Periodontal disease is a microbe-induced chronic inflammatory condition that leads to gingival inflammation, periodontal tissue destruction, and alveolar bone loss (108). Certain anaerobic gramnegative bacteria within the plaque biofilm are the major etiological agents of periodontal disease (62). However, as our understanding of the pathogenesis of periodontal diseases expands, it is becoming clear that most of the tissue damage that characterizes periodontal disease is caused indirectly by the host response to infection rather than directly by the infectious agent (121). Investigations into the mechanisms of the host-mediated response in periodontal disease have revealed that it involves the activation of the broad axis of innate immunity, specifically by upregulation of proinflammatory cytokines from monocytes/polymorphonuclear leukocytes, and downregulation of growth factors from macrophages (79). The host response in periodontal disease is characterized by the production of inflammatory mediators, cytokines, chemokines, and matrix metalloproteinases (prostaglandin E2, interleukin-1, interleukin-6, tumor necrosis factor-α, interleukin-8, and matrix metalloproteinase-1) (78). Lipopolysaccharide is one of the most important microbial virulence factors in generating host-mediated periodontal tissue destruction (62, 108). It has been shown that the level of a wide range of inflammatory mediators increases in response to lipopolysaccharide from periodontal pathogens (121).
Prominent inflammatory mediators associated with periodontal disease include the arachidonic acid-derived products leukotriene B4 and prostaglandin E2. Many of the pathophysiological events that occur in periodontal diseases can be explained to a large extent by the activities of lipid mediators (77, 118). Prostaglandin E2 is a potent stimulator of bone loss that is the hallmark of periodontal disease (118). Levels of prostaglandin E2 are significantly elevated in the crevicular fluid of patients with periodontal infections compared to that of healthy controls, and these levels correlate with disease severity and aggressiveness, and constitute a reliable indicator of ongoing periodontal tissue destruction (77).
Diabetes mellitus comprises a heterogeneous group of disorders characterized by altered glucose tolerance, and impaired lipid and carbohydrate metabolism. It is associated with a number of complications directly resulting from hyperglycemia (115). Most of these complications are vascular in nature such that macrovascular changes in diabetes lead to increased risk of myocardial infarction and stroke as a result of atherosclerosis (64), while diabetic microvascular pathology includes retinopathy, end-stage renal disease, a variety of debilitating neuropathies, poor wound healing, enhanced risk of infection, and periodontal disease (5, 6, 23, 63, 106). The hyperglycemic state is also associated with activated innate immunity, which is characterized by higher levels of inflammatory cytokines including tumor necrosis factor-α, interleukin-1β, and interleukin-6 (17, 21, 27, 32, 125). Moreover, specific inflammatory immune cell phenotypes are characterized by enhanced monocyte/polymorphonuclear leukocyte adhesion to endothelial cells, and expression of adhesion molecules (endothelial cell leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intracellular adhesion molecule-1) on endothelial cells and leukocytes (46, 82).
An abundance of information accumulated from studies on the complications of diabetes and periodontal disease has revealed that a hyperactive innate immune response may be the antecedent of both diseases, which probably have a synergistic effect when they coexist in the host (35). This relationship is illustrated by a number of studies demonstrating that the prevalence of periodontitis in diabetics is significantly higher, and that effective treatment of periodontitis may improve some diabetic complications and hyperglycemia. Moreover, the effective treatment of periodontal infection and the reduction in periodontal inflammation resulted in lower levels of glycated hemoglobin (34, 73). The mechanisms by which hyperglycemia can induce periodontal destruction have yet to be fully elucidated. Studies on diabetic complications suggest that the activation of the innate immune response may be mediated through several pathways. For example, the nonenzymatic reaction of glucose with the basic amino acids lysine and arginine in proteins leads to both extra- and intracellular accumulation of irreversible advanced glycated end products (AGE). Accumulation of AGE would in turn modulate the cellular function and alter tissue structure (89). Hyperglycemia can affect cell-signaling transduction, in particular activation of diacylglycerol and protein kinase C, which are important signaling molecules responsible for cell function. Hyperglycemia is also shown to increase the oxidative stress via protein kinase C-dependent activation of nicotinamide adenine dinuclotide diphosphate (NADPH) oxidase (an enzyme that catalyzes superoxide and peroxide generation) in neutrophils and other phagocytes (43, 52). Recent experimental evidence has established the role of these abnormalities in diabetics with periodontal disease (48). Additionally, impaired tissue healing in diabetes is a significant phenomenon that is associated with the establishment of persistent chronic inflammation.
Resolution of inflammation as an active natural process is a relatively new concept. Resolution is controlled by endogenous ‘pro-resolving’ lipid mediators that act as a ‘stop signal’ for leukocyte trafficking into the inflamed site, reversing vasodilatation and vascular permeability, and leading to restoration of the diseased tissue (7, 57, 72, 100, 101, 103, 104). These endogenous lipid mediators provide an opportunity for counterregulation of inflammatory responses based on manipulation of new pathways that may affect diabetic complications including periodontal disease. Our expanded understanding of the pathogenesis of periodontal disease in diabetics has led to the hypothesis that long-term hyperglycemia supports anaerobic infection in periodontal tissue in an environment of exacerbated innate immunity. Persistent hyperglycemia results in chronically activated innate immunity establishing chronic inflammation in various organ systems (vasculature, eye, and kidney) including the periodontium by either blocking or suppressing pathways of resolution. This paper examines the role of innate immunity, inflammation, and resolution of inflammation as essential components in the development of diabetic complications including periodontal disease.
Activation of innate immunity and inflammation in diabetes
The innate immune system is the first line of defense against infectious, chemical, or physical injury. Innate immunity is based on non-lymphoid tissue components, as well as a series of sentinel cells such as macrophages, mononuclear antigen-presenting cells, dendritic cells, endothelial cells, adipocytes, and neutrophils. Cells of innate immunity use a number of soluble and cellular receptors called pattern recognition receptors, which recognize harmful molecular structures (70). Toll-like receptors are the most studied pattern recognition receptors that are present at the cell surface as transmembrane receptors. The toll-like receptor that recognizes lipopolysaccharide on macrophages (toll-like receptor-4) and the receptor for AGE (RAGE) are members of this group (61). Binding to pattern recognition receptors activates the nuclear factor-κjB signaling pathway that induces immune response genes, especially those for inflammatory cytokines, which are the main initiators of inflammation and the acute-phase response (84). Another important function of innate immunity is to control the adaptive immune response through class II histocompatibility complex on antigen-presenting cells and costimulatory molecules (CD80 and CD86) (69).
Research has shown that inflammatory mechanisms are involved in the development of diabetic complications. Pickup and co-workers introduced the concept that chronic subclinical inflammation and activation of innate immunity are involved in the pathogenesis of the disease’s complications (85, 86). Inflammation comprises a pathophysiological series of events generated by the host in response to infection or other injuries. The primary events of inflammation are derived from vascular reactions at the site of injury leading to exudation of fluid and plasma proteins and recruitment of immune cells to the injury site. Inflammation serves as the first step in the initiation of the immune response, through which the infection is eliminated and the injury is repaired (57). In chronic inflammation, the persistence of the inflammatory response leads to host tissue destruction and may result in irreversible pathological changes. Several inflammatory mediators are important in the initiation and progression of inflammation. Recruitment of inflammatory cells, such as mast cells, platelets, and leukocytes, is the key event in the inflammatory response. These proinflammatory cells are capable of generating inflammatory mediators that can amplify the host immune response (57). Proteins such as cytokines and chemokines, low molecular weight lipids derived from arachidonic acid such as prostaglandin E2, gases like nitric oxide and carbon monoxide, reactive oxygen species, and nucleotides are recognized as important inflammatory mediators (102).
Inflammatory cytokines are the most studied aspect of inflammation in relation to hyperglycemia. Tumor necrosis factor-α, interleukin-6, and interleukin-1β have been shown to be markedly elevated in diabetes (87, 96, 110). The source of tumor necrosis factor-α in human plasma has yet to be determined; however, it has been shown that macrophages from diabetic patients release more tumor necrosis factor-α than healthy control macrophages (92). Furthermore, high glucose can activate monocytes and induce the expression of tumor necrosis factor-α via oxidant stress and nuclear factor-κB transcription factor (36). Other studies have indicated that there is a pattern of these cytokines associated with the increased risk for diabetes rather than an isolated elevation of the respective cytokine (20). Other indicators of inflammation have been reported to be elevated in diabetes. These include increase in acutephase proteins, cytokines, and mediators of endothelial activation (24). Prostaglandin E2 and nitric oxide were also reported to be elevated in diabetic retinopathy in animals, and may be associated with the diabetic microvascular complications (88).
Mechanisms of diabetic complications
Long-term complications of diabetes are mainly associated with prolonged hyperglycemia. Several studies have explained the biochemical pathways that lead to vascular complications. These include increased polyol pathway flux (sorbitol/aldose reductase) and oxidative stress, increased formation of AGE, and accumulation of diacylglycerol and activation of protein kinase C (53, 95, 105).
Oxidative stress
Through the polyol pathway, glucose is oxidized to sorbitol and then to fructose using aldose reductase and sorbitol dehydrogenase, respectively. The oxidation reaction uses NADPH as a cofactor. The over-consumption of NADPH may have a deleterious effect on cellular homeostasis by altering the redox balance, so causing increased intracellular oxidative stress (25, 76). Oxidative stress is an imbalance between the production of reactive oxygen species and antioxidant defense, leading to tissue damage. The produced reactive oxygen species, such as superoxide anion, hydroxyl radical, and peroxyl radical, result in damage to many biological molecules including DNA, lipids, and protein and prolonged existence of these reactive oxygen species promotes severe tissue damage and cell death (38, 65). Reactive oxygen species can act as second messengers and activate a number of serine/threonine and tyrosine protein kinases (19, 58, 67). Oxidative stress is thought to play a causative role in the pathogenesis of diabetes and its complications and has been shown to increase insulin resistance both in animal models and in diabetic patients (3, 22, 19, 58). It has been well demonstrated that hyperglycemia is a major factor responsible for the activation of oxidative stress (10, 11, 68, 74); however, little is known about the precise cellular mechanisms responsible for the generation of reactive oxygen species in the diabetic tissues (10, 19). Previously, it was postulated that high glucose could activate reactive oxygen species via multiple processes, such as enhanced formation of AGE, dysfunction of the mitochondrial electron transport chain, and activation of the plasma membrane NADPH oxidase (3, 10). Among these possibilities, recent attention has been focused on NADPH oxidase as a potential source of reactive oxygen species production in diabetic/hyperglycemic conditions (44, 58). This enzyme, which has been found primarily in phagocytic cells (2), was recently shown to exist in non-phagocytic cells, such as endothelial cells, vascular smooth muscle cells, fibroblasts, and adipocytes (37, 47, 49, 55, 66). Current evidence suggests that protein kinase C may regulate the activation of NADPH oxidase (4, 40, 44, 58). With this in mind, our group examined the superoxide-generating NADPH oxidase complex and protein kinase C activity in neutrophils in 50 diabetic patients, grouped according to glycemic control. Neutrophils from moderately or poorly controlled diabetic patients released significantly more superoxide than neutrophils from patients with good glycemic control and from non-diabetic healthy individuals. Moreover, neutrophils from these patients exhibited increased activity of protein kinase C, elevated amounts of diacylglycerol and enhanced NADPH oxidase activity. This suggests that hyperglycemia can lead to neutrophil priming (activation) where elevated protein kinase C activity results in increased oxidative stress (48).
Increased formation of AGE
Glucose reacts with proteins to form Schiff bases, which are then transformed into Amadori products (116). The Amadori products are degraded into highly active compounds that can react with proteins to form AGE. AGE can directly affect normal protein function, or indirectly act by reacting with RAGE on the cell membrane of a variety of cells (95). The potential importance of AGE in the pathogenesis of diabetic complications is indicated by the observation in animal models that two structurally unrelated AGE inhibitors prevented various manifestations of diabetic microvascular disease in retina, kidney, and nerve (38, 71, 109). AGE formation alters the functional properties of several important matrix molecules such as type I collagen and laminin (12, 112). In cell culture systems, AGE-RAGE interaction mediates long-term effects on key cellular targets of diabetic complications such as macrophages, glomerular mesangial cells, and vascular endothelial cells. These effects include the expression of cytokines and growth factors by macrophages and mesangial cells (interleukin-1, insulin-like growth factor-I, tumor necrosis factor-α, transforming growth factor-β, macrophage colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and plateletderived growth factor), and expression of endothelial cell adhesion molecules such as vascular cell adhesion molecule-1 (1, 15, 51, 94, 107, 122). RAGE has been shown to mediate signal transduction through the generation of reactive oxygen species, which activates transcription factor nuclear factor-κB (60, 124). Consistent with this concept, the blockade of RAGE in a murine model of diabetic periodontal disease diminished alveolar bone loss probably by blocking the activation of innate immunity and oxidative stress (59). These considerations suggest the possibility that the blockade of RAGE may provide an effective approach in a range of diabetic complications including periodontal disease.
Activation of protein kinase C
Protein kinase C and diacylglycerol are essential intracellular signaling molecules. Protein kinase C comprises a family of functional proteins derived from multiple genes and from alternative splicing of a single messenger ribonucleic acid transcript. Twelve isozymes of protein kinase C have already been cloned and characterized. Translocation of protein kinase C from the cytosol to the plasma membrane is the sign of protein kinase C activation, which occurs in response to an increase of diacylglycerol or exposure to exogenous tumor-promoting agents known as phorbol esters (42, 54). The diacylglycerol-protein kinase C intracellular signaling pathway is necessary for physiological cell responses to different extracellular signals. Physiologically, protein kinase C activation can occur through receptor-mediated phospholipase C or phospholipase D linked G-protein receptors, while pathological protein kinase C activation may occur in diabetes in response to hyperglycemia by increases in diacylglycerol levels primarily by de novo synthesis (42, 54), by binding of extracellular AGE to RAGE on the cell membrane, and by an increase in oxidative stress as a result of activation of the polyol pathway. The three pathways result in activation of protein kinase C (50). This activity may interfere with the normal cellular functions and may disrupt cellular responses to the environment. In early experimental diabetes, the activation of protein kinase C-β isoforms has been shown to mediate retinal and renal blood flow abnormalities (45), perhaps by depressing nitric oxide production and/or increasing endothelin-1 activity. Activation of protein kinase C has been implicated in the decreased glomerular production of nitric oxide induced by experimental diabetes (14), and in the decreased production of nitric oxide in smooth muscle cells that is induced by hyperglycemia (26). Activation of protein kinase C also inhibits insulin-stimulated expression of the mRNA for endothelial nitric oxide synthase in cultured endothelial cells (56). Hyperglycemia increases the activity of protein kinase C isoforms in glomerular mesangial cells (29). The increased permeability of endothelial cells induced by high glucose in cultured cells is mediated by activation of protein kinase C-α (41). However, activation of protein kinase C by elevated glucose induces expression of the permeability-enhancing factor vascular endothelial growth factor in smooth muscle cells (123).
In early work, although specific inhibitors of aldose reductase activity, AGE formation, and the protein kinase C activation pathway each abolished various diabetes-induced abnormalities in cell culture and animal models, there was no apparent common element linking these mechanisms of hyperglycemia-induced damage (18, 28, 38, 45, 71, 109). It has now been accepted that each of these different pathogenic mechanisms reflects a single hyperglycemia-induced process: overproduction of superoxide by the mitochondrial electron-transport chain (16, 75).
Relationship between periodontal inflammation and diabetes
Periodontal disease and other complications of diabetes are inflammatory processes characterized by activation of innate immunity and the subsequent host inflammatory response (78, 79, 121). Periodontal inflammation is initiated by gram-negative bacterial infection of the periodontal pocket (62, 108), whereas, diabetic inflammatory complications are mainly a result of hyperglycemia (115). Both diseases can modulate host immune response, such as upregulation of inflammatory cell phenotype, elevation of proinflammatory cytokines, and initiation of tissue damage. The outcome of activation of the inflammatory response in both diseases is similar in many aspects. The inflammatory response in diabetes is diverse, complicated, and affects a multitude of tissues and organs. The pathways of vascular tissue complications in diabetes are mediated through the formation of AGE, and increased production of reactive oxygen species by both activation of the diacylglycerol-protein kinase C pathway (42, 54, 76, 116) or increased activation of the polyol pathway (25, 76). The interaction between these mechanisms in the periodontium with pre-existing periodontal disease provides insight into the exacerbated periodontal destruction in diabetics, and also may explain why diabetic patients are at greater risk for periodontitis. Diabetes affects all periodontal parameters, including gingival bleeding, probing depth, and attachment loss (9). Alveolar bone loss is also greater in diabetic patients when compared with that in nondiabetic subjects (113). Additionally, periodontal disease occurs more frequently in diabetic patients with poor metabolic control compared with healthy subjects, and diabetic subjects with good metabolic control, respectively (73, 114, 117). These findings, coupled with the finding that increased metabolic control decreases the incidence of diabetic complications (111, 119, 120, 121), would suggest that control of the pathological mechanisms in the tissues of diabetic patients may be important in controlling periodontal destruction. However, some well-controlled diabetics still experience some degree of periodontal damage (117). This suggests that the diabetes-induced mechanisms of tissue destruction sometimes may not be reversible. On the other hand, the effective treatment of periodontitis may improve diabetic complications and glycemic status in type 2 diabetes (73), and effective treatment of periodontal infection is associated with lower levels of glycated hemoglobin (34). Investigation into specific pathological mechanisms of periodontal destruction in diabetics is underway. We have demonstrated that a positive relationship/correlation exists between elevated levels of glycated hemoglobin, enhanced superoxide release by neutrophils, and the severity of periodontitis in diabetics (48). It was interesting to note that there were no differences amongst the diabetic groups in other laboratory values, such as mean cholesterol, triglyceride, or high-density lipoprotein, suggesting that the increased risk for inflammatory diseases, such as periodontitis, in diabetes is linked to increased inflammation and oxidative stress mediated by the neutrophil. Our finding is consistent with previous studies suggesting a reciprocal relationship between diabetes and periodontal disease (34, 35, 73).
Tissue repair and resolution of inflammation in diabetes: potential for therapy
Tissue repair is a complex coordinated sequence of events involving migration of cells into the healing site, inflammation, proliferation of different cell types, angiogenesis, formation of matrix components, remodeling, and complete tissue repair. Impaired tissue healing is a significant phenomenon in diabetic patients. Several molecular mechanisms have been proposed to account for these pathophysiological effects. Two mechanisms, in particular, have an important influence on the process of tissue repair in diabetes: the accumulation of AGE in cells exposed to chronic hyperglycemia, and oxidative damage resulting from overproduction of reactive oxygen species (4, 12, 15, 38, 40, 51, 71, 83, 94, 107, 109, 112, 122). The inflammatory response following injury is an important event for rapid wound healing. In diabetes, initially there is delayed influx of inflammatory cells into a wound site; however, when these cells become established, then a state of chronic inflammation prevents deposition of matrix components, remodeling, and eventual repair of the wound (31). This sustained inflammatory response occurs after the accumulation of AGE in tissue and immune cells enhances the release of proinflammatory molecules such as tumor necrosis factor-α and the production of destructive matrix metalloproteinases, which impede tissue repair (31). The fibroblast is central to the process of extracellular matrix deposition and remodeling. It functions both as a synthetic cell, depositing collagen-rich matrix, and as a signaling cell, secreting growth factors that are important for cell-cell communication during the repair process. Any impediment to fibroblast function prevents normal healing and results in chronic, unhealed tissue. In a hyperglycemic environment, the interaction of AGE with RAGE on fibroblasts may cause reduced deposition of the collagen that is required for normal tissue repair (81). Bone healing is also reduced in diabetes as reported in recent studies which demonstrated that 40% reduction in bone healing was associated with increased RAGE expression in diabetic mice compared with non-diabetic animals (91). Application of an AGE blocker to these lesions reduced bone healing in the non-diabetic mice, providing further support for the role of AGE in tissue repair. In another study, it was reported that diabetes reduced bone formation and enhanced apoptosis of osteoblasts in bacteria-stimulated bone loss in an animal model (83).
A deficiency or alteration in growth factor activity may contribute towards the delayed healing seen in diabetes mellitus (80). Decreased growth factor (keratinocyte growth factor, vascular endothelial growth factors, and platelet-derived growth factor) production, excess protease activity, and/or increased microbial load are other aspects that may lead to impaired tissue healing in diabetes mellitus. For example, in diabetic mice fibroblast growth factor-2 accelerated wound repair by stimulating angiogenesis and granulation tissue formation (80). Diabetic wounds are well known to be slow to close, poor at forming granulation tissue, and prone to sustained inflammation. It was found that administration of sRAGE (a decoy receptor for AGE) in a murine diabetic wounding model led to accelerated wound closure, most likely by decreasing the expression of proinflammatory cytokines and matrix metalloproteinases, and by augmenting levels of angiogenic factors (31).
Research into the mechanisms of the resolution of inflammation as an active process controlled by ‘proresolving’ mediators is a growing field. These mediators switch off leukocyte trafficking to the inflamed site, reversing vasodilatation and vascular permeability and lead to the restoration of inflamed tissue to its previous physiological function. Pro-resolving mediators are derived from the lipoxygenase metabolism of arachidonic acid. Of particular interest are a group of lipid mediators termed the lipoxins (98). Lipoxins are bioactive eicosanoids derived from membrane arachidonic acid by the combined action of 5-lipoxygenase and 12-lipoxygenase or 15-lipoxygenase (31). A number of recent in vitro and in vivo studies have revealed that lipoxins, and specifically lipoxin A4, function as innate ‘stop signals’, serving to control local inflammatory processes (102). Recently, we demonstrated that inflammation is reduced in transgenic rabbits overexpressing 15-lipoxygenase that generate enhanced levels of lipoxin. These rabbits are protected from the inflammatory bone loss of periodontal disease while leukocytes from 15-lipoxygenase transgenic rabbits exhibited enhanced lipoxin production. Moreover, the findings also suggested that lipoxin has potential for novel approaches to disease therapy, such as in periodontitis complicated by diabetes where the inflammatory response is activated and endogenous resolution of inflammation is apparently suppressed. Further research is needed to characterize the mechanisms whereby these pro-resolving mediators may be affected by hyperglycemia.
Chronic periodontitis in diabetics is a disease that is greatly affected by the inflammatory host response as a result of gram-negative infection and by hyperglycemia-associated cellular/molecular change. Therapeutic approaches dealing with chronic periodontitis in diabetics should consider the amplified nature of the inflammatory response in this disease. Two main approaches are considered: the antibacterial approach directed towards the gram-negative infection, and the host modulation approach directed towards the inflammatory host products. Host modulation therapy has considered the inhibition of matrix metalloproteinases with antiproteinases such as doxycycline (30), blocking the production of the proinflammatory cytokines and arachidonic acid metabolites with non-steroidal anti-inflammatory drugs (8), and inhibiting osteoclastic activities with bone-sparing agents such as bisphosphonates (93).
A therapeutic approach to limit inflammation in diabetic vascular complications has targeted the specific inflammatory mechanisms in diabetes (accumulation of AGE and increased oxidative stress) (33, 39, 94, 105, 122). Interventions for each inflammatory mechanism have had great efficacy in animal models but disappointing outcomes in clinical trials (10). It was concluded that a cocktail of inhibitors might be necessary to effectively block these deleterious cellular responses in a complex human model of fluctuating hyperglycemia (105). The observation that inflammatory pathways reflect a single hyperglycemia-induced process, namely oxidative stress, suggested that anti-oxidants could serve as a single agent in the prevention of diabetes complications. Conventional antioxidants scavenge free radicals in an inefficient stoichiometric manner so that one molecule of the antioxidant would be needed to neutralize each free radical generated. Novel small molecular weight compounds that function as superoxide dismutase mimetics may offer more reliable benefits because of the catalytic properties that could permit enzymatic detoxification (90). Administration of sRAGE in a murine diabetic wound model leads to accelerated wound closure, most likely by decreasing the expression of proinflammatory cytokines and matrix metalloproteinases, and by augmenting levels of angiogenic factors (122). It diminishes the loss of alveolar bone by blocking the destructive inflammatory process, and containing the gram-negative infection (10). The blockage of RAGE on different immune cells could also provide an effective therapeutic approach that can affect the development and progression of diabetic periodontitis.
Another therapeutic approach based on the function of ‘pro-resolving’ mediators such as lipoxin is developing (104). Synthetic lipoxin analogs exhibit greater potency for these actions than the native compound, likely because of decreased metabolism (97). Recently, synthetic analogs [e.g. 15-epi-16- (para-fluoro)-phenoxy-lipoxin A4 (aspirin-triggered lipoxins, ATLa)] have been modeled on 15-epi-lipoxin A4, a native lipoxin generated in vivo in the presence of aspirin via cyclooxygenase-2 acetylation (13). We have recently reported that lipoxin and their stable analogs, which block neutrophil chemotaxis and superoxide production in vitro, dramatically reduce periodontitis in a rabbit model (99). Clinical studies are now under way to evaluate the long-term usefulness of these compounds in humans. It will be important to determine if treatment methods targeted at limiting the inflammatory response would reduce the severity of periodontitis in diabetics without compromising the antimicrobial properties of phagocytic leukocytes in these patients.
Conclusion
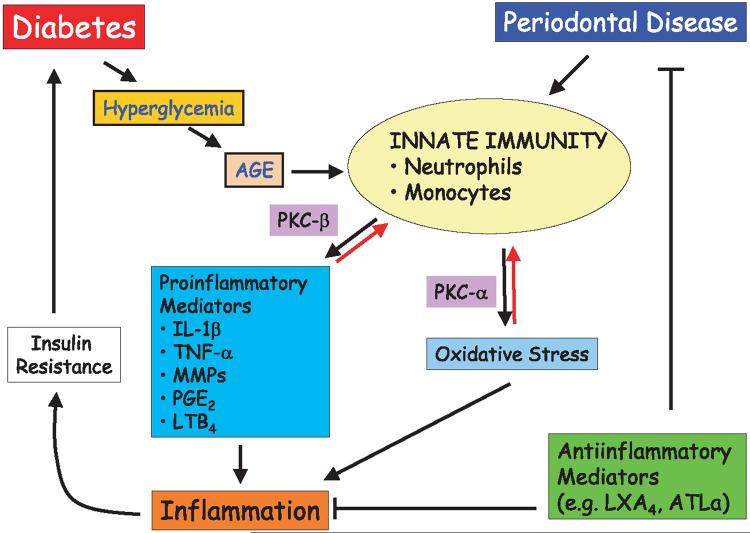
The dynamics of the inflammatory response that leads to tissue destruction in the periodontal disease of diabetic patients are complex. As demonstrated in Fig. 1, innate immunity in diabetes plays a central role in determining the extent and the magnitude of diabetic complications, including periodontitis. Activation of innate immunity is the common mechanism in both diseases. In diabetes, as a result of hyperglycemia, the inflammatory response is triggered primarily through the action of AGE, resulting in increased production of reactive oxygen species and proinflammatory mediators. Interestingly, while protein kinase C is the predominant activation pathway for these proinflammatory responses, different isoforms of protein kinase C seem to regulate different pathways. In designing therapeutic approaches to dampen the protein kinase C-mediated responses, it is clear that a cocktail of inhibitors will be necessary to block both the NADPH oxidase-mediated superoxide generation (protein kinase C-α) and cytokine secretion (protein kinase C-β).
Fig. 1.
Pathways of pathogenesis in diabetic periodontitis. Diabetes initiates a series of innate immune responses through production of advanced glycated end-products (AGEs) as a result of hyperglycemia. Innate immune responses via neutrophils and monocytes lead to proinflammatory cytokine production and will generate oxidative stress through the activities of protein kinase C beta (PKC-β) and PKC-α, respectively. These mediators of host response, in turn, result in inflammatory processes, which would further complicate the glycemic control through insulin resistance. Periodontal disease has a direct effect on the deterioration of diabetic state through the chronic inflammatory changes. All these processes could be potentially blocked through the use of anti-inflammatory mediators. AGE, advanced glycated end-products; PKC, protein kinase C; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; MMPs, matrix metalloproteinases; PGE2, prostaglandin E2; LTB4, leukotriene B4; LXA4, lipoxin A4; ATL-α, aspirin-triggered lipoxin-α.
Another theoretically promising approach for the prevention of diabetic complications can be found in a new approach to therapy. That is, rather than attempting to inhibit the onset of inflammation, actively promote the resolution of inflammation. Naturally occurring resolving molecules like lipoxin have been demonstrated to prevent establishment of chronic periodontal lesions, stroke, reperfusion injury, and in inflammatory bowel disease (98) in add-back experiments, essentially using endogenous mediators as pharmacological agents. Promotion of resolution of the innate immune response in periodontitis has the net effect of preventing the onset and progression of tissue destruction. The efficacy of pro-resolution strategies in the prevention of other complications of diabetes remains to be evaluated.
Acknowledgments
This work was supported by USPHS DE13499, DE15566, RR00533, and DE16191.
References
- 1.Abordo EA, Westwood ME, Thornalley PJ. Synthesis and secretion of macrophage colony stimulating factor by mature human monocytes and human monocytic THP-1 cells induced by human serum albumin derivatives modified with methylglyoxal and glucose-derived advanced glycation end products. Immunol Lett. 1996;53:7–13. doi: 10.1016/0165-2478(96)02601-6. [DOI] [PubMed] [Google Scholar]
- 2.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 3.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 4.Bouin AP, Grandvaux N, Vignais PV, Fuchs A. p40(phox) Is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase c-type kinase in the phosphorylation process. J Biol Chem. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- 5.Boulton AJM. Pathogenesis of diabetic neuropathy. In: Marshall SM, Home PD, Alberti KG, Krall LP, editors. The Diabetes Annual 7. Elsevier; Amsterdam: 1993. pp. 192–210. [Google Scholar]
- 6.Bower G, Brown DM, Steffes MW, Vernier RL, Mauer SM. Studies of the glomerular mesangium and the juxtaglomerular apparatus in the genetically diabetic mouse. Lab Invest. 1980;43:333–341. [PubMed] [Google Scholar]
- 7.Brady HR, Serhan CN. Lipoxins: putative braking signals in host defense, inflammation and hypersensitivity. Curr Opin Nephrol Hypertens. 1996;5:20–27. [PubMed] [Google Scholar]
- 8.Bragger U, Muhle T, Formousis I, Lang NP, Mombelli A. Effect of the NSAID flurbiprophen on remodeling after periodontal surgery. J Periodontal Res. 1997;32:575–582. doi: 10.1111/j.1600-0765.1997.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 9.Bridges RB, Anderson JW, Sax SR, Gregory K, Bridges SR. Periodontal status of diabetic and non-diabetic men: effect of smoking, glycemic control, and socioeconomic factors. J Periodontol. 1996;67:1158–1192. doi: 10.1902/jop.1996.67.11.1185. [DOI] [PubMed] [Google Scholar]
- 10.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49:27–29. doi: 10.1016/s0026-0495(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 12.Charonis AS, Reger LA, Dege JE, Kouzi-Koliakos K, Furcht LT, Wohlhueter RM, Tsilibary EC. Laminin alterations after in vitro nonenzymatic glycosylation. Diabetes. 1990;39:807–814. doi: 10.2337/diab.39.7.807. [DOI] [PubMed] [Google Scholar]
- 13.Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 14.Craven PA, Studer RK, DeRubertis FR. Impaired nitric oxide-dependent cyclic guanosine monophosphate generation in glomeruli from diabetic rats. Evidence for protein kinase C-mediated suppression of the cholinergic response. J Clin Invest. 1994;93:311–320. doi: 10.1172/JCI116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi T, Vlassara H, Kirstein M, Yamada Y, Striker GE, Striker LJ. Receptor specific increase in extracellular matrix productions in mouse mesangial cells by advanced glycosylation end products is mediated via platelet derived growth factor. Proc Natl Acad Sci U S A. 1992;89:2873–2877. doi: 10.1073/pnas.89.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elner S, Elner V, Jaffe G, Stuart A, Kunkel S, Strieter R. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14:1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- 18.Engerman RL, Kern TS, Larson ME. Nerve conduction and aldose reductase inhibition during 5 years of diabetes or galactosaemia in dogs. Diabetologia. 1994;37:141–144. doi: 10.1007/s001250050084. [DOI] [PubMed] [Google Scholar]
- 19.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 20.Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Real J, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin-6 levels, blood pressure and insulin resistance in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 22.Fiala E, Westendorf J, West IC. Radicals and oxidative stress in diabetes. Toxicology. 2000;146:83–92. doi: 10.1016/s0300-483x(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 23.Frank RN. Diabetic retinopathy. Prog Ret Eye Res. 1995;14:361–392. [Google Scholar]
- 24.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 25.Gabbay KH. Hyperglycemia, polyol metabolism, and complications of diabetes mellitus. Ann Rev Med. 1975;26:521–536. doi: 10.1146/annurev.me.26.020175.002513. [DOI] [PubMed] [Google Scholar]
- 26.Ganz MB, Seftel A. Glucose-induced changes in protein kinase C and nitric oxide are prevented by vitamin E. Am J Physiol. 2000;278:E1460–E152. doi: 10.1152/ajpendo.2000.278.1.E146. [DOI] [PubMed] [Google Scholar]
- 27.Geerlings S, Brouwer E, Van Kessel K, Gaastra W, Stolk R, Hoepelman A. Cytokine secretion is impaired in women with diabetes mellitus. Eur J Clin Invest. 2000;30:995–1001. doi: 10.1046/j.1365-2362.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 28.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 29.Glogowski EA, Tsiani E, Zhou X, Fantus IG, Whiteside C. High glucose alters the response of mesangial cell protein kinase C isoforms to endothelin-1. Kidney Int. 1999;55:486–499. doi: 10.1046/j.1523-1755.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 30.Golub LM, Sorsa T, Lee H-M, Ciancio S, Sorbi D, Ramamurthy NS, Gruber B, Salo T, Konttinen YT. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinase in human adult periodontitis gingiva. J Clin Periodontol. 1995;22:100–109. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 31.Goova MT, Li J, Kislinger T, Qu W, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, Stern DM, Schmidt AM. Blockade of receptors for advanced glycation endproducts restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graves DT, Oskoui M, Volejnikova S, Naguib G, Cai S, Desta T, Kakouras A, Jiang Y. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J Dent Res. 2001;80:1875–1879. doi: 10.1177/00220345010800100301. [DOI] [PubMed] [Google Scholar]
- 33.Greene DA, Arezzo JC, Brown MB. Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Zenarestat Study Group. Neurology. 1999;53:580–591. doi: 10.1212/wnl.53.3.580. [DOI] [PubMed] [Google Scholar]
- 34.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 35.Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, Genco RJ. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713–719. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 36.Guha M, Bai W, Nadler J, Natarajan R. Molecular mechanisms of TNF-alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress dependent and independent pathways. J Biol Chem. 2000;275:17728–17739. doi: 10.1074/jbc.275.23.17728. [DOI] [PubMed] [Google Scholar]
- 37.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Cir Res. 2000;86:E85–E90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 38.Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Natl Acad Sci USA. 1991;88:11555–11559. doi: 10.1073/pnas.88.24.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 40.Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RA, MacHarzina R, Brasen JH, Meinertz T, Munzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int. 1999;55:252–260. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- 41.Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, Luft FC, Haller H. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res. 1997;81:363–371. doi: 10.1161/01.res.81.3.363. [DOI] [PubMed] [Google Scholar]
- 42.Idris I, Gray S, Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44:659–673. doi: 10.1007/s001250051675. [DOI] [PubMed] [Google Scholar]
- 43.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 44.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 45.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 46.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes. 2000;49:485–491. doi: 10.2337/diabetes.49.3.485. [DOI] [PubMed] [Google Scholar]
- 47.Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol Heart Circ Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 48.Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam B-H, Malabanan A, Trackman PC, Badwey JA, Van Dyke TE. Enhanced superoxide release and elevated protein Kinase C activity in neutrophil from diabetic patients: association with periodontitis. J Leukocyte Biol. 2005;78:1–9. doi: 10.1189/jlb.1004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kashiwagi A, Shinozaki K, Nishio Y, Maegawa H, Maeno Y, Kanazawa A, Kojima H, Haneda M, Hidaka H, Yasuda H, Kikkawa R. Endothelium-specific activation of NAD(P)H oxidase in aortas of exogenously hyperinsulinemic rats. Am J Physiol Endocrinol Metab. 1999;277:E976–E983. doi: 10.1152/ajpendo.1999.277.6.E976. [DOI] [PubMed] [Google Scholar]
- 50.Keogh RJ, Dunlop ME, Larkins RG. Effect of inhibition of aldose reductase on glucose flux, diacylglycerol formation, protein kinase C, and phospholipase A2 activation. Metabolism. 1997;46:41–47. doi: 10.1016/s0026-0495(97)90165-7. [DOI] [PubMed] [Google Scholar]
- 51.Kirstein M, Aston C, Hintz R, Vlassara H. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest. 1992;90:439–446. doi: 10.1172/JCI115879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitada M, Koya D, Sugimoto T, Isono M, Araki S, Kashiwagi A, Haneda M. Translocation of glomerular p47phox and p67phox by protein kinase C activation is required for oxidative stress in diabetic nephropathy. Diabetes. 2003;52:2603–2614. doi: 10.2337/diabetes.52.10.2603. [DOI] [PubMed] [Google Scholar]
- 53.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 54.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 55.Krieger-Brauer HI, Medda PK, Kather H. Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem. 1997;272:10135–10143. doi: 10.1074/jbc.272.15.10135. [DOI] [PubMed] [Google Scholar]
- 56.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo a specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 57.Kumar V, Abbas AK, Fausto N, Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th edn. Elsevier Saunders; Philadelphia, PA: 2005. pp. 47–86. [Google Scholar]
- 58.Kuroki T, Isshiki K, King GL. Oxidative stress: the lead or supporting actor in the pathogenesis of diabetic complications. J Am Soc Nephrol. 2003;14:S216–S220. doi: 10.1097/01.asn.0000077405.07888.07. [DOI] [PubMed] [Google Scholar]
- 59.Lalla E, Lamster IB, Feit M, Huang L, Spessot A, Qu W, Kislinger T, Lu Y, Stern DM, Schmidt AM. Blockade of RAGE suppresses periodontitis-associated alveolar bone loss in diabetic mice. J Clin Invest. 2000;105:1117–1124. doi: 10.1172/JCI8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 61.Li YM, Mitsuhashi T, Wojciechowicz D, Shimizu N, Li J, Stitt A, He C, Banerjee D, Vlassara H. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90-80K-H membrane proteins. Proc Natl Acad Sci U S A. 1996;93:11047–11052. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liljenberg B, Lindhe J, Berglundh T, Dahlen G, Jonsson R. Some microbiological, histopathological and immunohistochemical characteristics of progressive periodontal disease. J Clin Periodontol. 1994;21:720–727. doi: 10.1111/j.1600-051x.1994.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 63.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111:850–857. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 64.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 66.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulinstimulated hydrogen peroxide reversibly inhibits proteintyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 67.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marfella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J Clin Invest. 2001;108:635–636. doi: 10.1172/JCI13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 70.Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura S, Makita Z, Ishikawa S, Yasumura K, Fujii W, Yanagisawa K, Kawata T, Koike T. Progression of nephropathy in spontaneous diabetic rats is prevented by OPB-9195, a novel inhibitor of advanced glycation. Diabetes. 1997;46:895–899. doi: 10.2337/diab.46.5.895. [DOI] [PubMed] [Google Scholar]
- 72.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 73.Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, Knowler WC. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1998;13:836–840. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- 74.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 75.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 76.Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol. 2002;50:325–392. doi: 10.1016/s0074-7742(02)50082-9. [DOI] [PubMed] [Google Scholar]
- 77.Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 78.Offenbacher S, Odle BM, Gray RC, Van Dyke TE. Crevicular fluid prostaglandin E levels as a measure of the periodontal disease status of adult and juvenile periodontitis patients. J Periodontal Res. 1984;19:1–13. doi: 10.1111/j.1600-0765.1984.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 79.Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 80.Okumura M, Okuda T, Nakamura T, Yajima M. Acceleration of wound healing in diabetic mice by basic fibroblast growth factor. Biol Pharm Bull. 1996;19:530–535. doi: 10.1248/bpb.19.530. [DOI] [PubMed] [Google Scholar]
- 81.Owen WF, Hou FF, Stuart RO, Kay J, Boyce J, Chertow GM, Schmidt AM. b2-microglobulin modified with advanced glycation endproducts modulates collagen synthesis by human fibroblasts. Kidney Int. 1998;53:1365–1371. doi: 10.1046/j.1523-1755.1998.00882.x. [DOI] [PubMed] [Google Scholar]
- 82.Park K, Ahn K, Chang JS, Lee SE, Ryu SB, Park YI. TGF-b1 down-regulates inflammatory cytokine-induced VCAM-1 expression in cultured human glomerular endothelial cells. Nephrol Dial Transplant. 2000;15:596–604. doi: 10.1093/ndt/15.5.596. [DOI] [PubMed] [Google Scholar]
- 83.Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, Li Z, Croitoru A, Thung S, Vlassara H. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003;52:2805–2813. doi: 10.2337/diabetes.52.11.2805. [DOI] [PubMed] [Google Scholar]
- 84.Pickup JC, Crook M. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 85.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 86.Pickup JC, Chusney GC, Thomas SM, Burt D. Plasma interleukin-6, tumor necrosis factor alpha and blood cytokine production in types 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 87.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 88.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–39S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez-Manas L, Angulo J, Vallejo S, Peiro C, Sanchez-Ferrer A, Cercas E, Lopez-Doriga P, Sanchez-Ferrer CF. Early and intermediate Amadori glycosylation adducts, oxidative stress, and endothelial dysfunction in the streptozotocin-induced diabetic rats vasculature. Diabetologia. 2003;46:556–566. doi: 10.1007/s00125-003-1056-1. [DOI] [PubMed] [Google Scholar]
- 90.Ross AD, Sheng H, Warner DS, Piantadosi CA, Batinic-Haberle I, Day BJ, Crapo JD. Hemodynamic effects of metalloporphyrin catalytic antioxidants: structure-activity relationships and species specificity. Free Radic Bio Med. 2002;33:1657–1669. doi: 10.1016/s0891-5849(02)01140-1. [DOI] [PubMed] [Google Scholar]
- 91.Santana RB, Xu L, Chase HB, Amar S, Graves DT, Trackman PC. A role for advanced glycation endproducts in diminished bone healing in type 1 diabetes. Diabetes. 2003;52:1502–1510. doi: 10.2337/diabetes.52.6.1502. [DOI] [PubMed] [Google Scholar]
- 92.Sartippour MR, Renier G. Upregulation of macrophage lipoprotein lipase in patients with type 2 diabetes: role of peripheral factors. Diabetes. 2000;49:597–602. doi: 10.2337/diabetes.49.4.597. [DOI] [PubMed] [Google Scholar]
- 93.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation end-products interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice: a potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracey RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 96.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Serhan CN. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim Biophys Acta. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 98.Serhan CN. Eicosanoids in leukocyte function. Curr Opin Hematol. 1994;1:69–77. [PubMed] [Google Scholar]
- 99.Serhan CN, Fiore S. Lipoxin recognition sites of human neutrophils. Adv Prostaglandin Thromboxane Leukotriene Res. 1994;22:317–326. [PubMed] [Google Scholar]
- 100.Serhan CN, Oliw E. Unorthodox routes to prostanoid formation: new twists in cyclooxygenase-initiated pathways. J Clin Invest. 2001;107:1481–1489. doi: 10.1172/JCI13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serhan CN, Fiore S, Levy BD. Cell-cell interactions in lipoxin generation and characterization of lipoxin A4 receptors. Ann NY Acad Sci. 1994;744:166–180. doi: 10.1111/j.1749-6632.1994.tb52734.x. [DOI] [PubMed] [Google Scholar]
- 102.Serhan CN, Maddox JF, Petasis N, Papayianni A, Brady HR, Colgan SP, Madara J. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 103.Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokine. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 104.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 105.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 106.Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. 1990;121:532–536. doi: 10.14219/jada.archive.1990.0211. [DOI] [PubMed] [Google Scholar]
- 107.Skolnik EY, Yang Z, Makita Z, Radoff S, Kirstein M, Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodeling and diabetic nephropathy. J Exp Med. 1991;174:931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 109.Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes. 1991;40:1328–1334. doi: 10.2337/diab.40.10.1328. [DOI] [PubMed] [Google Scholar]
- 110.Spranger J, Kroke A, Möhlig M, Hoffman K, Bergman MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 111.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tanaka S, Avigad G, Brodsky B, Eikenberry EF. Glycation induces expansion of the molecular packing of collagen. J Mol Biol. 1988;203:495–505. doi: 10.1016/0022-2836(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 113.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67:1085–1093. doi: 10.1902/jop.1996.67.10s.1085. [DOI] [PubMed] [Google Scholar]
- 114.Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol. 1998;69:76–83. doi: 10.1902/jop.1998.69.1.76. [DOI] [PubMed] [Google Scholar]
- 115.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 116.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 117.Tsai CC, Hong YC, Chen CC, Wu YM. Measurement of prostaglandin E2 and leukotriene B4 in the gingival crevicular fluid. J Dent. 1998;26:97–103. doi: 10.1016/s0300-5712(96)00084-x. [DOI] [PubMed] [Google Scholar]
- 118.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Commun Dent Oral Epidemiol. 2002;30:182–192. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 119.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 120.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 121.Van Dyke TE, Lester MA, Shapira L. The role of the host response in periodontal disease progression: implication for future treatment strategies. J Periodontol. 1993;64:792–806. doi: 10.1902/jop.1993.64.8s.792. [DOI] [PubMed] [Google Scholar]
- 122.Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- 123.Williams B, Gallacher B, Patel H, Orme C. Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes. 1997;46:1497–1503. doi: 10.2337/diab.46.9.1497. [DOI] [PubMed] [Google Scholar]
- 124.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- 125.Zykova S, Jenssen T, Berdal M, Olsen R, Myklebust R, Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes. 2000;49:1451–1458. doi: 10.2337/diabetes.49.9.1451. [DOI] [PubMed] [Google Scholar]