Summary
The uterine sarcoma human cell line MES-SA/D×5 overexpresses the MDR1 gene product, P-glycoprotein (Pgp). Pgp is a heavily glycosylated, ATP-dependent drug efflux pump expressed in many human cancers. There are more than 50 known isoforms of Pgp, which complicates the characterization of Pgp glycans because each isoform could present a different glycome. The contribution of these oligosaccharides to the structure and function of Pgp remains unclear. We identified distinct Pgp glycans recognized by the lectins in the digoxigenin (DIG) glycan differentiation kit from Roche Allied Science, all of which were N-glycans. Pgp was isolated using both slab and preparative gel elution. The monoclonal antibody C219 was used to identify the presence of Pgp and Pgp treated with PNGase F on our blots. Pgp isolated from MES-SA/D×5 cells contains at least two different complex N-glycans—one high mannose tree, detected by GNA, and one branched hybrid oligosaccharide—capped with terminal sialic acids, detected by SNA and MAA. DSA, specific for biantennary oligosaccharides possessing β(1-4)-N-acetyl-D-glucosamine residues, also recognized the blotted Pgp and is probably detecting the core Galβ(1-4) – GlcNAcx component found in other Pgps.
Keywords: P-glycoprotein, multidrug resistance, MES-SA/D×5 cell line, DIG glycan detection, lectin, gel elution
1. Introduction
P-glycoprotein (Pgp) is a heavily glycosylated membrane protein that is a member of the superfamily of ATP-binding cassette (ABC) transporters [1,2]. Malignant tissue overexpression of Pgp, or an altered expression of Pgp in tumor cells, can result in resistance to a broad range of anticancer drugs during chemotherapy, commonly referred to as multidrug resistance [3]. Pgp is an energy-driven efflux pump and consists of 1,280 amino acids with 12 α-helix transmembrane domains and two cytoplasmic nucleotide-binding domains. This configuration represents the 6+6 helix model [4] or the more recent double β-barrel model described by Jones and George [5].
Glycosylation occurs on the three N-linked recognition sites (Asn 91, 94, and 99) [6] in the first extracellular loop. These sites are the only extracellular glycosylation sites of the 10 putative N-link sites [7]. Glycosylation in this first extracellular loop ranges from one N-glycan to all three sites having unique oligosaccharides [8–11]. Treatment of Pgp with either peptide-N-glycosidase F (PNGase F) or endo-β-N-acetylglucosaminidase (Endo F) reduces the apparent molecular weight from 170,000 to the predicted core weight of 140,000 [7,12].
The oligosaccharides of glycoconjugates have varied effects on the properties of proteins to which they are attached, specifically on biosynthesis, folding, solubility, stability, subcellular trafficking, turnover, and half-life [13]. The oligosaccharide content of Pgp has been shown to be important in the normal plasma membrane expression of the protein. Mammalian Pgp has been successfully expressed without its sugars in Escherichia coli [14], insect cells [15], and yeast [16,17]. The sugarless Pgp reaches the plasma membrane as an active pump; however, it is expressed in much lower numbers and does not model all the properties of Pgp observed in mammalian cells. Thus, the sugar moieties are purported to play an important role in the plasma membrane expression and efficient function of Pgp.
More than 50 single-nucleotide polymorphisms (SNP) have been identified for Pgp [18,19], allowing cells to create “super” pumps with variation in expressed polymorphic and glycosylation schemes. One can see this microheterogeneity of Pgp in the broad banding it creates in electrophoretic gels. It is thought that these polymorphisms contribute to variability in Pgp function [20]. If key Pgp sugars can be identified on polymorphic forms of Pgp, more effective strategies in drug design and targeting can be devised to shut down these “super” pumps during the treatment of multidrug resistance in tumors. For example, bisecting N-acetylglucosamines are present on many complex glycoproteins. Rebbaa et al. [21], found that the bisecting GlcNAc sugars in human Pgp, expressed in pediatric brain tumors, is correlated with tumor progression and may have a potential relevance as a tumor marker. Thus, glioma response to chemotherapy may be modulated using the bisecting GlcNAc as targeted lectin therapy.
Galactose and GlcNAc were found in the N-glycans of Pgps in multidrug-resistant Chinese hamster cells in complex hybrid trees [22]. In another study using Karnal bunt (KB) cell lines, there was no evidence of fucose or mannose, and little or no detected sialic acids attached to the Pgp [7]. There is no evidence of O-linked glycosylation of Pgp [23–26].
Here, we show that the overexpressed human Pgp isolated from MES-SA/D×5 cells [27] contains at least two different complex N-glycans—one high mannose tree and one branched hybrid oligosaccharide—capped with terminal sialic acids. Moreover, the second sialylated oligosaccharide is a complex glycan with terminal N-acetylneuraminic acids having α(2-3) and/or α(2-6) linkage. There is a possibility that there are two distinct sialylated N-glycans, one having Neu5Ac α(2-6)-Galβ(1-4) termini, identified with SNA; the other capped with Neu5Ac α(2-3)-Galβ(1-4), recognized by MAA. These proposed N-glycans must be attached to the first extracellular loop.
2. Materials and Methods
2.1 Materials
The human uterine sarcoma cell line, multidrug-resistant variant line MES-SA/D×5, was purchased from the American Type Culture Collection (ATCC; Rockville, MD). The other materials and their suppliers were as follows:
McCoy’s 5A modified medium—Sigma-Aldrich, St. Louis, MO
Fetal bovine serum—HyClone Laboratories, Inc., Logan, UT
Monoclonal antibody C219 [28]—Signet Laboratories, Inc., Dedham, MA
Gel electrophoresis and immunoblotting chemicals, membranes, and equipment—Bio Rad Laboratories, Inc., Hercules, CA
Digoxigenin (DIG) glycan detection kit (Cat. no. 1 142 372), DIG glycan differentiation kit (Cat. no. 11 210 238 001), and peptide-N-glycosidase (PNGase F)— Roche Applied Science, Indianapolis, IN
The Amicon Ultrafiltration device and 50K cutoff membranes—Amicon, Inc., Beverly, MA
QIAamp spin columns—Qiagen, Inc., Chatsworth, CA
Tissue culture flasks, Dounce tissue grinder, Bright-Line Hemacytometer (0.1 mm), and all other chemicals—Fisher Scientific, Pittsburgh, PA
2.2 MES-SA/D×5 cell line and media
The variant subline of MES-SA cells (MES-SA/D×5) overexpress human Pgp and have developed a 100-fold resistance to doxorubicin, an anticancer agent [29]. MES-SA/D×5 cells were grown in monolayers in 150 cm2 plastic tissue culture flasks at 37 °C under 5% C02 in McCoy’s 5A modified medium, supplemented with 10% fetal bovine serum and 1.0% penicillin (50 IU/ml) – streptomycin (50μg/ml). Cells were grown to confluence at 37 °C with changes of media and three additions of doxorubicin (2.0 μg/ml) at P19, P21, and P22, sufficient for MDR1 gene expression of overexpressed P-glycoprotein. Cells were harvested with 0.8 mM EDTA collection solution, which contained 137 mM NaCl, 6.7 mM NaHCO3, 5.6 mM dextrose, and 5.4 mM KCl, pH 7.3.
2.3 Solubilization of MES-SA/D×5 membrane proteins
Cells (2.04 × 108 on average) were pelleted at low-speed centrifugation (7,000 × g; Sorvall Superspeed RC2-B centrifuge; Newton, CT) for 10 min. Cells were then resuspended in lysing buffer containing 5.0 mM EDTA, 5.0 mM HEPES, pH 7.5, with the addition of protease inhibitors: 1.0 mM PMFS, 1.0 mM 1,10 phenanthroline, 1.0 μg/ml aprotinin, 1.0 μg/ml leupeptin, and 0.02% NaN3. The lysed cell suspension was centrifuged at 42,000 × g at 4 °C for 20 min and resuspended in solubilization buffer containing 5.0% LDS, 2.0% β-mercaptoethanol, 60 mM Tris-HCl, pH 6.8, and the aforementioned protease inhibitors. Cells were homogenized with a Dounce tissue grinder and again centrifuged. The supernatant, containing the solubilized membranes, was heated at 80 °C for 3 min and allowed to cool to room temperature (RT). The solubilized membranes were filtered through Qiagen spin columns (Cat. No. 29306) to remove nucleic acids and debris. Glycerol (10% final concentration) was added to the pooled filtrates to increase density. Protein concentrations were determined using the direct procedure of Peterson’s modification of the micro-Lowry method (Protein Assay Kit, Sigma Diagnosis, St. Louis, MO). Samples were stored at 80 °C until use.
2.4 Bio Rad Whole Gel elution
For separation of Pgp using Bio Rad’s Whole Gel Eluter (slab gel separation), a 4.0-ml aliquot of solubilized membrane protein (4.30 × 108 cells) was electrophoresed in the first dimension using a Protean II (Bio Rad) gel system with a 7.0% SDS-PAGE (3.0 mm thick) run for 19.5 h at RT (40 mA constant current) [30]. The gel was placed on the Whole Gel Eluter and transferred in the second dimension at 250 mA constant current for 1.5 h at RT [31]. The eluant was collected into 30 (12 × 75 mm) glass tubes. Aliquots were analyzed using Bio Rad 7.0% SDS-PAGE mini gels.
2.5 Bio Rad 491 Prep Cell elution
Preparative column electrophoresis of the solubilized membrane proteins was performed in a Bio Rad Model 491 Prep Cell [32]. A 2.0-ml aliquot of solubilized membrane protein was electrophoresed on a 6.5% LDS-PAGE column gel (37 mm diameter × 4.5 cm), with a 4.0% LDS-PAGE stacking gel (1.0 cm). Electrophoresis was conducted for 21 h at 4 °C using 40 mA constant current. The proteins were eluted with 0.1% LDS running buffer (25 mM Tris-base, 192 mM glycine, 3.5 mM LDS, pH 8.3) at 1.12 ml/min. Fraction size was 14.0 ml, with a total of 75 fractions collected. Fractions 23–67 were pooled in groups of three (42 ml) and concentrated by Amicon ultrafiltration (50K M r cutoff) to 1.5 ml. Aliquots (70μl) of the pooled fractions were mixed with 5× sample buffer, heated for 2 min at 80 °C, then electrophoresed on a 7.0% SDS-PAGE mini gel (1.5 mm at RT, 40 mA constant). Gels were silver-stained using the procedure outlined by Bollag et al. [33].
LDS-PAGE Western blot transfer to nitrocellulose was conducted for 1 h 10 min at 4 °C using 200 mA constant current following the method of Towbin et al. [34]. Transfer buffer was 25 mM Tris-base, 192 mM glycine, 20% methanol, pH 8.3. Immunoblots were blocked with 5.0% dry milk or blocking solution provided in the DIG glycan detection kits. The dry milk blocking solution was 150 mM NaCl and 25 mM Tris-HCl, pH 7.5, while subsequent antibody incubations were carried out in this solution containing 1.0% dry milk. The blots were challenged (RT) with the monoclonal antibody C219 (1:200), and immunostained with GAM-AP (goat anti-mouse alkaline phosphatase; 1:2000) at RT for 2 h. From the results of the immunostaining, fractions containing Pgp were selected for DIG glycan studies [35].
2.6 PNGase F treatment of P-glycoprotein for DIG lectin analysis
Each selected aliquot received additions of 7.5% NP-40 (nondinet-40) and EDTA to final concentrations of 0.5% and 10 mM, respectively. Samples were heated at 70 °C for 3.0 min, cooled to RT, then equally divided between two microcentrifuge tubes. PNGase F was added to one tube (1.0 U/5.0 μl resuspended enzyme) and both tubes were incubated in a 37 °C water bath for 21.5 h. Sample buffer (5×) was added to each tube, and to aliquots of positive and negative DIG lectin controls listed in Table 1. The samples and controls, grouped according to experimental parameters, were electrophoresed on 7.0% LDS-PAGE gels, and then transferred to nitrocellulose. The nitrocellulose strips were either challenged directly using DIG-labeled specific lectins (DIG Glycan Differentiation Kits, Roche Allied Science) or sugars with reactive hydroxyl groups that were chemically modified with the attachment of DIG according to the protocols included in the glycan detection kit. Both DIG-lectin and DIG-labeled sugar groups on our blots were detected with polyclonal sheep anti-DIG Fab fragments conjugated to AP, using color detection. All DIG glycan detection and DIG glycan differentiation reactions were carried out in accordance with kit instructions.
Table 1.
Characteristics of the lectins used in this study.*
| Lectin | Control Protein(s) | Hapten and sugar specificity |
|---|---|---|
| GNA:
Galanthus nivalis agglutinin [44] |
Carboxypeptidase Y | Mannose:
α(1-2), α(1-3), or α(1-6) linked to mannose |
| SNA:
Sambucus nigra agglutinin [45] |
Transferrin and fetuin | Sialic acid:
α(2-6) to GalNAc |
| MAA:
Maackia amurensis agglutinin [46] |
Fetuin | Sialic acid:
α(2-3) to galactose N-linked α(2-3) to galactose O-linked |
| PNA:
Peanut (Arachis hypogaea) agglutinin [43] |
Asialofetuin | Core and terminal galactose:
Gal-β(1-3)-N-acetylgalactosamine |
| DSA:
Datura stramonium agglutinin [47] |
Asialofetuin | Core galactose:
Gal-β(1-4)-N-acetylglucosamine |
The specificity includes the control proteins used to validate the lectin binding on our blots and does not include the monosaccharide or other chemical variations of the lectin haptens (e.g., PNA also has side reactivities to LacNAc) [69].
3. Results
3.1. Isolation of human MES-SA/D×5 Pgp using Bio Rad Whole Gel Eluter
P-glycoprotein has been isolated from many sources using a variety of isolation techniques, including immunoprecipitation [36], detergent solubilization [37], sucrose density gradient [38], and lectin chromatography [39]. Regardless of how Pgp is isolated, ultimately it is visualized on an electrophoretic gel system. The heavily glycosylated Pgp runs as a diffuse band (microheterogeneity) in electrophoretic gels, making it difficult to purify or identify as a single form of P-glycoprotein in gels and on blots [40]. Purification is generally confirmed as seeing no additional banding except the identified Pgp band in a Coommassie-stained gel [41]. Here, we used silver-stained gels for observing the purity of Pgp after gel isolation.
In order to achieve consistent Pgp gel banding for our gel runs and subsequent DIG lectin labeling on our blots, we compared two gel elution techniques to isolate the human uterine sarcoma Pgp. We include both isolation approaches here to show the variability experienced in detecting ‘purified’ Pgp on blots from these two different gel elution systems.
First, human Pgp was isolated from the membranes of solubilized MES-SA/Dx5 cells using slab gel elution. Solubilized MES-SA/D×5 membranes were loaded in the first dimension using a 7.0% SDS-PAGE Protean II slab gel. This gel was then transferred to the Bio Rad Whole Gel Eluter for electro elution. The eluted fractions were run on a 7.0% LDS-PAGE mini gel and silver-stained. Variability was seen in the silver-stained gel banding patterns of the collected fractions, usually producing a doublet in each lane as shown in Figure 1a, lanes 2–9. To identify Pgp on the blot transfer, we used the monoclonal antibody C219, which recognizes an internal cytoplasmic sequence, six residues away from the ATP-binding domain of Pgp [28, 42]. Immunostained blots of these gel-eluted fractions, containing the purified Pgp, proved unsatisfactory for the lectin challenges due to the variable banding patterns seen in the blotted proteins (Figure 1b; lane 4).
Figure 1.
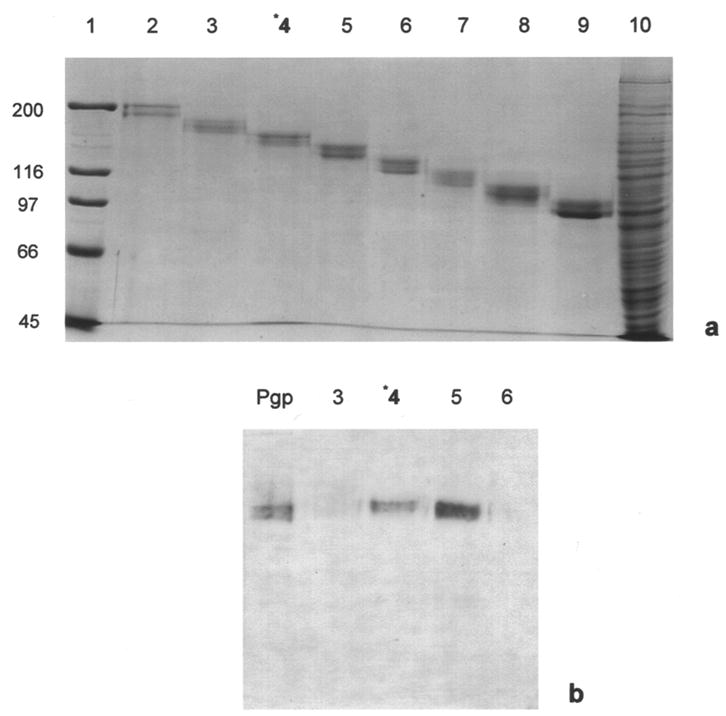
3.2. Isolation of human Pgp using Bio-Rad Prep Cell
We next isolated human Pgp using Bio Rad’s 491 Prep Cell with a 6.5% LDS-polyacrylamide preparative gel column. Although not as rapid a procedure as the whole gel elution process, this preparative gel system gave a more consistent banding pattern with satisfactory yields (105 μg) of isolated Pgp. For the DIG lectin studies, we choose the fractions from the prep cell that gave consistent banding patterns of Pgp (Figures 2a and 2b, lane 5).
Figure 2.

3.3. DIG-labeled lectin analysis of Pgp N-glycans
Blots containing the gel-purified Pgp were challenged in Figure 3 with the DIG-labeled lectins GNA, SNA, MAA, PNA, and DSA (Table 1). The heavily glycosylated Pgp was recognized by all the lectins except PNA. PNA is specific for the core disaccharide galactose β(1-3) N-acetylgalactosamine, found in the core units of O-glycans, and recognizes β-glycosidically linked D-galactosyl residues [43]. PNA staining on our blots (Figure 3, panel V) gave a high background, presumably due to the nonspecific binding of PNA to the blocking agent included in the DIG kit. All of the lectins recognized their appropriate control proteins (Figure 3, lanes 5, 8, 11, 14, and 17), ruling out false positives and supporting their specific binding activity on the blots.
Figure 3.
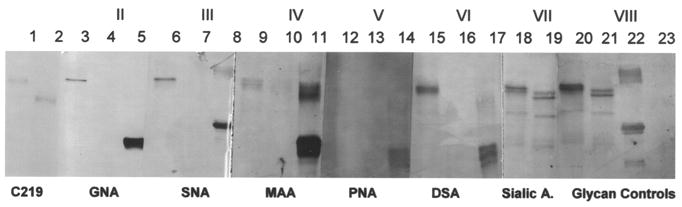
Only Pgp was detected by the lectins, no additional glycoproteins were detected in the lanes containing the isolated Pgp. However, periodate labeling of the hydroxyl groups of all reactive sugars showed additional lower molecular weight proteins on the blots (Figure 3, panels VII and VIII). None of these lower molecular weight proteins were Pgp, because Rf values did not correspond to the untreated Pgp band or the PNGase F deglycosylated band of Mr 140,000, both recognized with C219. Specifically, the glycan control blot (panel VIII, lanes 20–23) was chemically treated at RT with sodium periodate, oxidizing available hydroxyl groups of the sugars and converting them to aldehydes. The aldehyde groups were then labeled with the steroid hapten digoxigenin (DIG). These were subsequently immunostained with anti-DIG Fab fragments conjugated to AP. Terminal sialic acids were detected by the lectins, SNA and MAA, and by the periodate treatment of glycoconjugates to detect only reactive sialic acids (Figure 3, panels III, IV, and VII). Panel VII (total sialic acid) was treated using a modified DIG-periodate chemical labeling procedure to specifically label sialic acids with DIG, incubated at 0 °C in 1.0 mM sodium periodate.
PNGase F was used to specifically remove all N-linked oligosaccharides [48]. The control blot (Panel I) was immunostained with C219 and detected the untreated and PNGase F-treated Pgp. After PNGase F treatment, the Mr of Pgp was 140,000, in agreement with other studies of Pgp treated with PNGase F or Endo-F [7, 12, 49]. None of the lectins used in this study recognized Pgp without its accompanying N-glycans. GNA and SNA (Panels II and III) have a sharp banding profile on the blots. A doublet was seen in the Pgp lane treated with MAA. This doublet pattern was seen again in panels VII and VIII, where chemical labeling detected all sugars, including sialic acids. DSA gave a broader banding pattern in panel VI.
Only N-glycans were detected on the overexpressed human Pgp found in MES-SA/D×5 cells using the DIG-labeled panel of lectins. One glycan was high in mannose (GNA) with no terminal sialic acids. The other detected oligosaccharides contained terminal sialic acids, detected by SNA and MAA. DSA detects the core Galβ(1-4)–GlcNAcx component found in many complex N-glycans [21]. All the lectins gave strong banding reactions to their corresponding control proteins, including PNA.
4. Discussion
Glycosylation is important for the proper transport and, in many cases, the proper function of major membrane glycoproteins. The heavily glycosylated ion channels are a good example: N-linked glycosylaton of potassium channels is required for their transport to the cell surface [50]. Glycosylation is required of calcium channels for current stimulation [51] and proper glycosylation is necessary for the maintenance of functional sodium channels in neuroblastoma cells [52]. It is not a surprise that glycosylation is required also for the proper targeting and function of Pgp.
Bentley et al. [53] showed that inhibition of N-linked glycosylation of Pgp, using tunicamycin, produced hypersensitive KB-C1 cells, but did not alter Pgp activity. Using deletion mutants of human MDR1 Pgp, Schinkel et al. [40] showed that N-glycosylation contributed to the proper routing and stability of Pgp in BRO human melanoma cells, but not to drug transport. Gribar et al. [6] used N-linked glycosylation-deficient mutants of human P-glycoprotein to study the effects of glycosylation on Pgp’s plasma membrane incorporation, cellular half-life, and function using a Vaccinia virus expression system in HeLa cells. The deglycosylated Pgp was expressed and reached the cell surface with proper function, although at much lower levels.
It is clear that Pgp N-glycans are important for targeting and function in the plasma membrane. To help characterize these necessary oligosaccharides, we isolated the overexpressed human Pgp from MES-SA/D×5 cells, comparing whole gel elution and preparative gel elution. We isolated similar quantities to Harker and Sikic [54], who recovered approximately 3.0% of the expressed human Pgp from solubilized MES-SA/D×5 membranes. The gel-purified Pgp ran as a diffuse band on our silver-stained gels.
No other non-Pgp proteins were detected in the silver-stained gels (Figure 2, lane 5). However, additional non-Pgp glycoproteins were detected after Pgp and PNGase F-treated Pgp were incubated overnight at 37 °C (Figure 3, lanes 18–21). A comparison of Rf values confirmed that these additional lower molecular weight bands were neither Pgp nor deglycosylated Pgp, because they were not detected using C219, which binds the polypeptide chain [28]. Since these bands were not detected until the overnight incubation (unpublished results), they were either degraded comigrating glycoproteins or non-Pgp proteins detected with the chemical derivitization of the blot contents and subsequent detection using anti-DIG Fab fragments conjugated to AP (see Methods). It has been shown previously by Ivey et al. [55] that small amounts of contaminating proteins are all but impossible to detect and to remove from a preparation of solubilized electroplax membrane proteins, even using preparative gel electrophoresis. Care should therefore be taken when identifying membrane protein samples as “pure.” Fortunately, these non-Pgp bands did not interfere with the DIG-lectin labeling results (Figure 3), where we detected several Pgp-associated N-glycans.
Four of the five lectins used recognized Pgp on our blots. Two of these, SNA and MAA, are specific for terminal sialic acids. MAA (Maackia amurensis agglutinin) recognizes only terminal sialic acid residues with alpha 2,3 linkage to penultimate galactose residues [46]. MAA columns favored complex type triantennary and tetraantennary asparagine-linked oligosaccharides containing this 2,3 linked terminal neuraminic acid.
MAA does not recognize oligosaccharides with only alpha 2,6-linked sialic acids. MAA has been shown to agglutinate the mouse lymphoma cell line BW5147 [56], possibly binding cell-surface Pgp glycans containing terminal sialic acids. However, MAA binding to the human Pgp on our blots was weak (Figure 3, lane 9).
Because lectin studies can be a qualitative and not a quantitative measure of oligosaccharide content, the intensities of the lectin detection reactions cannot be a measure of how much of a particular sugar chain is present. In theory, minor structures cannot be distinguished from predominate structures.
SNA, the elderberry (Sambucus nigra L.) bark lectin, recognizes terminal sialic acids with an alpha 2,6 linkage to galactose residues [45]. This is distinct from the MAA recognition site. There is a possibility that these two distinct linkages exist on a single multibranched oligosaccharide; however it is more likely that there are two distinct oligosaccharides with different linkages of terminal sialic acids, as postulated in Figure 4. The presence of sialic acid residues on Pgp is rarely reported. Fiala et al. [57] detected sialic acids in the murine leukemic cell line L1210/VCR, though indirectly by the histochemical staining of cell surfaces with ruthenium red, which predictably detected the overexpressed sialylated Pgp in the presence of vincristine.
Figure 4.
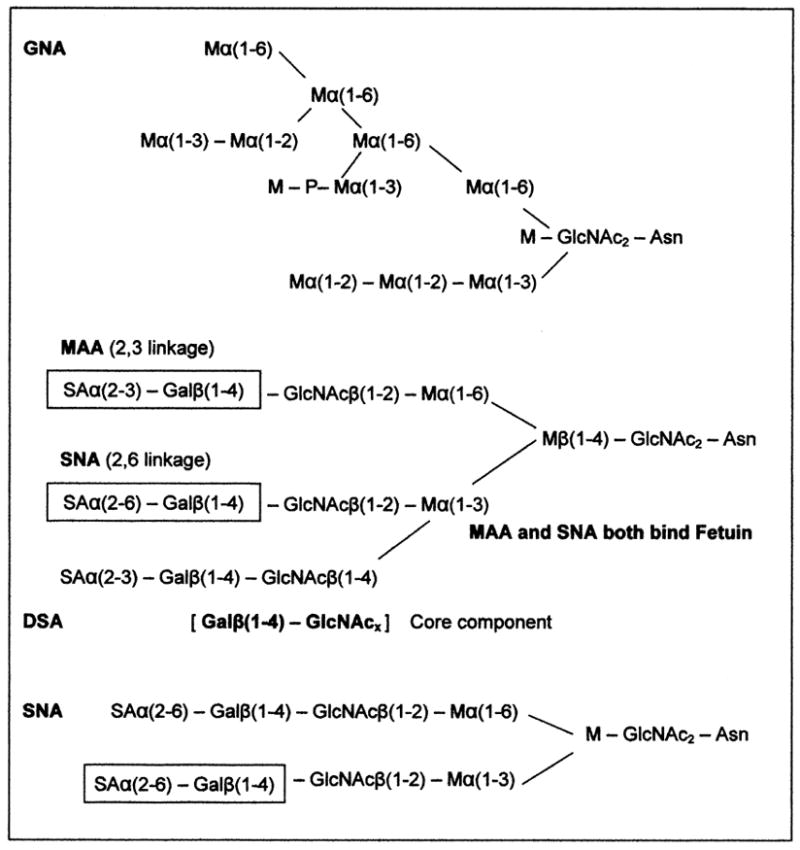
The DSA lectin (Datura stramonium agglutinin) also recognized Pgp and gave a broad banding on our blots, an indication of a high affinity for the human Pgp. DSA is specific for biantennary oligosaccharides possessing β(1-4)-N-acetyl-D-glucosamine residues [47]. These residues are probably in the two predicted sialylated glycans recognized by SNA and MAA. Rebbaa et al. [21] has shown the significance of bisecting GlcNAc as a tumor marker in pediatric brain tumors (gliomas), where the expression of the bisecting GlcNAc was correlated with tumor progression. The tumor GlcNAc sugars were detected using the lectin E-PHA (erythroagglutinating phytohemagglutinin) isolated from Phaseolus vulgaris [58].
From the binding profiles of GNA, SNA, MAA, and DSA on our blots, we have provided possible structures of the MES-SA/D×5 Pgp N-glycans (Figure 4). These generic lectin-bound N-glycan structures are consistent with the glycoprotein glycan structures associated with cancers, and include the α6 sialyl terminal groups and β1-6 branching glycans, which are highly expressed in cancers due to the up-regulation of acetylglucosaminyltransferase V, which could include the heavy glycosylation of Pgp [59].
Pgp’s first extracellular loop carries three extracellular N-linked sites [4]. We detected at least two lectin-distinct glycans present on the human uterine sarcoma Pgp. One glycan is high in branched terminal mannoses, as detected by the binding of GNA, which has a strict binding requirement for nonreducing end mannose residues [44]. Glycoproteins having the Man(α1,3)Man residue linkage have the highest affinity for GNA. Interestingly, the presence of additional linkages of this type, which would elongate the oligosaccharide branches, do not enhance its binding. A generalized structure of one of the possible high mannose branched oligosaccharides present on MES/DA/D×5 human Pgp is presented in Figure 4. With similar results to this study, Kerboeuf et al. [60] used LCA specific also for α-mannosyl residues to bind Pgp expressed in Haemonchus contortus eggs (nematode).
Other GlcNAc or galactose-specific lectins WGA (wheat germ agglutinin), RCA-1 (Ricinus communis agglutinin), and lentil lectin (Lens culinaris agglutinin [LCA]) have been used to isolate Pgps from multidrug-resistant Chinese hamster ovary cells and KB cells [22,61]. Surprisingly, tomato lectin (Lycopersicon esculentum) specific for polylactosamine chains was used to detect and study rat brain capillary endothelial and multidrug-resistant tumor cells expressing Pgp [62], supporting the heterogeneity of Pgp glycosylation. Such differential glycosylation has been reported with many lectin studies of Pgp, including the Ichikawa et al. [61] study in which Pgps from KB-C2 cells, kidney, and adrenal gland all had different lectin-binding properties. Ambudkar et al. [39] also saw altered elution patterns from lectin columns, probably due to differences in the glycomes of human Pgp expressed in mouse NIH 3T3 cells and Pgp purified from Chinese hamster cells.
O-glycans were not detected. MAA recognizes sialic acid α(2,3)-Gal linkages found terminally on O-glycans, and PNA binds core Galβ(1-3)–GalNAc O-glycans. DSA can also detect GlcNAc linked to serine or threonine residues. None of the five lectins used here recognized N-glycan deglycosylated Pgp on our blots, which would leave O-glycans for lectin detection. As reported by others [4,7], we saw no shift in Pgp’s molecular weight on our gels after O-glyconase or neuraminidase treatments (unpublished results).
Hounsell et al. [63] stated that the “tumor-associated oligosaccharide changes should be revisited” and represent “a renaissance in clinical applications.” The burgeoning field of glycomics supports this statement. Unfortunately, the glycome of human P-glycoproteins is not categorized in the Functional Glycomics Gateway (www.functionalglycomics.org), a comprehensive and regularly updated resource for functional glycomics research.
To identify and sequence glycans released from glycoproteins, many groups turn to the use of enzyme-linked lectin assays (ELLBA), surface plasmon resonance (SPR), MALDI-MS/MS sequencing, or the novel glyco-catch method for a comprehensive analysis of glycoproteins [64–68]. Encouraged by this study, our laboratory is using the Prep Cell gel elution technique presented here to isolate Pgp from several cancer cell lines for MALDI-MS sequencing of their N-glycans.
We realize there are inherent challenges when using established cell lines to postulate the glycan structures in human tumors. Nonetheless, we have shown, using Western blotting coupled with the DIG Glycan Differentiation Kit from Roche Allied Science, that key N-glycans are bound to the first extracellular loop of human Pgp overexpressed in MES/SA-D×5 cells. The techniques presented here offer a good model for isolating sufficient quantities of relatively “pure” Pgp to use for glycan studies with the techniques outlined above.
Acknowledgments
Special thanks to Dr. Leonard Davis for his help in the preparation of this manuscript. This study was supported by grants from NIH/NCI CA68992-03 and The Cancer Federation.
Abbreviations
- DIG
digoxigenin
- DSA
Datura stramonium agglutinin
- EDTA
ethylenediaminetetraacetic acid
- Endo F
endo-β-N-acetylglucosaminidase
- GNA
Galanthus nivalis agglutinin
- KB
Karnal bunt
- LCA
Lens culinaris agglutinin
- LDS
lithium dodecyl sulfate
- MAA
Maackia amurensis agglutinin
- Pgp
P-glycoprotein
- PNA
peanut (Arachis hypogaea) agglutinin
- PNGase F
peptide-N-glycosidase F
- RCA
Ricinus communis agglutinin
- RT
room temperature
- SDS
sodium dodecyl sulfate
- SNA
Sambucus nigra agglutinin
- SNP
single-nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A. Mechanisms of resistance to anticancer drugs: The role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics. 2005;6:115–138. doi: 10.1517/14622416.6.2.115. [DOI] [PubMed] [Google Scholar]
- 2.Ambudkar SV, Kim IW, Sauna ZE. The power of the pump: Mechanisms of action of P-glycoprotein (ABCB1) Eur J Pharm Sci. 2006;27:392–400. doi: 10.1016/j.ejps.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Ling V. P-glycoprotein: Its role in drug resistance. Amer J Med. 1995;99:6A31S–6A34. doi: 10.1016/s0002-9343(99)80283-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen SC, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Robinson IB. Internal duplication and homology with bacterial transport proteins in the MDR1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 5.Jones PM, George AM. Symmetry and structure in P-glycoprotein and ABC transporters. Eur J Biochem. 2000;267:5298–5305. doi: 10.1046/j.1432-1327.2000.01628.x. [DOI] [PubMed] [Google Scholar]
- 6.Gribar JJ, Ramachandra M, Hrycyna CA, Dey S, Ambudkar SV. Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system. J Mem Biol. 2000;173:203–214. doi: 10.1007/s002320001020. [DOI] [PubMed] [Google Scholar]
- 7.Richert ND, Aldwin L, Nitecki D, Gottesman MM, Pastan I. Stability and covalent modification in multidrug-resistant KB cells. Biochem. 1988;27:7607–7613. doi: 10.1021/bi00420a006. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein RS, Grogan TM, Kuszak JR, Jakate SM, Kluskens LF, Coon JS. Lab Med. Mosby-Year Book, Inc; 1991. Multidrug resistance gene product (P-glycoprotein) in normal tissue and tumors, Advances Path; pp. 207–234. [Google Scholar]
- 9.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 10.Loo TW, Clark DM. Covalent modifications of human P-glycoprotein mutants containing a single cysteine in either nucleotide-binding fold abolishes drug-stimulated ATPase activity. J Biol Chem. 1995;270:22957–22961. doi: 10.1074/jbc.270.39.22957. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg MF, Callaghan R, Modok S, Higgins CF, Ford RC. Three-dimensional structure of P-glycoprotein. J Biol Chem. 2005;280:2857–2862. doi: 10.1074/jbc.M410296200. [DOI] [PubMed] [Google Scholar]
- 12.Loo TW, Bartlett MC, Clarke DM. Processing mutations located throughout the human multidrug resistance P-glycoprotein disrupt interactions between the nucleotide binding domains. J Biol Chem. 2004;279:38395–38401. doi: 10.1074/jbc.M405623200. [DOI] [PubMed] [Google Scholar]
- 13.Dwek RA. Glycobiology: Towards understanding the functions of sugars. Biochem Soc Trans. 1995;23:1–25. doi: 10.1042/bst0230001. [DOI] [PubMed] [Google Scholar]
- 14.Béjà O, Bibi E. Functional expression of mouse MDR1 in an outer membrane permeability mutant of Escherichia coli. Proc Natl Acad Sci USA. 1996;93:5969–5974. doi: 10.1073/pnas.93.12.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Germann UA, Willingham MC, Pastan I, Gottesman MM. Expression of the human multidrug transporter in insect cells by a recombinant baculovirus. Biochemistry. 1990;29:2295–2303. doi: 10.1021/bi00461a013. [DOI] [PubMed] [Google Scholar]
- 16.Kuchler K, Thorner J. Functional expression of human MDR1 in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:2302–2306. doi: 10.1073/pnas.89.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans GL, Ni B, Hrycyna CA, Chen D, Ambudkar SV, Pastan I, Germann UA, Gottesman MM. Heterologous expression systems for P-glycoprotein: E.coli, yeast, and baculovirus. J Bioenergetics Biomem. 1995;27:43–52. doi: 10.1007/BF02110330. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa T, Onishi Y, Hirano H, Oosumi K, Nagakura M, Tarui S. Pharmacogenomics of drug transporters: A new approach to functional analysis of the genetic polymorphisms of ABCB1 (P-glycoprotein/MDR1) Biol Pharm Bull. 2004;27:939–948. doi: 10.1248/bpb.27.939. [DOI] [PubMed] [Google Scholar]
- 19.Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicology Appl Pharm. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Leschziner GB, Andrew T, Pirmohamed M, Johnson MR. ABCB1 genotype and PGP expression, function, and therapeutic drug response: A critical review and recommendations for future research. Pharmacogenomics J (Epub) 2006 Sep 12; doi: 10.1038/sj.tpj.6500413. [DOI] [PubMed] [Google Scholar]
- 21.Rebbaa A, Chou PM, Vucic I, Mirkin BL, Tomita T, Bremer EG. Expression of bisecting GlcNAc in pediatric brain tumors and its association with tumor cell response to vinblastine. Clin Can Res. 1999;5:3661–3668. [PubMed] [Google Scholar]
- 22.Doige CA, Sharom FJ. Strategies for the purification of P-glycoprotein from multidrug-resistant Chinese hamster cells. Protein Expr Pur. 1991;2:256–265. doi: 10.1016/1046-5928(91)90081-s. [DOI] [PubMed] [Google Scholar]
- 23.Bakos É, Hegedüs T, Holló Z, Welker E, Tusnády GE, Zaman GJR, Flens MJ, Váradi A, Sarkadi B. Membrane topology and glycosylation of the human multidrug resistance-associated protein. J Biol Chem. 1996;271:12322–12326. doi: 10.1074/jbc.271.21.12322. [DOI] [PubMed] [Google Scholar]
- 24.Downs AR, Greer DA, Schroeder RD, Ivey S. Characterization of P-glycoprotein carbohydrates in the uterine sarcoma cell line MES-SA/D×5. FASEB J. 1997;11:A538. [Google Scholar]
- 25.Ivey S, Downs AR, Greer DA. Characterization of N-linked human MES-SA/D×5 P-glycoprotein sugars using fluorophore-assisted carbohydrate electrophoresis. Mol Biol Cell. 1998;9:76a. [Google Scholar]
- 26.Greer DA, Downs AR, Shroeder R, Ivey S. Using lectins to detect terminal sugars on human P-glycoprotein overexpressed in MES-SA/D×5 cells: Possible O-linked sialic acids. AACR. 2005;95:A102. [Google Scholar]
- 27.Wesolowska O, Paprocka M, Kozlak J, Motohashi N, Dus D, Michalak K. Human sarcoma cell lines MES-SA and MES-SA/D×5 as a model for multidrug resistance modulaters screening. Anticancer Res. 2005;25:383–389. [PubMed] [Google Scholar]
- 28.Kokubu N, Cohen D, Watanabe T. Functional modulation of ATPase of P-glycoprotein by C219, a monoclonal antibody against P-glycoprotein. Biochem Biophy Res Comm. 1997;230:398–401. doi: 10.1006/bbrc.1996.5970. [DOI] [PubMed] [Google Scholar]
- 29.Hua J, Mutch DG, Herzog TJ. Stable suppression of MDR-1 gene using siRNA expression vector to reverse drug resistance in a human uterine sarcoma cell line. Gynecologic Oncology. 2005;98:31–38. doi: 10.1016/j.ygyno.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 30.Fairbanks G, Steck TL, Wallach DFH. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 31.Gustafsson E, Thoren K, Larsson T, Davidsson P, Karlsson KA, Nilsson CL. Identification of proteins from Escherichia coli using two-dimensional semi-preparative electrophoresis and mass spectrometry. Rapid Comm Mass Spec. 2001;15:428–432. doi: 10.1002/rcm.243. [DOI] [PubMed] [Google Scholar]
- 32.Mulvey C, Ohlendieck K. Use of continuous-elution gel electrophoresis as a preparative tool for blot overlay analysis. Anal Biochem. 2003;319:122–130. doi: 10.1016/s0003-2697(03)00321-x. [DOI] [PubMed] [Google Scholar]
- 33.Bollag DM, Rozycki MD, Edelstein SJ. Protein Methods. 2. Wiley-Liss; New York: 1996. [Google Scholar]
- 34.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4450–4454. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo SH, Goloubeva O, Webby R, Webster RG. Characterization of a porcine lung epithelial cell line suitable for influenza studies. J Virology. 2001;75:9517–9525. doi: 10.1128/JVI.75.19.9517-9525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JM, Chin KV, Hait WN. Interaction of P-glycoprotein with protein kinase C in human multidrug resistant carcinoma cells. Can Res. 1996;56:3490–3494. [PubMed] [Google Scholar]
- 37.Romsicki Y, Sharom FJ. The ATP-ase and ATP-binding functions of P-glycoprotein modulation by interaction with defined phospholipids. Eur J Biochem. 1998;256:170–178. doi: 10.1046/j.1432-1327.1998.2560170.x. [DOI] [PubMed] [Google Scholar]
- 38.Diociaiuti M, Molinari A, Ruspantini I, Gaudiano MC, Ippoliti R, Lendaro E, Bordi F, Chistolini P, Arancia G. P-glycoprotein inserted in planar lipid bilayers formed by liposomes opened on amorphous carbon and Langmuir-Blodgett monolayer. Biochim Biophys Acta. 2002;1559:21–31. doi: 10.1016/s0005-2736(01)00425-4. [DOI] [PubMed] [Google Scholar]
- 39.Ambudkar SV, Lelong IH, Zhang J, Cardarelli C. Purification and reconstitution of human P-glycoprotein. Meth Enzymol. 1998;292:492–504. doi: 10.1016/s0076-6879(98)92038-9. [DOI] [PubMed] [Google Scholar]
- 40.Schinkel AH, Kemp S, Dollé M, Rudenko G, Wagennar E. N-glycosylation and deletion mutants of the human MDR1 P-glycoprotein. J Biol Chem. 1993;268:7474–7481. [PubMed] [Google Scholar]
- 41.Cai J, Gros P. Overexpression, purification, and functional characterization of ATP-binding cassette transporters in the yeast Pichia pastoris. Biochim Biophys Acta. 2003;1610:63–76. doi: 10.1016/s0005-2736(02)00718-6. [DOI] [PubMed] [Google Scholar]
- 42.Georges E, Bradley G, Gariepy J, Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci USA. 1990;87:152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein IJ, Hayes CE. The lectins: Carbohydrate-binding proteins of plants and animals. Adv Carbo Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- 44.Shibuya N, Goldstein IJ, Van Damme EJM, Peumans WJ. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem. 1988;263:728–734. [PubMed] [Google Scholar]
- 45.Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 46.Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- 47.Crowley JF, Goldstein IJ, Arnarp J, Lönngren J. Carbohydrate binding studies on the lectin from Datura stramonium seeds. Arch Biochem Biophys. 1984;231:524–533. doi: 10.1016/0003-9861(84)90417-x. [DOI] [PubMed] [Google Scholar]
- 48.Tarentino AL, Plummer TH. Enzymatic deglycosylation of asparagine-linked glycans: Purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Meth Enzymol. 1994;230:44–57. doi: 10.1016/0076-6879(94)30006-2. [DOI] [PubMed] [Google Scholar]
- 49.Loo TW, Bartlett MC, Clarke DM. The dileucine motif at the COOH terminus of human multidrug resistance P-glycoprotein is important for folding but not activity. J Biol Chem. 2005;28:2522–2528. doi: 10.1074/jbc.M411483200. [DOI] [PubMed] [Google Scholar]
- 50.Santacruz-Toloza L, Huang Y, John SA, Papazian DM. Glycosylation of shaker potassium channel protein in insect cell culture and in Xenopus oocytes. Biochemistry. 1994;33:5607–5613. doi: 10.1021/bi00184a033. [DOI] [PubMed] [Google Scholar]
- 51.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2σ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 52.Waechter CJ, Schmidt JW, Catterall WA. Glycosylation is required for maintenance of functional sodium channels in neuroblastoma cells. J Biol Chem. 1983;258:5117–5123. [PubMed] [Google Scholar]
- 53.Bentley J, Quinn DM, Pitman RS, Warr JR, Kellent GL. The human KB multidrug-resistant cell line KB-C1 is hypersensitive to inhibitors of glycosylation. Cancer Letters. 1997;115:221–227. doi: 10.1016/s0304-3835(97)04739-3. [DOI] [PubMed] [Google Scholar]
- 54.Harker WG, Sikic BI. Multidrug (pleiotropic) resistance in doxorubicin-selected variants of the human sarcoma cell line MES-SA. Canc Res. 1985;45:4091–4095. [PubMed] [Google Scholar]
- 55.Ivey S, Thornhill WB, Levinson SR. Detection of 180 kDa proteins in Electroplax sodium channel preparations. Comp Biochem Physiol. 1993;106:551–556. doi: 10.1016/0305-0491(93)90131-n. [DOI] [PubMed] [Google Scholar]
- 56.Knibbs RN, Goldstein IJ, Ratcliffe RM, Shibuya N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. J Biol Chem. 1991;266:83–88. [PubMed] [Google Scholar]
- 57.Fiala R, Sulová Z, El-Saggan AH, Uhrík B, Liptaj T, Dovinová I, Hanušovská E, Drobná Z, Baranik M, Breier A. P-glycoprotien-mediated multidrug resistance phenotype of L1210/VCR cells is associated with decreases of oligo- and/or polysaccharide contents. Biochim Biophys Acta. 2003;1639:213–224. doi: 10.1016/j.bbadis.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Green ED, Baenziger JU. Oligosaccharide specificities of Phaseolus vulgaris leukoagglutinating and erythroagglutinating phytohemagglutinins. J Biol Chem. 1987;262:12018–12029. [PubMed] [Google Scholar]
- 59.Krueger KE, Srivastava S. Posttranslational protein modifications: Current implications for cancer detection, prevention, and therapeutics. Mol Cellular Proteomics. 2006;5.10:1799–1810. doi: 10.1074/mcp.R600009-MCP200. [DOI] [PubMed] [Google Scholar]
- 60.Kerboeuf D, Guegnard F, Vern YL. Analysis and partial reversal of multidrug resistance to anthelmintics due to P-glycoprotein in Haemonchus contortus eggs using Lens culinaris lectin. Parasitology Res. 2002;88:816–821. doi: 10.1007/s00436-002-0654-z. [DOI] [PubMed] [Google Scholar]
- 61.Ichikawa M, Yoshimura A, Furukawa T, Sumizawa T, Nakazima Y, Akiyama S. Glycosylation of P-glycoprotein in a multidrug-resistant KB cell line, and in the human tissues. Biochim Biophys Acta. 1991;1073:309–315. doi: 10.1016/0304-4165(91)90136-5. [DOI] [PubMed] [Google Scholar]
- 62.Fakla I, Hever A, Molnár J, Fischer J. Tomato lectin labels the 180 kD glycoform of P-glycoprotein in rat brain capillary endothelia and MDR tumor cells. Anticancer Res. 1998;18:3107–3112. [PubMed] [Google Scholar]
- 63.Hounsell EF, Young M, Davies MJ. Glycoprotein changes in tumours: A renaissance in clinical applications. Clin Sci. 1997;93:287–293. doi: 10.1042/cs0930287. [DOI] [PubMed] [Google Scholar]
- 64.Kiyoko F, Kinoshita A, Kanehisa M. Bioinformatics approaches in glycomics and drug discovery. Curr Opin Mol Therapeutics. 2006;8:514–520. [PubMed] [Google Scholar]
- 65.Drickamer K, Taylor ME. Glycan arrays for functional glycomics. Genome Biology. 2002;3:1034.1–1034.4. doi: 10.1186/gb-2002-3-12-reviews1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirabayasbi J, Hasbidate T, Kasai KI. Glyco-catch method: A lectin affinity technique for glycoproteomics. J Biomolecular Techniques. 2002;13:205–218. [PMC free article] [PubMed] [Google Scholar]
- 67.Kovarova A, Mislovicova D, Sulova Z, Masarova J, Skrabana R, Breier A. Study of surface glycoproteins in development of multidrug resistance of murine leukemic cell line L1210 by lectins. 13th European Carbohydrate Symposium; 2005. [Google Scholar]
- 68.Haslam SM, North SJ, Dell A. Mass spectrometric analysis of N- and O-glycosylation of tissues and cells. Curr Opin Structural Bio. 2006;16:584–591. doi: 10.1016/j.sbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Chacko BK, Appukuttan PS. Peanut (Arachis hypogaea) lectin recognizes alpha-linked galactose, but not N-acetyl lactosamine in N-linked oligosaccharide terminals. J Biol Macromol. 2001;28:365–71. doi: 10.1016/s0141-8130(01)00139-8. [DOI] [PubMed] [Google Scholar]


