Gly-Asp photochemically grafted in polyethylene glycol modified polyurethane. Artif Organs. 2001 Aug;25(8):617–621. doi: 10.1046/j.1525-1594.2001.025008617.x. [DOI] [PubMed] [Google Scholar]
- 30.Sagnella SM, Kligman F, Anderson EH, King JE, Murugesan G, Marchant RE, et al. Human microvascular endothelial cell growth and migration on biomimetic surfactant polymers. Biomaterials. 2004 Mar-Apr;25(78):1249–1259. doi: 10.1016/s0142-9612(03)00634-3. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe EA. Physiologic functions of normal endothelial cells. Ann N Y Acad Sci. 1985;454:279–291. doi: 10.1111/j.1749-6632.1985.tb11868.x. [DOI] [PubMed] [Google Scholar]
- 32.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]