Abstract
Background
Treatment side effects after radical prostatectomy include urinary, sexual, and bowel dysfunction. These functional declines, coupled with the bother associated with these dysfunctions, lead to a complicated pattern of change in quality-of-life and decreased self-efficacy.
Methods
In this study, 72 men who underwent radical prostatectomy 6-weeks prior were randomly assigned to usual health care control group or peer-to-peer support (dyadic support) group. The dyadic meetings were held once a week for 8 weeks. Measured pre- and post-test was general health-related quality-of-life (SF-36), prostate cancer-specific quality-of-life (UCLA Prostate Cancer Index), and self-efficacy (Stanford Inventory of Cancer Patient Adjustment).
Results
By 8 weeks, self-efficacy significantly improved for men in the experimental group, but not for men in the control group. A series of logistic regression analyses showed that the dyadic intervention significantly accounted for changes in physical role functioning, bowel function, mental health, and social function. Age, education, and self-efficacy had significant interaction effects and increased the effects of the dyadic intervention on several outcomes.
Conclusions
The intervention had a significant impact on how men react socially and emotionally to the side effects of radical prostatectomy.
Keywords: Function and bother, Prostate cancer, Self-efficacy, Support intervention, Radical prostatectomy, Treatment side effects
Introduction
All men undergoing radical prostatectomy for localized prostate cancer endure clinically significant declines in urinary, sexual, and, to varying degrees, bowel dysfunction [1,2]. These functional declines, coupled with the bother associated with these dysfunctions, lead to a complicated pattern of change in quality-of-life and also negatively affect the psychosocial wellbeing of men, even after adjusting for pretreatment demographic and psychosocial factors [3,4].
General health-related quality-of-life, which is a multidimensional construct that assesses patients' physical, mental and social well-being, among others, returns to pre-surgical levels within 3–6 months following radical prostatectomy. However, some disease-specific quality-of-life indicators are less resilient and can lead to additional disease-specific worries [5,6]. The magnitude of these effects varies from minor transient inconveniences to major morbidity. For those with unremitting symptoms related to prostatectomy, quality-of-life can be adversely affected for long periods of time [5,6].
‘One size fits all’ programs of social support focus on meeting the educational and informational needs of men and improving quality-of-life, yet, they may not satisfy the emotional needs of men after radical prostatectomy, and do not support strategies that can be included in individually tailored programs of peer-to-peer (dyadic) support [7–10]. Despite these differences between group social support and dyadic support, older age and low education enhance the effect of these two support interventions on quality-of-life outcomes [8,11,12].
Other factors also affect quality-of-life. According to Bandura [13] high self-efficacy can lead to good social functioning that is essential to quality-of-life, and this is supported by several studies involving cancer survivors [9,10,13–16]. Although age, education, and self-efficacy are important elements for quality-of-life for many cancer patients, the underlying factors that bring about changes in quality-of-life for men after radical prostatectomy are not well defined.
The purposes of this study were to determine the effect of a dyadic support intervention on quality-of-life for men after radical prostatectomy, and to assess the moderating effects of self-efficacy, age, and education on the change associated with the dyadic intervention.
Materials and methods
Sample and data collection
Following Institutional Review Board approval, 72 men diagnosed with prostate cancer (aged 45 years and older) who were recently treated by radical prostatectomy were recruited from the urology clinics at two tertiary care medical centers in the Southeastern US. Potential participants were identified by their urologists, who agreed to have patients enrolled in the study. Those men who were eligible, and who agreed to participate, were randomized to either a control (usual health care) or experimental group (usual health care plus peer support intervention (dyads)). Recruitment took place 6 weeks after surgery when treatment side effects commonly include urinary incontinence and erectile dysfunction. Information from their medical records (time since treatment and comorbidity) and self-report (bereavement) was used to screen participants. Excluded were men who had a prior history of another cancer, current diagnosis of clinical depression, and the death of a loved one within the past year. Since only men whose cancer is confined to the prostatic capsule are eligible for surgery, the stage of disease was controlled by study design. None of the men received neoadjuvant or adjuvant hormone therapy while participating in the study. Data were collected by telephone interview at baseline and at 8 weeks.
Intervention
Men in the control group received usual health care provided by their urologist. In addition to usual health care, the men in the experimental group were paired (to form a dyad) with a former patient (hereafter referred to as the support partner) who had had a radical prostatectomy at least 3 years prior to the study and had experienced similar treatment side effects as the participants. The dyads met eight times during an 8-week period when the support partner was able to vicariously relate successful coping and recovery strategies after prostatectomy. The meetings were held at gourmet-style coffee shops where the men were able to discuss issues and concerns in a semi-private environment that had living room style seating. This setting provided an opportunity for the men to talk in a relaxed atmosphere without feeling rushed. Additional detailed information about the intervention in general, and about the dyadic meetings in particular, have been published elsewhere [9,10].
Measures of quality-of-life
General health-related quality-of-life
General health-related quality-of-life was measured using the 36-item Rand Medical Outcomes Study Short Form (SF-36). The SF-36 has been used extensively in a variety of populations of various age groups and among those with cancer. A principal reason for selecting the SF-36 was in order to attempt to compare the study results across other studies. The measure is a multi-purpose health survey that has four physical subscales (physical function, physical role functioning, bodily pain, general health) and four emotional subscales (vitality, social functioning, emotional role, mental health) that are scored separately. Participants responded to the 36 items on what were either dichotomous or 3–6-point scales. Total scores for the measure ranged from 0–100 with higher scores indicating better quality-of-life. Cronbach's alpha reliability for the subscales ranged from 0.63–0.94 [17].
Prostate cancer-specific quality-of-life
Prostate cancer-specific quality-of-life was measured using the 20-item UCLA Prostate Cancer Index that was originally developed with input from men with prostate cancer and their spouses to assess outcomes in the care of men with prostate cancer. Six scales measured unique domains of concern for men with prostate cancer. The domains were urinary, sexual, and bowel function, and urinary, sexual, and bowel bother by dysfunction. The test-retest reliability of the UCLA Prostate Cancer Index incontinence subscale is high (r = 0.93) and internal consistency is good (a = 0.87). The test-retest reliability of the impotence subscale is high (r = 0.92) and internal consistency is good (a = 0.93).[18] The sensitivity of the disease-specific measure was greater than for general measures of sexual well being, when compared to the Cancer Rehabilitation Evaluation System [19].
Self-efficacy
Self-efficacy was measured using the 38-item Stanford Inventory of Cancer Patient Adjustment. This scale indicated a man's belief in his functional abilities when presented with problems related to cancer (not prostate-specific), including medical treatment, communication, activity, personal management, affective state, and self-satisfaction. Likert ratings ranged from 0 (not at all confident) to 10 (completely confident). The ratings were summed (total scores range from 0 to 380), and a higher score indicated higher self-efficacy. The psychometric properties of this measure have previously been reported [10].
Statistics
To identify potential covariates for the multivariate analyses, Pearson correlations between quality-of-life at post-intervention and particular variables (age, education, self-efficacy, and baseline measures of each of the outcomes of interest such as urinary function) were computed at pre-test to determine the utility of the covariates. The dependent variables were general quality-of-life in terms of the physical (physical function, physical role functioning, bodily pain, general health) and emotional (vitality, social functioning, emotional role, mental health) domains measured by the SF-36, and disease-specific quality-of-life as measured by the UCLA Prostate Cancer Index (urinary function/bother, sexual function/bother, and bowel function/bother). Most measures were significantly skewed and transformation did not correct the problems. Rose et al [20] report that data from the SF-36 that is skewed makes it difficult to analyze using parametric tests. They, along with Streiner [21], suggest that if transformation does not resolve the non-normal distribution problem, then the data should be dichotomized. Thus, scatter plots and histograms were used to identify naturally occurring cut-points in the data, which were used as referents to dichotomize the data into high versus low outcomes for each variable. Logistic regression was then used to estimate the effect that the experimental intervention, age, education, and baseline self-efficacy had on the dichotomized quality-of-life outcomes while controlling for pre-test measures on the outcomes at 8 weeks. Significant interactions were graphed to interpret the interaction of effect (see Figures 1–5, below)[22,23].
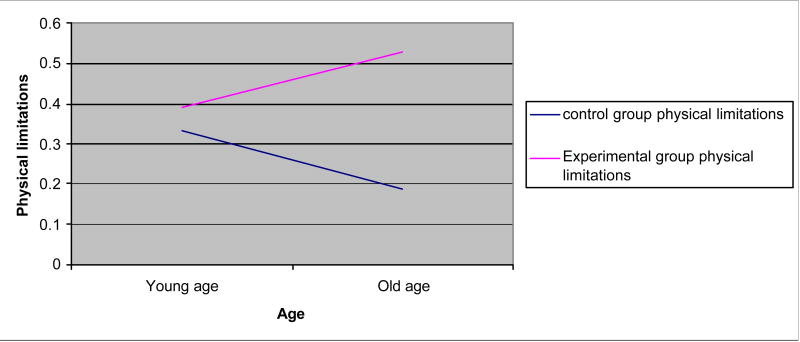
Figure 1.
Interaction effects: physical limitations × age.
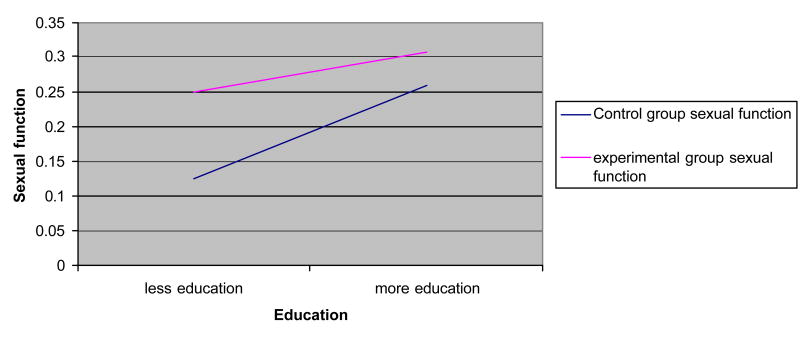
Figure 5.
Interaction effects: sexual function × education.
Results
Demographic characteristics
In all, 152 men met the eligibility criteria, and 81 agreed to participate. Fifty (33%) failed to respond to written or telephone invitations or refused due to lack of interest and 21 (14%) were excluded due to geographic location. During the study, seven men (2 experimental and 5 controls) were lost to follow-up and two relocated resulting in an attrition rate of 8.6 %. Complete data were available on 72 men.
Participants ranged in age from 47 to 74 years (meanage = 60; standard dviation (SD) = 7). Of the sample, 82% was white (n = 52) and 45 % worked full time (n = 33). Education ranged from 34.9% having at least high school education or technical training (n = 25), 32% having some college education (n = 23), and 34 % having a 4-year degree or higher (n = 24). None of the men in either the control or experimental group participated in any non-study support groups during the study period.
General and prostate cancer-specific quality-of-life
There were no significant differences on measures of physical or emotional general quality-of-life between groups at baseline (Table 1). However, baseline role function and vitality were low in both groups indicating poor quality-of-life in these domains. For prostate cancer-specific quality-of-life, the experimental group had significantly better baseline urinary and bowel function than the control group. There were no significant differences on self-efficacy at pre-test.
Table 1.
General quality-of-life group differences at baseline versus post-test
| Variable | Control group | Experimental group | ||
|---|---|---|---|---|
| Baseline Mean (SD) | Post-test Mean (SD) | Baseline Mean (SD) | Post-test Mean (SD) | |
| General quality-of-life | ||||
| Physical function | 69.0 (21.9) | 69.7 (30.0) | 74.7 (22.6) | 75.9 (22.7) |
| Physical role function | 26.4 (34.3) | 41.9 (42.1) | 38.6 (39.9) | 55.7 (44.6) |
| Bodily pain | 64.0 (27.9) | 72.6 (23.2) | 67.2 (28.5) | 69.1 (29.9) |
| General health | 76.9 (18.2) | 76.8 (18.1) | 72.4 (20.8) | 73.6 (16.6) |
| Vitality | 55.4 (20.1) | 63.2 (18.7) | 62.4 (17.5) | 65.6 (16.4) |
| Social function | 71.1 (25.7) | 70.3 (26.4)* | 73.2 (24.8) | 81.8 (22.8) |
| Emotional role | 80.0 (35.4) | 84.7 (31.0) | 71.4 (39.8) | 72.4 (40.8) |
| Mental health | 80.0 (18.1) | 79.7 (15.3) | 79.2 (17.0) | 80.6 (16.2) |
| Prostate cancer-specific quality-of-life | ||||
| Urinary function | 29.5 (18.9)*** | 43.7 (26.7)* | 49.3 (28.8) | 56.9 (24.4) |
| Urinary bother | 32.1 (31.6) | 53.4 (37.3) | 46.4 (35.9) | 53.6 (31.6) |
| Sexual function | 10.4 (10.5) | 13.1 (10.7) | 13.3 (18.6) | 17.0 (20.6) |
| Sexual bother | 41.4 (42.0) | 38.5 (40.4) | 42.9 (39.1) | 38.6 (40.4) |
| Bowel function | 70.2 (20.3)* | 82.1 (20.1) | 80.0 (17.7) | 82.1 (16.2) |
| Bowel bother | 73.6 (26.4) | 77.0 (27.2) | 81.4 (24.5) | 82.1 (24.7) |
P<0.05
P<0.001 for t-tests of group differences
A series of logistic regression analyses (Table 2) were used to explore potential differences in predictor variables, i.e. dyadic intervention, age, education, self-efficacy, and baseline measures of the quality-of-life outcome of interest on quality-of-life at 8 weeks post-test. However, by 8 weeks, self-efficacy had significantly increased for men in the experimental group from baseline to 8-weeks (mean score = 305 and 329, respectively) and significantly declined for the control group over the same time period (mean score = 309 and 300, respectively).
Table 2.
Logistic regression results
| β | SE | P- | Odds | |
|---|---|---|---|---|
| Model 1 (Physical functioning) | ||||
| Baseline | 0.072 | 0.017 | 0.0001 | 1.075 |
| Model 2 (Physical role functioning) | ||||
| Baseline | 0.02 | 0.009 | 0.0099 | 1.023 |
| Group | - | 8.691 | 0.0058 | 0.000 |
| Group * Age | 0.208 | 0.105 | 0.0475 | 1.232 |
| Group* Self-efficacy | 0.040 | 0.018 | 0.0233 | 1.041 |
| Model 3 (Pain) | ||||
| Baseline | 0.047 | 0.015 | 0.0017 | 1.048 |
| Group*Age | 0.218 | 0.109 | 0.0458 | 1.243 |
| Model 4 (General health) | ||||
| Baseline | 0.085 | 0.023 | 0.0002 | 1.088 |
| Model 5 (Sexual function) | ||||
| Baseline | 0.122 | 0.045 | 0.0071 | 1.129 |
| Group*Education | −0.060 | 0.026 | 0.0245 | 0.942 |
| Group*Self-efficacy | −0.050 | 0.023 | 0.0457 | 0.956 |
| Model 6 (Urinary function) | ||||
| Baseline | 0.043 | 0.015 | 0.0056 | 1.044 |
| Model 7 (Bowel function) | ||||
| Group | - | 11.482 | 0.0271 | 0.000 |
| Model 8 (Vitality) | ||||
| Baseline | 0.087 | 0.031 | 0.0058 | 1.091 |
| Model 8 (Social function) | ||||
| Baseline | 0.068 | 0.022 | 0.0023 | 1.070 |
| Group | - | 12.733 | 0.0448 | 0.000 |
| Group*Age | 0.330 | 0.157 | 0.0348 | 1.392 |
| Model 9 (Emotional role) | ||||
| Baseline | 0.029 | 0.009 | 0.0025 | 1.030 |
| Urinary function | 0.033 | 0.016 | 0.032 | 1.034 |
| Model 10 (Sexual bother) | ||||
| Baseline | 0.045 | 0.012 | 0.0001 | 1.046 |
| Education | −0.040 | 0.020 | 0.0478 | 0.991 |
| Model 11 (Urinary bother) | ||||
| Baseline | 0.038 | 0.017 | 0.0276 | 1.039 |
| Urinary function | 0.150 | 0.041 | 0.0003 | 1.162 |
| Model 12 (Bowel bother) | ||||
| Baseline | 0.053 | 0.017 | 0.0021 | 1.055 |
| Bowel function | 0.063 | 0.022 | 0.0048 | 1.065 |
| Model 13 (Mental health) | ||||
| Baseline | 0.097 | 0.031 | 0.0017 | 1.102 |
| Group | - | 13.620 | 0.0056 | 0.000 |
| Age | −0.258 | 0.094 | 0.0058 | 0.773 |
| Group*Age | 0.459 | 0.164 | 0.0051 | 1.583 |
As indicated in Table 2, except for bowel function, the baseline measures of each quality-of-life outcome variable were significant predictors of 8-week outcomes. Next, the dyadic intervention significantly accounted for physical role functioning (odds ratio (OR) < 0.001), bowel function (OR < 0.001), mental health (OR = 0.001), and social function (OR = 1.070).
Interaction effects
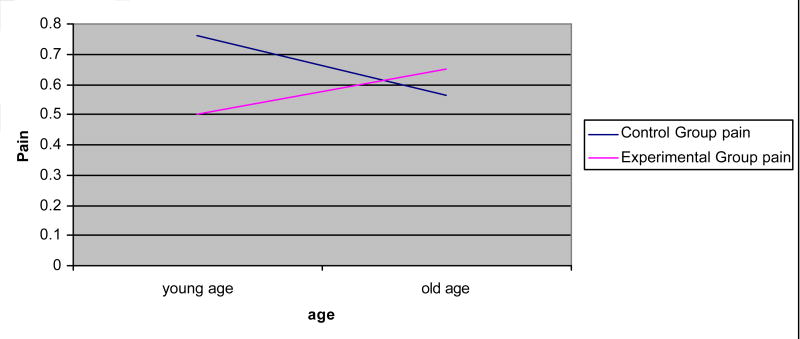
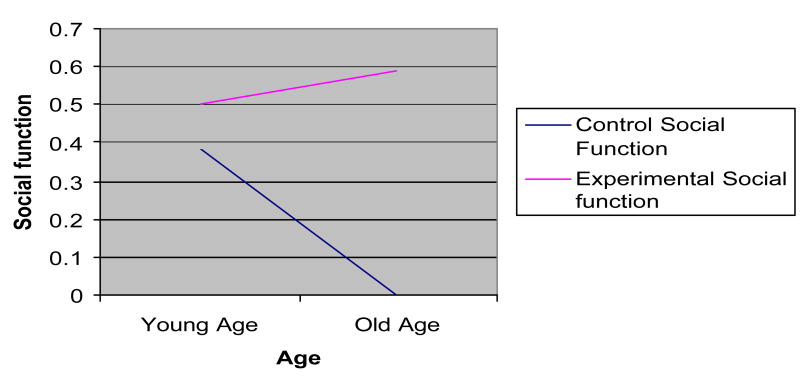
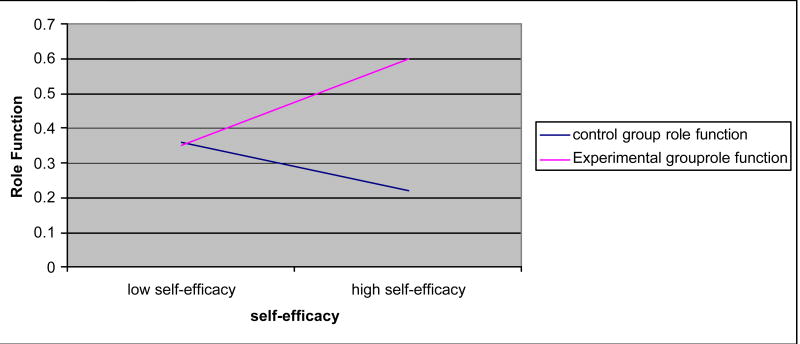
Age, education, and self-efficacy had significant interaction effects for some outcomes (Figures 1–5). Age increased the effects of the dyadic intervention on physical role function, pain, and social functioning, with those being older experiencing fewer physical limitations associated with their role (OR = 1.232), less pain (OR = 1.243), and greater social functioning (OR = 1.392) than younger aged men. Self-efficacy also increased the effect of the intervention. Men who started the intervention with high self-efficacy were better able to function in role-specific activities (OR = 1.041) than men with low self-efficacy. Lastly, education had an effect on the intervention. Men with higher education were less likely to benefit from the dyadic intervention (OR = 0.942) than did men with low education.
Discussion
The effect of dyadic support on quality-of-life
Treatment side effects after radical prostatectomy include urinary, sexual, and bowel dysfunction. These iatrogenic outcomes can undermine a man's self-efficacy since they are new and unfamiliar experiences that he may not know how to manage [16]. Findings from this study show that dyadic support is effective at raising self-efficacy. The dyadic support intervention provided the opportunity to see others successfully manage the aftermath of prostatectomy. Thus, they were able to gain new skills and master similar problems [16,24]. Moreover, the intervention had a significant impact on how men reacted socially and emotionally, thus significantly improving their quality-of-life.
Factors that moderate the intervention effect
Age
Younger men had better overall mental health than older men. However, the intervention was most effective for older men. The dyadic intervention reduced the social isolation, which is commonplace for those of advancing years [25–28].
Education
In this study, men with higher education were less bothered by sexual symptoms after radical prostatectomy. Higher education is associated with patients who are socially advantaged and who have greater access to and utilization of health care services that may have allowed them to receive treatment for sexual dysfunction using strategies such as phosphodiesterase-5 inhibitors (drugs like Viagra), penile implants, pumps, rings, and injections and suppositories [29,30]. Moreover, men with high education may be more likely to receive encouragement from health care providers to try strategies like pelvic floor exercises that improve local blood supply and sexual functioning [31,32].
Self-efficacy
Consistent with Bandura's self-efficacy theory, the effects of the dyadic intervention were enhanced by self-efficacy. Men with high self-efficacy had greater physical role function that may have provided an important resource to deal with the challenges of the side effects of radical prostatectomy. In contrast, men with high self-efficacy had significantly decreased sexual function but the same was true at baseline when there was little to no variation in the men's confidence in their ability to function sexually. Although self-efficacy was expected to enhance the intervention on other outcomes (e.g. urinary and bowel dysfunction), the self-efficacy measure used only captured sexual function and not the urinary and bowel function common in this population. The omission of self-efficacy in relation to the urinary and bowel domains, and no variation on the sexual domain, limited the ability to assess the influence that self-efficacy had on the effects of the intervention on urinary and bowel function and bother.
More than 90% of men in the present study reported moderate to high confidence in discussing their cancer concerns and fears with a close friend or family member. The interaction with their social network may have confounded the support from the dyadic intervention. Several authors have reported that individuals prefer to utilize their own social networks rather than talk to strangers when confronted with traumatic events such as cancer diagnosis and treatment [33–35]. Friends and family members are viewed as better able to relate to individual cancer fears and concerns within the context of the patient's life. In spite of support from a man's social network, the dyadic intervention may have provided additional support that was firmly rooted in the prostate cancer experience [9,10].
Limitations and implications
In this study, the findings must be viewed with caution because of the small sample size that compromises generalizability and the over-representation of educated White men who enrolled in this study. Given the unique circumstances encountered by minority patients in the health care setting, it remains unclear if these findings adequately represent minorities.
Quality-of-life measured with the SF-36 was expected to provide results that could be compared across studies, however, data from the SF-36 in this study were significantly skewed. This was a sample recovering from radical prostatectomy and the subjects may be experiencing similar things captured by the SF-36. Hence, they look different than other healthier populations that may have greater variability in this measure. Transformation did not fix the skewness problem, so the data was dichotomized. According to Streiner [21] and Rose et al. [20], a great deal of information is lost when continuous data is dichotomized, but it is also pointed out that, as in this study, where transformation did not correct the problem, dichotomizing is often necessary. The moderating effects of self-efficacy may have been limited because the Stanford Inventory of Cancer Patient Adjustment reflected overall cancer self-efficacy rather than that specific to prostate cancer.
Additional research is needed that focuses on the short- and long-term emotional consequences of prostate cancer and the effect they have on quality-of-life after prostate cancer treatment. This study assessed the effect of a dyadic support intervention shortly after surgery and the long-term effects of earlier or later implementation were not assessed.
Clinicians should provide comprehensive psychosocial assessment for men who are at greatest risk for developing negative emotional outcomes following radical prostatectomy. Those at greatest risk of adverse outcomes are young men, those without adequate support systems, and men not accustomed to changes in alterations in physical function (e.g. erectile dysfunction associated with comorbid conditions and medications) that define their masculine role.
Figure 2.
Interaction effects: pain × age.
Figure 3.
Interaction effects: social function × age.
Figure 4.
Interaction effects: role function × self-efficacy.
Acknowledgments
Funding support was provided by the National Cancer Institute (R03CA96204). Furthermore, this material is the result of work supported with resources and the use of facilities at the VA HSR&D/RR&D Rehabilitation Outcomes Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bryan A. Weber, University of Florida, Gainesville, FL, USA.
Beverly L. Roberts, University of Florida, Gainesville, FL, USA.
Hossein Yarandi, Wayne State University, Detroit, MI, USA.
Terry L. Mills, University of Florida, Gainesville, FL, USA.
Neale R. Chumbler, VA HSR&D/RR&D Rehabilitation Outcomes Research Center, North Florida/South Georgia Veterans Health System, Gainesville, FL and University of Florida, Gainesville, FL, USA.
Chester Algood, University of Florida, Gainesville, FL, USA.
References
- 1.Arredondo SA, Elkin EP, Marr PL, Latini DM, DuChane J, Litwin MS, et al. Impact of comorbidity on health-related quality of life in men undergoing radical prostatectomy: data from CaPSURE. Urology. 2006;67(3):559–65. doi: 10.1016/j.urology.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Melmed GY, Nakazon T. Life after radical prostatectomy: a longitudinal study. J Urol. 2001;166(2):587–92. [PubMed] [Google Scholar]
- 3.Schapira MM, Lawrence WF, Katz DA, McAuliffe TL, Nattinger AB. Effect of treatment on quality of life among men with clinically localized prostate cancer. Med Care. 2001;39(3):243–53. doi: 10.1097/00005650-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Clark JA, Rieker P, Propert KJ, Talcott JA. Changes in quality of life following treatment for early prostate cancer. Urology. 1999;53(1):161–8. doi: 10.1016/s0090-4295(98)00457-9. [DOI] [PubMed] [Google Scholar]
- 5.Deimling GT, Kahana B, Bowman KF, Schaefer ML. Cancer survivorship and psychological distress in later life. Psycho-oncology. 2002;11(6):479–94. doi: 10.1002/pon.614. [DOI] [PubMed] [Google Scholar]
- 6.Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psycho-oncology. 2006;15(4):306–20. doi: 10.1002/pon.955. [DOI] [PubMed] [Google Scholar]
- 7.Lepore SJ, Helgeson VS. Psychoeducational support group enhances quality of life after prostate cancer. Cancer Res Ther Cont. 1999;8:81–91. [Google Scholar]
- 8.Lepore SJ, Helgeson VS, Eton DT, Schulz R. Improving quality of life in men with prostate cancer: a randomized controlled trial of group education interventions. Health Psychol. 2003;22(5):443–52. doi: 10.1037/0278-6133.22.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber BA, Roberts BL, Resnick M, Deimling G, Zauszniewski JA, Musil C, et al. The effect of dyadic intervention on self-efficacy, social support, and depression for men with prostate cancer. Psycho-oncology. 2004;13(1):47–60. doi: 10.1002/pon.718. [DOI] [PubMed] [Google Scholar]
- 10.Weber BA, Roberts BL, Yarandi H, Mills TL, Chumbler N, Wajsman Z. The impact of dyadic social support on self-efficacy and depression after radical prostatectomy. Aging Health. 2007 doi: 10.1177/0898264307300979. In press. [DOI] [PubMed] [Google Scholar]
- 11.Yang BK, Crisci A, Young MD, Silverstein AD, Peterson BL, Dahm P. Cross-sectional survey of long-term quality of life after radical perineal prostatectomy. Urology. 2005;65(1):120–5. doi: 10.1016/j.urology.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 12.Jayadevappa R, Chhatre S, Whittington R, Bloom BS, Wein AJ, Malkowicz SB. Health-related quality of life and satisfaction with care among older men treated for prostate cancer with either radical prostatectomy or external beam radiation therapy. BJU Int. 2006;97(5):955–62. doi: 10.1111/j.1464-410X.2006.06128.x. [DOI] [PubMed] [Google Scholar]
- 13.Bandura A. Self-efficacy: The Exercise of Control. New York: W. H. Freeman and Company; 1997. [Google Scholar]
- 14.Holahan CK, Holahan CJ. Self-efficacy, social support, and depression in aging: a longitudinal analysis. J Gerontol. 1987;42(1):65–8. doi: 10.1093/geronj/42.1.65. [DOI] [PubMed] [Google Scholar]
- 15.Helgason AR, Dickman PW, Adolfsson J, Steineck G. Emotional isolation: prevalence and the effect on well-being among 50–80-year-old prostate cancer patients. Scand J Urol Nephrol. 2001;35(2):97–101. doi: 10.1080/003655901750170407. [DOI] [PubMed] [Google Scholar]
- 16.Roberts KJ, Lepore SJ, Helgeson VS. Social-cognitive correlates of adjustment to prostate cancer. Psycho-oncology. 2006;15(3):183–92. doi: 10.1002/pon.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHorney CA, Ware JE, Jr, Rogers W, Raczek AE, Lu JF. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992;30 5:MS253–65. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 18.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health related-quality of life measure. Med Care. 1998;36(7):1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Schag CA, Heinrich RL. Development of a comprehensive quality of life measurement tool: CARES. Oncology. 1990;4(5):135–8. [PubMed] [Google Scholar]
- 20.Rose MS, Koshman ML, Spreng S, Sheldon R. Statistical issues encountered in the comparison of health-related quality of life in diseased patients to published general population norms: problems and solutions. J Clin Epidemiol. 1999;52(5):405–12. doi: 10.1016/s0895-4356(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 21.Streiner DL. Breaking up is hard to do: the heartbreak of dichotomizing continuous data. Can J Psychiatry. 2002;47(3):262–6. doi: 10.1177/070674370204700307. [DOI] [PubMed] [Google Scholar]
- 22.Hosemer DW, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley & Sons; 2000. [Google Scholar]
- 23.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousands Oaks, CA: Sage; 1991. [Google Scholar]
- 24.Maddux JE, Lewis J. Self-efficacy and adjustment: basic principles and issues. In: Maddux JE, editor. Self-Efficacy Adaptation, and Adjustment Theory, Research, and Application. New York: Plenum Press; 1995. pp. 37–68. [Google Scholar]
- 25.Dunkle RE, Roberts BL, Haug M, Raphelson M. An examination of coping resources of very old men and women: their association to the relationship between stress, hassles and function. J Women Aging. 1992;4(3):79–104. [Google Scholar]
- 26.McPherson M, Smith-Lovin L, Brashears M. Social isolation in America: changes in core discussion networks over two decades. Am Sociol Rev. 2006;71:353–75. [Google Scholar]
- 27.Avlund K, Due P, Holstein BE, Heikkinen RL, Berg S. Changes in social relations in old age. Are they influenced by functional ability? Aging Clin Exp Res. 2002;14 3:56–64. [PubMed] [Google Scholar]
- 28.Bosse R, Aldwin CM, Levenson MR, Spiro A, III, Mroczek DK. Change in social support after retirement: longitudinal findings from the Normative Aging Study. J Gerontol. 1993;48(4):210–7. doi: 10.1093/geronj/48.4.p210. [DOI] [PubMed] [Google Scholar]
- 29.Steenland K, Rodriguez C, Mondul A, Calle EE, Thun M. Prostate cancer incidence and survival in relation to education (United States) Cancer Causes Control. 2004;15(9):939–45. doi: 10.1007/s10552-004-2231-5. [DOI] [PubMed] [Google Scholar]
- 30.Greenwald HP, Borgatta EF, McCorkle R, Polissar N. Explaining reduced cancer survival among the disadvantaged. Milbank Q. 1996;74(2):215–38. [PubMed] [Google Scholar]
- 31.Dorey G, Speakman M, Feneley R, Swinkels A, Dunn C, Ewings P. Pelvic floor exercises for treating post-micturition dribble in men with erectile dysfunction: a randomized controlled trial. Urol Nurs. 2004;24(6):490–7. 512. [PubMed] [Google Scholar]
- 32.Dorey G, Speakman MJ, Feneley RC, Swinkels A, Dunn CD. Pelvic floor exercises for erectile dysfunction. BJU Int. 2005;96(4):595–7. doi: 10.1111/j.1464-410X.2005.05690.x. [DOI] [PubMed] [Google Scholar]
- 33.Wessely S, Deahl M. Psychological debriefing is a waste of time. Br J Psychiatry. 2003;183:12–4. doi: 10.1192/bjp.183.1.12. [DOI] [PubMed] [Google Scholar]
- 34.Kangas M, Henry JL, Bryant RA. The course of psychological disorders in the 1st year after cancer diagnosis. J Consult Clin Psychol. 2005;73(4):763–8. doi: 10.1037/0022-006X.73.4.763. [DOI] [PubMed] [Google Scholar]
- 35.Kangas M, Henry JL, Bryant RA. Predictors of posttraumatic stress disorder following cancer. Health Psychol. 2005;24(6):579–85. doi: 10.1037/0278-6133.24.6.579. [DOI] [PubMed] [Google Scholar]