Abstract
Background and Purpose
To examine the radiosensitivity of skin cells obtained directly from the irradiated skin of patients undergoing fractionated radiation treatment prior to surgery for treatment of soft tissue sarcoma (STS) and to determine if there was a relationship with the development of wound healing complications associated with the surgery post-radiotherapy.
Methods
Micronucleus (MN) formation was measured in cells (primarily dermal fibroblasts) obtained from human skin at their first division after being removed from STS patients during post radiotherapy surgery (2-9 weeks after the end of the radiotherapy). At the time of radiotherapy (planned tumor dose - 50 Gy in 25 daily fractions) measurements were made of surface skin dose at predetermined marked sites. Skin from these sites was obtained at surgery and cell suspensions were prepared directly for the cytokinesis-blocked MN assay. Cultured strains of the fibroblasts were also established from skin nominally outside the edge of the radiation beam and DNA damage (MN formation) was examined following irradiation in vitro for comparison with the results from the in situ irradiations.
Results
Extensive DNA damage (MN) was detectable in fibroblasts from human skin at extended periods after irradiation (2-9 weeks after the end of the 5-week fractionated radiotherapy). Analysis of skin receiving a range of doses demonstrated that the level of damage observed was dose dependent. There was no clear correlation between the level of damage observed after irradiation in situ and irradiation of cell strains in culture. Similarly, there was no correlation between the extent of MN formation following in situ irradiation and the propensity for the patient to develop wound healing complications post surgery.
Conclusions
Despite the presence of DNA damage in dermal fibroblasts weeks after the end of the radiation treatment, there was no relationship between this damage and wound healing complications following surgery post irradiation. These results suggest that factors other than the radiosensitivity of the skin fibroblasts likely also play a role in wound healing in deep wound sites associated with surgery for STS following radiation therapy.
Keywords: fibroblasts, radiosensitivity in situ, micronuclei, wound healing, bystander effects
INTRODUCTION
Preoperative irradiation is the preferred treatment for most patients with large soft tissue sarcomas (STS) treated at the Princess Margaret Hospital/Mount Sinai Hospital in Toronto. However, a significant proportion of patients treated with this procedure develop wound healing complications (WHC) [40]. The alternative of giving the radiation treatment post-surgery has led to a significant incidence of late radiation-induced fibrosis and bone fracture; an incidence that is much reduced in preoperatively-treated patients, presumably because of the smaller total radiation dose ordinarily delivered in the preoperative setting [11, 22]. Methods for predicting patient-specific potential for WHC following radiotherapy would be valuable in therapy selection for STS patients. Attempts to establish in vitro predictive assays for normal tissue response to radiotherapy have been based on fibroblast clonogenic survival, differentiation and DNA damage [3, 9, 42, 44]. Results of these studies have been mixed with conclusions ranging from relationships between in vitro endpoints and normal tissue reactions [5, 25] to no useful associations [42, 45]. Some reviews emphasize the importance of analyzing orchestrated response (i.e., cytokines dynamics and genes expression) rather than target-cell approach for prediction of normal tissue reactions [4, 44]. It has also been suggested that most normal tissue complications arise in patients with normal radiosensitivity simply because every patient has a certain probability of responding severely [12]. However, animal studies have suggested that the effects of radiation on the clonogenic capacity of fibroblasts may be responsible for the delay in wound healing following radiotherapy and surgery [10, 17].
Radiation leads to reduced fibroblast proliferative capability and function and this might be expected to impact negatively on the normal tissue response [10, 17]. In a previous study we demonstrated that skin fibroblasts from individuals who developed wound healing complications following pre-operative radiotherapy had no detectable differences in radiosensitivity in vitro but tended to show a smaller reduction in early proliferation after irradiation [2]. A recent ‘validation’ study showed a similar trend although the number of new patients studied was small [1]. We hypothesized that increased proliferative potential may render the fibroblasts less capable of differentiating to produce the collagen necessary for effective wound healing. However, we could not rule out the possibility that the radiosensitivity of the fibroblasts was affected by in vivo conditions that were not reflected in our studies done with fibroblast strains irradiated and analyzed in culture. Consequently in the current study we analyzed DNA damage (micronuclei) in fibroblasts obtained directly from the irradiated skin of 31 STS patients and compared the results with the extent of DNA damage induced by irradiation in vitro of fibroblast strains derived from the skin of the same patients.
MATERIALS AND METHODS
Patients, clinical background
After Institutional Research Ethics Board approval, human skin biopsies were obtained from 31 patients undergoing treatment (radiation and surgery) for STS at the time of surgery (at 2-9 weeks after the end of radiation therapy). The cohort included 18 males and 13 females, with mean age: 53.2 (range: 19-87 years), 12 cases of upper limb and 17 cases of lower limb STS. Biopsies were obtained from skin being discarded at the time of surgery from regions in the irradiation field adjacent to the wound margin, at the edge of the irradiation field and outside the irradiation field, and stored in cold Hanks salt solution until processing within 2-5 hours. These regions had been predetermined based on the radiation treatment plan and marked on a life-size mold made of the limb involved. Doses received at the skin surface at the marked points were measured directly (see below) and the mold was placed on the patient during the surgical procedure to identify the location of the biopsies. The planned radiation dose (to tumor) was 50 Gy in 25 fractions over 5 weeks. For wound healing morbidity, patients were categorized into two groups namely: WHC—wound healing complications requiring either further surgery or prolonged deep wound packing; and No WHC—no detectable WHC as described previously [2, 40]. Although these criteria are objective they inevitably include a degree of subjectivity in the decision of the particular surgeon involved.
Dose measurements at different depths
Radiation treatment planning (conformal, non-IMRT) for each patient was performed jointly by the radiation oncologists and surgeons and in particular the location of the planned incision site was identified. A mold of the treatment site of the patient was prepared prior to CT scan and the surgeon marked the planned surgical incision on the mold. Thermo-Luminescent Dosimeters (TLDs) LiF:Mg,Ti were then used during the radiotherapy treatment to measure in-vivo surface doses on the patient in the region of the planned incision. The TLDs had 3.15mm2 square area and 0.14mm thickness and were calibrated on 6 MV X-rays, the same energy used for the radiation treatment of the patient. For in-vivo surface dose measurement seven to eight points of interest were selected on the marked area. Before radiation treatment the TLDs were placed on the patient’s skin on each of the selected points. The TLDs were removed after radiation treatment and the doses were read. This process was repeated for three consecutive days of radiation treatment. For a given point an average of the three days readings was recorded as the in vivo dose for that point. Since the TLDs could not be physically inserted several phantoms were used to determine the dose at the near-skin depths of 1mm, 4 mm and 10 mm in relation to the surface dose. One of them was an anthropomorphic leg phantom in which the TLDs were inserted at the near-skin depths and doses were measured. The leg phantom was also used to simulate patient treatment geometry and validate the calculated dose with the delivered dose. The calculated near-skin dose agreed with the near-surface measurements on the leg phantom within ±3%. Since the treatment was delivered as a fractionated schedule with an equal number of fractions there were different fraction sizes in different regions of the skin so we also calculated equivalent single (E. S.) doses based on the linear-quadratic relationship assuming values for α/β of 3 or 10 Gy [20].
Isolation of in situ fibroblasts and micronucleus assay
Fibroblast isolation from skin biopsies and the cytokinesis-blocked micronucleus (MN) assay were performed as described previously [1, 2, 26, 27], with minor modifications. Briefly each biopsy (about 0.75 cm2) was minced with sterile scissors and treated with an enzyme cocktail of 0.06 mg/ml collagenase and 0.5 mg/ml DNAse (Sigma, St Louis, USA) in αMEM with 2000 mg/ml glucose, 100 mg/ml streptomycin and 100 mg/ml penicillin at 37°C for 1.5 hours (mixing every 30 minutes). The digest was then strained into 50 ml tubes containing 5 ml of ice-cold αMEM supplemented with 20% fetal bovine serum (CanSera, Etobicoke, Canada) (termed fibroblast isolation medium) on ice via a 70 μm nylon strainer (BD Falcon, Bedford, USA). Remaining tissue was further digested in 1 mg/ml trypsin (Sigma, St Louis, USA) at 37°C for 30 minutes (mixing every 10 minutes). Digestion was stopped by adding an equal amount of fibroblast isolation medium plus 200 μl of DNAse stock solution (20 mg/ml), and the cells were strained into the same tube. After centrifugation, the cell pellet was resuspended in fibroblast isolation medium and plated into chamber slides (Nunc, Rochester, USA) for the micronucleus assay and/or placed into 25cm2 culture flasks (Nunc, Rosklide, Denmark) for primary culture. The chamber slides were washed and fresh medium containing 3.2 μg/ml cytochalasin B (Sigma, St.-Louis, USA) was added after 24 hrs and 72 hrs later the slides were washed with PBS, incubated in hypotonic KCl (5.56 mg/l) for 10 min. and fixed in cold methanol for 1.5 min. Just prior to scoring of MN, the slides were stained with Acridine orange (BD, Sparks, USA) for 2 min., washed in PBS and mounted in PBS. Micronuclei were scored in up to 1000 binucleate cells and were defined as rounded bodies, no more than 1/3 of the size of the nucleus, having staining color and intensity identical to the staining of nuclei and completely detached from nuclei [16]. The small number of binucleate cells apparently containing more than 6 MN were not scored because it is very difficult to confidently interpret them as micronuclei in a single binucleated cell. The total MN score per 1000 binucleated cells as well as per cent of cells containing MN were calculated. Personnel conducting these studies were blinded to the doses received by the skin biopsies knowing only that they had come from regions nominally ‘in-field’, ‘edge of field’, or ‘out-of-field’.
Establishment of primary culture
Primary cultures were established from patients skin samples that were nominally out of the treatment field. Measured (fractionated) doses given to these skin samples ranged from 0.19 to 14.02 Gy. The cells were grown in the 25cm2 culture flasks until confluency and then trypsinized and transferred to 75cm2 culture flasks until almost confluent. They were then trypsinized, resuspended in FCS+10% DMSO and stored cryogenically until future use. Cells from the different patients required 16-37 days to establish these frozen cell strains. Of the 31 patients in the study, only 23 provided skin samples that could be used to establish these primary cultures.
Irradiation and Analysis of fibroblasts in vitro
Cultures were thawed and grown in 25cm2 culture flasks in αMEM, supplemented with 10% fetal bovine serum until confluent, then trypsinized and divided onto two 25cm2 culture flasks. Upon confluence, the flasks were incubated overnight in 15 cc of fresh αMEM. The flasks were sealed, irradiated at room temperature using a Gammacell 40 Exactor 137Cs γ–irradiator to a dose of 2.4 Gy (dose rate 0.94 Gy/min), incubated for 4-5 hours at 37°C to allow for recovery, trypsinized and plated onto 12×12 mm cover slips in 35 mm culture dishes. At 3 hours after plating, medium was replaced with fresh medium containing 3.2 μg/ml of cytochalasin B. After 40-42 hours the cells were incubated in hypotonic (5.56 mg/l) KCl for 10 minutes and fixed in cold methanol for 1.5 minutes. This procedure is similar to that described previously [2]. For scoring of MN, the fixed cover slips were stained with Acridine orange for 2 minutes, washed in PBS, mounted in PBS on glass microscopic slides and sealed by Cytoseal-60 (Richard-Allan Scientific, Kalamazoo, USA). Upon scoring of MN, binucleated and mononucleated cells were counted, and BNI was calculated as the percent of binucleated cells in the total population of binucleated and mononucleated cells. Studies were also performed with non confluent fibroblasts plated onto 12×12 mm cover slips in 35 mm culture dishes. The cells were allowed to attach for 3-4 hours and then irradiated on ice using Gammacell 100 Elite 137Cs γ–irradiator to doses of 0.25-6 Gy (dose rate 2.7 Gy/min). Immediately after irradiation, medium was replaced with fresh medium containing cytochalasin B, and the micronucleus assay was conducted as described for confluent fibroblasts.
Statistical analysis
For associations, linear and non-linear regression analysis was used. For comparison of two independent samples, the Mann–Whitney rank sum test was used; for comparison of matched samples, Wilcoxon signed-ranks test for matched pairs was used (Prizm 4 for Windows software, version 4.03). Association between wound healing complications and various parameters was assessed using logistic regression for numeric data [23; statpages.org/logistic.html] and Fisher’s exact test for nominal data (www.matforsk.no/ola/fisher.htm). P-values were from two-sided tests. The dose response curves were fitted to three different models (linear; [MN/1000BN = a D + b], linear quadratic; [MN/100 BN = aD2 + bD + c]; and exponential; [MN/1000BN = a ekD], where a,b and c are constants). Confidence intervals for correlation coefficients of these models were obtained using Fisher’s z’ transformation: (http://icp.giss.nasa.gov/education/statistics/page3.html), (http://davidmlane.com/hyperstat/B8544.html).
For comparison of the fitted models, the Akaike information criterion (AIC) was used (http://www.theses.ulaval.ca/2004/21842/apa.html). AIC compares fitting quality while accounting for the different number of coefficients in the models and thus compensating for over-fitting by a model with more coefficients. The difference (delta) between AIC values for different fitting models determines whether one model can be chosen in favor of another. Delta exceeding 10 suggests a strong preference for the model with the lesser AIC value, whereas delta less than 2 suggests no preference for model [6].
RESULTS
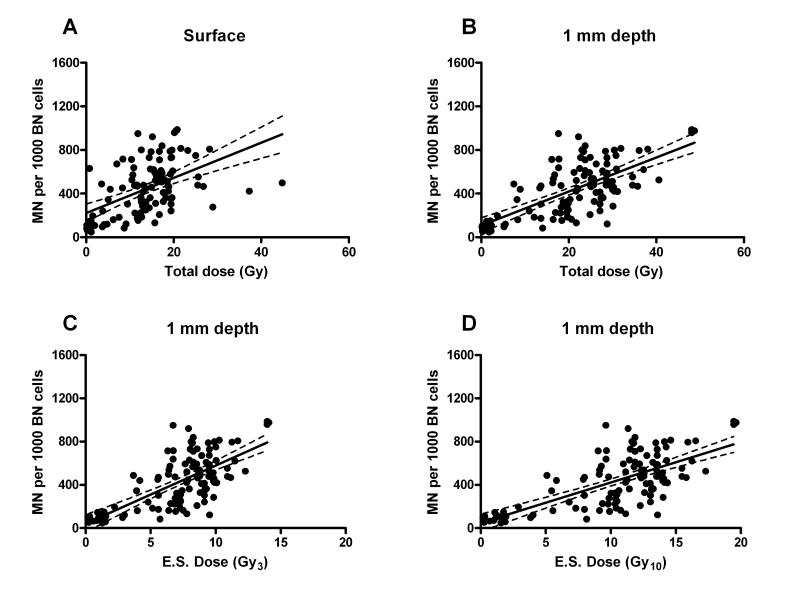
Taken together the total MN scores for fibroblasts irradiated in situ showed a significant correlation with dose measured at 0 mm and calculated at 1 mm depth (Fig. 1 A,B) but not at 4 mm or 10 mm (data not shown). The dose at 1 mm depth is likely to be the biologically-relevant dose because the depth of the dermis (from where fibroblasts are primarily being analyzed) is approximately 0.1 to 1.5 mm from the surface [14, 18, 24, 30, 41]. Furthermore, a depth of 1-2 mm has been reported in the literature as relevant for skin irradiation [19, 37]. Consequently we have used the dose at 1 mm depth for our further analyses.
Fig. 1.
MN in freshly isolated in situ fibroblasts in all available biopsies. MN number per 1000 of BN cells is plotted against fractionated dose at depth of 0 mm (A) and 1 mm (B) and against equivalent single dose (E.S.) at 1 mm depth for α/β=3 (C) (Gy3) or α/β=10 (D) (Gy10). The line from the linear fit model with 95% confidence intervals is shown on each plot.
Replacing the actual total fractionated dose with calculated equivalent single dose did not change the pattern of the plot (Fig. 1 C,D). The two equivalent single dose calculations for α/β=3 or 10 Gy demonstrated very similar correlations ( compared to 0.481 respectively). Since the α/β value for skin is generally expected to be in the region of 10 for an early responding tissue [20] and wound healing is an early response, the equivalent single dose calculated for α/β=10 was used for further analysis.
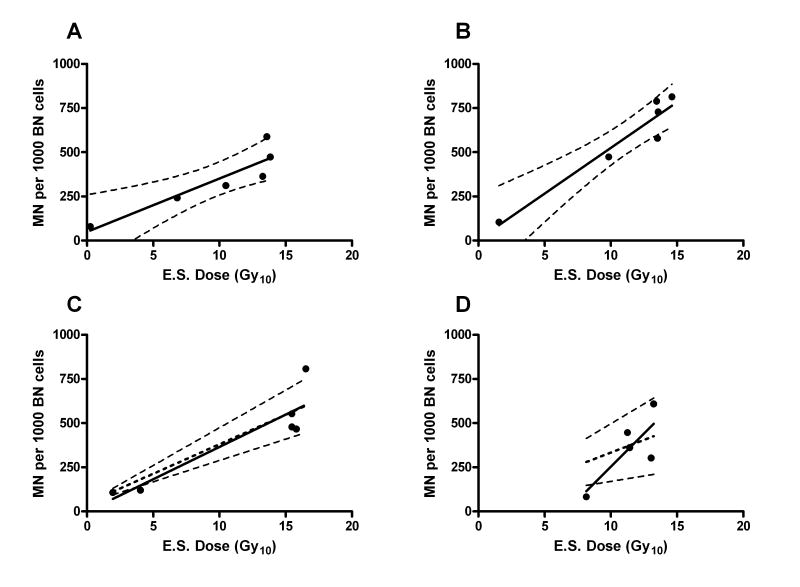
For the total dataset, both the linear fit and the exponential fits were significant (P<0.01), (unlike the linear-quadratic fit), but the linear fit was favored by the AIC test (delta AIC >10). For 2 of the 31 patients, the generation of individual dose response curves was not possible because of limited data. For the remaining 29 patients individual dose-response curves were generated (see examples in Fig. 2) and these were also fitted to a linear model. If the fitting produced a negative intercept, making no biological sense, a forced intercept was set equal to average intercept for the total dataset (47.4 MN/1000 BN). Otherwise, actual values of the intercept and the linear slope were used. The intercept (for in situ fibroblasts) was subtracted from the response values, and the dose required to achieve 400 MN/1000BN was then determined from the fitted line (see Fig. 3).
Fig. 2.
MN in freshly isolated in situ fibroblasts, individual curves for different patients. MN number per 1000 of BN cells is plotted against equivalent single (E.S.) dose (Gy10 for calculation based on α/β=10). A line from a linear fit model with 95% confidence intervals is shown on each plot. Dashed linear trend on panels C and D shows linear fit using intercept forced to the average value of the whole dataset (Fig 1D).
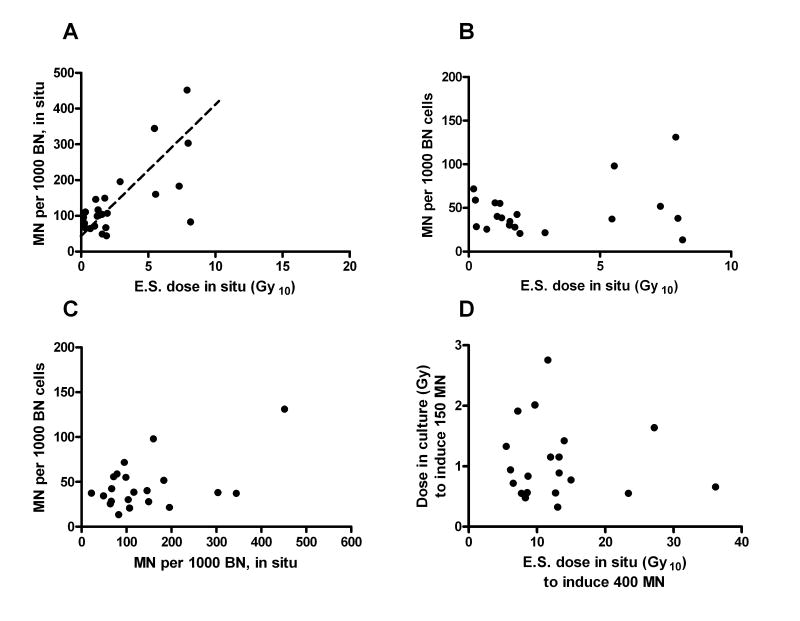
Fig. 3.
(A) MN in in situ fibroblasts used for culturing plotted against pre-op equivalent single (E.S.) dose (Gy10 for calculation based on α/β=10) (regression trend from fig 1D for the total dataset in situ is shown as a dashed line); (B) background MN in confluent cultured fibroblasts plotted against pre-op equivalent single (E.S.) dose (α/β=10); (C) background MN in confluent cultured fibroblasts plotted against corresponding values for in situ fibroblasts; (D) dose required to induce 150 MN per 1000 BN cells in confluent cultured fibroblasts vs. equivalent single (E.S.) dose (α/β=10) required to induce 400 MN per 1000 BN cells in in situ fibroblasts (in cultured fibroblasts, background was subtracted from the response values).
The MN burden of in situ fibroblasts, that were used for culturing, correlated with the received dose in situ ( , P<0.01) (see Fig. 3A), and was considered a part of the overall in situ dose-response curve (Fig. 1). However, background MN for the cultured fibroblasts did not correlate either with MN burden for the corresponding in situ fibroblasts (Fig. 3B) or with the dose the skin sample received in situ. Also, the irradiation-induced MN in culture did not correlate with the background MN level in culture (Fig. 3C). Finally, no correlation was found between the radiation response in culture (equivalent single dose to induce 150 MN above background estimated by linear fitting of the data) and that in situ (equivalent single dose to induce 400 MN determined from the linear fit to the data as described above) (Fig. 3D). This finding was not different for confluent vs non-confluent cultures (data not shown).
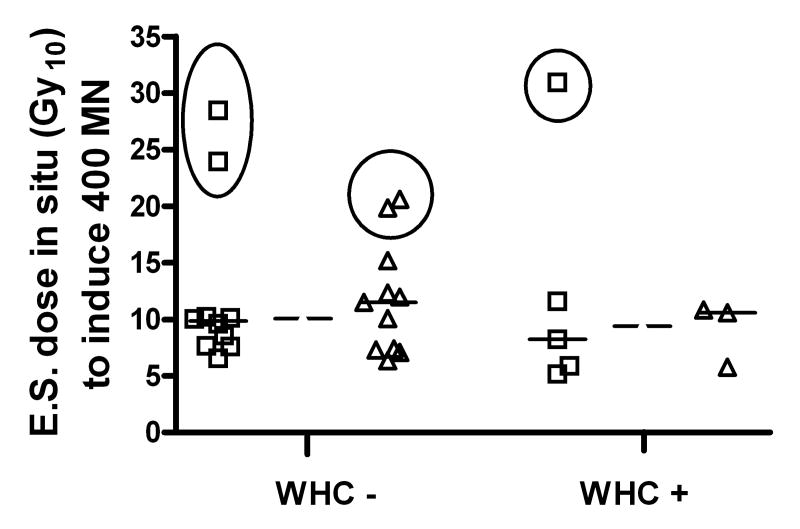
Wound healing complications (WHC) following surgery occurred in 8 of the 31 patients (26%) included in this study; a percentage which is similar to that reported by our group previously for similarly treated patients [40] and in a recent retrospective analysis [48]. The median age and sex profile of the patients and median time between irradiation and surgery did not differ between WHC- and WHC+ groups. The previous studies [40, 48] identified a higher level of WHC in patients with lower limb sarcomas but the number of patients involved in the current study was too small to detect such a difference. Maximum measured skin dose was also not different between WHC- and WHC+ groups. Importantly, there was no significant difference in dermal fibroblast radiosensitivity between WHC- and WHC+ groups of patients (Fig. 4) even upon removal of the high values where extrapolation of the linear fit was necessary to obtain the dose required to induce 400 MN and may therefore contain more error. Similarly, no significant differences were observed between WHC- and WHC+ groups for other analyzed characteristics of MN and BNI, in situ and in culture (Table 1). The data was also examined by stratification according to in situ radiosensitivity (defined as the dose to induce 400 MN/1000BN cells) consistent with suggestions of Dikomey et al [12]. The frequency of WHC did not differ in patients with in situ radiosensitivity above and below the median value (Table 2).
Fig. 4.
Relationship between wound healing complications (WHC) and induced MN response in in situ fibroblasts. Equivalent single dose (Gy10 for calculation based on α/β=10) required to induce 400 MN per 1000 BN cells using actual linear fit intercepts (◻) and using a forced intercept equal to the average intercept for the total dataset (△) are shown separately. Data in circles are the cases when extrapolation beyond the data points needed to be done to obtain the value. Medians are marked for each group separately (solid lines) and for pooled data for the patients without WHC or with WHC (dashed lines).
Table 1.
Relationship between occurrence of wound healing complications (WHC) and micronuclei (MN) and binucleation index (BNI) in fibroblasts in situ used for culturing and in cultured fibroblasts
| No WHC | N | WHC | N | |
|---|---|---|---|---|
| MN Burden in in situ fibroblasts used for culturing | 99 (22 - 452) | 17 | 93 (44 - 196) | 6 |
| 1Induced MN response in confluent cultures (Gy10) | 0.9 (0.6 - 2.8) | 14 | 0.7 (0.3 - 1.9) | 7 |
| 2Induced BNI response in confluent cultures (%) | 84 (65 - 91) | 15 | 84 (79 - 90) | 5 |
| 1Induced MN response in non-confluent cultures (Gy10) | 0.9 (0.6 - 2.6) | 15 | 1.0 (0.7 - 1.3) | 2 |
| 2Induced BNI response in non-confluent cultures (%) | 59 (31 - 84) | 17 | 65 (47 - 81) | 6 |
Medians are shown (range in brackets).
Induced MN response is expressed as equivalent single dose (α/β=10, Gy10) required to induce 150 MN per 1000 BN cells for cultured fibroblasts.
Induced BNI response is expressed as per cent decrease in BNI at 1 Gy in non-confluent cultures and as per cent decrease in BNI at 2.4 Gy in confluent cultures.
Table 2.
Relationship between occurrence of wound healing complications (WHC) and stratified micronuclei (MN) response in fibroblasts in situ
| No WHC | WHC | |
|---|---|---|
| Induced MN response (Gy10) at or below median | 11 | 4 |
| Induced MN response (Gy10) above median | 10 (7) | 4 (3) |
Induced MN response is expressed as equivalent single dose (α/β=10, Gy10) required to induce 400 MN per 1000 BN. Values in brackets represent occurrence of WHC after exclusion of extrapolated cases of MN response.
DISCUSSION
The present work follows up our previous studies examining possible relationships between fibroblast radiosensitivity and the development of wound healing complications observed in soft tissue sarcoma patients treated with pre operative radiotherapy [1, 2]. Our previous work studied fibroblasts in culture and demonstrated a trend for a relationship with the proliferative capacity of the fibroblasts (as measured by the BNI) following irradiation but found no evidence for a correlation with radiosensitivity measured by clonogenic assay or MN formation. It is possible, however, that the in situ microenvironment of the dermal fibroblast might affect their radiosensitivity by a mechanism not present in culture. Recent work has suggested that integrin signaling, such as may occur in interaction between cells and the extracellular matrix can affect radiosensitivity [8]. Thus we were interested to study the radiosensitivity of the dermal fibroblasts irradiated in situ.
For this purpose we isolated cells directly from the skin of patients undergoing surgery following radiotherapy and subjected them to a cytokinesis-blocked MN assay within a few hours after removal from the patient, so that we observed the DNA damage present in the cells at their first division in culture. Since this analysis was only possible at the time of surgery (2-9 weeks but mostly 4-8 weeks after the end of the 5-week course of irradiation) it was of considerable interest that we were still able to detect substantial DNA damage in the skin fibroblasts at this time. This result is consistent with our studies in rat skin, which have demonstrated that damage can be detected out to at least 9 months after irradiation (Kaspler and Hill in preparation 2007). This finding suggests that, because skin fibroblasts are largely quiescent, the DNA damage caused by the irradiation has remained in the cells throughout the time between irradiation and assay after surgery and/or that damage is being repaired and regenerated post irradiation. Our previous work using a MN assay with rat lung fibroblasts has indicated that repeated regeneration of DNA damage can occur in lung tissue following irradiation in situ [7, 28]. Such results are consistent with work on the bystander effect in vitro that suggests that following irradiation there can be chronic production of ROS that could cause cellular DNA damage [35, 36]. Recent studies in human skin have demonstrated evidence for prolonged oxidative stress following irradiation [29].
In our study, we had an opportunity to compare MN formation in fibroblasts directly from the patient and in fibroblast strains after culturing the cells from the same patient in vivo. No correlation was observed between radiosensitivity in situ and in culture (Fig 3D) but we did observe that higher equivalent single doses were required in situ to induce MN in the skin than in fibroblasts irradiated in culture. The dose required to induce 150 MN per 1000 BN cells was about 5 times lower for the cultures than for the corresponding in situ fibroblasts (P<0.0001). Because the time between irradiation and MN analysis in situ and in culture was very different (weeks in situ and hours in culture), it is possible that DNA damage repair occurring in the quiescent in situ fibroblasts resulted in lower numbers of MN. However, we did not observe any difference in the in situ dose-response curves between the patients with short post-irradiation time (14 – 35 days) and those with long post-irradiation time (50 – 65 days) which suggests that MN levels could be stable at least from 2 weeks to 2 months after irradiation. Similarly we have observed in rat skin that the level of DNA damage after irradiation does not decline until at least 1 month after irradiation (Kaspler and Hill, in preparation, 2007). This suggests that comparison between MN irradiation response of in situ and cultured fibroblasts can be relevant despite the difference in time between irradiation and analysis. It is possible that the higher MN response in culture than in situ at a given dose may be due to higher proliferation of cultured cells following irradiation, in contrast to quiescent cells in situ.
The “out-of-field” biopsies, the source of cultured fibroblasts, received some (scattered) dose during radiotherapy (0.19-14.02 Gy fractionated dose or 0.19 – 8.16 Gy10 equivalent single dose), and this generated variability in MN score (burden), which correlated with pre-op dose (R2 = 0.4729, P<0.01). However, a relationship between the background MN in culture and the corresponding MN burden in situ was not observed for confluent cultures (Fig. 3C). The effects of the confluent state may be due to the effect of cell synchronization into G0/G1 phase, which occurs with fibroblasts under such conditions. The lack of correlation between in situ and in culture values was not due to damage depletion during culturing because the background MN in confluent cultures did not correlate with the time or number of passages needed to establish primary cultures (similar to the studies of O’Driscoll et al. [39]), nor with the time needed to grow thawed fibroblasts for in culture studies. In addition, no decrease in background MN burden was observed when five strains of the fibroblasts were cultivated for four passages (up to 4-6.5 weeks of culture) without irradiation (data not shown). These results suggest that genomic damage is persisting during culture consistent with studies by others of surviving descendants of irradiated cells in vitro and in vivo [13, 15, 33, 34, 38, 49, 50], even after a dose as low as 0.5 Gy [43], although only a fraction of the cell progeny (about 30-60%, depending on cell type and dose) may carry this damage [21, 32]. Such persistent instability is also consistent with work on the bystander effect and could be due to factors released into the medium during culturing. [31, 35, 36, 38, 47].
We observed no significant correlation between the radiosensitivity of the dermal fibroblasts irradiated in situ and the development of WHC in the patients regardless of whether we split the group according to WHC (Fig. 4) or according to median radiosensitivity (Table 2). Since the number of patients (29) contributing to this part of the study was relatively small this negative finding has low power but the overlap of the data indicates that even if a significant correlation did exist the difference in radiosensitivity is too small (or the assay error is too large) for it to be used as a predictive factor. We also observed no relation between WHC and the maximum dose measured on the irradiated skin for the individual patients since these in-field skin samples were taken from skin near the wound margin. However we have no direct information to indicate whether the doses measured represented the maximum dose received by the skin in the wound site.
One of the reasons of these negative results may be that not all actual DNA damage is translated into micronuclei. Even in ideal populations, the MN frequency always will be less than the frequency of acentric fragments, so MN inevitably under-estimates actual DNA fragmentation damage; this discrepancy grows with dose [46]. In addition, MN score may be affected by different exclusion of DNA fragments from nuclei [46]. Some lethal damage may not be expressed as MN at the first division, only in later ones (though many are eliminated at the first mitosis) [50]. Unfortunately, it was not possible to assess the BNI accurately for fibroblasts being assayed directly from skin samples, thus we could not examine for a relationship between BNI in the in situ samples and WHC, however, the BNI for the treated fibroblast strains also did not correlate with WHC in the limited group of 23 patients for whom fibroblast strains were derived.
Another reason may be that healing of the skin may not in itself be sufficient to prevent the development of a wound complication in situations where fluid accumulates in the wound cavity inevitably developing after the removal of a large tumor mass. We have shown previously that injection of non-irradiated autologous fibroblasts (or bone marrow stromal cells) into an irradiated superficial wound site in rats can result in improved wound strength but using a deep wound model in rat hind limb we found that such injections are of limited value in improving wound strength [10, 17, 51]. Overall our results suggest that factors other than the radiosensitivity of the skin fibroblasts must play a critical role in wound healing in deep wound sites associated with surgery for STS.
Acknowledgments
This study was supported by an IHRT grant from the Canadian Institutes of Health Research and by funds from NIH grant number U19 AI06773302. The authors acknowledge Dr John Akudugu for advice in analyzing the results of the micronucleus assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akudugu JM, Bell RS, Catton C, et al. Wound healing morbidity in STS patients treated with preoperative radiotherapy in relation to in vitro skin fibroblast radiosensitivity, proliferative capacity and TGF-beta activity. Radiother Oncol. 2006;78:17–26. doi: 10.1016/j.radonc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Akudugu JM, Bell RS, Catton C, et al. Clonogenic survival and cytokinesis-blocked binucleation of skin fibroblasts and normal tissue complications in soft tissue sarcoma patients treated with preoperative radiotherapy. Radiother Oncol. 2004;72:103–112. doi: 10.1016/j.radonc.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Begg AC, Sprong D, Balm A, Martin JM. Premature chromosome condensation and cell separation studies in biopsies from head and neck tumors for radiosensitivity prediction. Radiother Oncol. 2002;62:335–343. doi: 10.1016/s0167-8140(01)00498-4. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 5.Brock WA, Tucker SL, Geara FB, et al. Fibroblast radiosensitivity versus acute and late normal skin responses in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 1995;32:1371–1379. doi: 10.1016/0360-3016(95)00068-A. [DOI] [PubMed] [Google Scholar]
- 6.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: a practical information-theoretic approach. 2. New York: Springer-Verlag; 2002. [Google Scholar]
- 7.Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005;81:887–899. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- 8.Cordes N, Blaese MA, Plasswilm L, Rodemann HP, Van Beuningen D. Fibronectin and laminin increase resistance to ionizing radiation and the cytotoxic drug Ukrain in human tumour and normal cells in vitro. Int J Radiat Biol. 2003;79:709–720. doi: 10.1080/09553000310001610240. [DOI] [PubMed] [Google Scholar]
- 9.Dahm-Daphi J, Dikomey E, Brammer I. DNA-repair, cell killing and normal tissue damage. Strahlenther Onkol. 1998;174(Suppl 3):8–11. [PubMed] [Google Scholar]
- 10.Dantzer D, Ferguson P, Hill RP, et al. Effect of radiation and cell implantation on wound healing in a rat model. J Surg Oncol. 2003;83:185–190. doi: 10.1002/jso.10242. [DOI] [PubMed] [Google Scholar]
- 11.Davis AM, O’Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Dikomey E, Borgmann K, Peacock J, Jung H. Why recent studies relating normal tissue response to individual radiosensitivity might have failed and how new studies should be performed. Int J Radiat Oncol Biol Phys. 2003;56:1194–1200. doi: 10.1016/s0360-3016(03)00188-3. [DOI] [PubMed] [Google Scholar]
- 13.Durante M, Grossi GF, Yang TC. Radiation-induced chromosomal instability in human mammary epithelial cells. Adv Space Res. 1996;18:99–108. doi: 10.1016/0273-1177(95)00796-h. [DOI] [PubMed] [Google Scholar]
- 14.Eatough JP. Alpha-particle dosimetry for the basal layer of the skin and the radon progeny 218-Po and 214-Po. Phys Med Biol. 1997;42:1899–1911. doi: 10.1088/0031-9155/42/10/004. [DOI] [PubMed] [Google Scholar]
- 15.Falt S, Holmberg K, Lambert B, Wennborg A. Long-term global gene expression patterns in irradiated human lymphocytes. Carcinogenesis. 2003;24:1837–1845. doi: 10.1093/carcin/bgg134. [DOI] [PubMed] [Google Scholar]
- 16.Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson PC, Boynton EL, Wunder JS, et al. Intradermal injection of autologous dermal fibroblasts improves wound healing in irradiated skin. J Surg Res. 1999;85:331–338. doi: 10.1006/jsre.1999.5664. [DOI] [PubMed] [Google Scholar]
- 18.Gagnon WF. Measurement of surface dose. Int J Radiat Oncol Biol Phys. 1979;5:449–450. doi: 10.1016/0360-3016(79)91231-8. [DOI] [PubMed] [Google Scholar]
- 19.Halm EA, Tamri A, Bridier A, Wibault P, Eschwege F. Influence of thermoplastic masks on the absorbed skin dose for head and neck tumor radiotherapy. Cancer Radiother. 2002;6:310–319. doi: 10.1016/s1278-3218(02)00206-8. [DOI] [PubMed] [Google Scholar]
- 20.Hill R, Bristow RG. The Scientific Basis of Radiotherapy. In: Tannock I, Hill RP, Bristow RG, Harrington L, editors. Basic Science of Oncology. 4. New York: McGraw-Hill Professional; 2005. pp. 289–321. [Google Scholar]
- 21.Holmberg K, Falt S, Johansson A, Lambert B. Clonal chromosome aberrations and genomic instability in X-irradiated human T-lymphocyte cultures. Mutat Res. 1993;286:321–330. doi: 10.1016/0027-5107(93)90197-n. [DOI] [PubMed] [Google Scholar]
- 22.Holt GE, Griffin AM, Pintilie M, et al. Fractures following radiotherapy and limb-salvage surgery for lower extremity soft-tissue sarcomas. A comparison of high-dose and low-dose radiotherapy. J Bone Joint Surg Am. 2005;87:315–319. doi: 10.2106/JBJS.C.01714. [DOI] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 24.ICRP. ICRP (International Commission on Radiological Protection) ICRP Publication 60. Oxford: Pergamon; 1991. 1990 Recommendations of the International Commission on Radiological Protection; pp. 149–153. [Google Scholar]
- 25.Johansen J, Bentzen SM, Overgaard J, Overgaard M. Evidence for a positive correlation between in vitro radiosensitivity of normal human skin fibroblasts and the occurrence of subcutaneous fibrosis after radiotherapy. Int J Radiat Biol. 1994;66:407–412. doi: 10.1080/09553009414551361. [DOI] [PubMed] [Google Scholar]
- 26.Khan MA, Hill RP, Van Dyk J. Partial volume rat lung irradiation: an evaluation of early DNA damage. Int J Radiat Oncol Biol Phys. 1998;40:467–476. doi: 10.1016/s0360-3016(97)00736-0. [DOI] [PubMed] [Google Scholar]
- 27.Khan MA, Van Dyk J, Yeung IW, Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol. 2003;66:95–102. doi: 10.1016/s0167-8140(02)00325-0. [DOI] [PubMed] [Google Scholar]
- 28.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79:231–238. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Laurent C, Voisin P, Pouget JP. DNA damage in cultured skin microvascular endothelial cells exposed to gamma rays and treated by the combination pentoxifylline and alpha-tocopherol. Int J Radiat Biol. 2006;82:309–321. doi: 10.1080/09553000600733150. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Hwang K. Skin thickness of Korean adults. Surg Radiol Anat. 2002;24:183–189. doi: 10.1007/s00276-002-0034-5. [DOI] [PubMed] [Google Scholar]
- 31.Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Prot Dosimetry. 2002;99:169–172. doi: 10.1093/oxfordjournals.rpd.a006753. [DOI] [PubMed] [Google Scholar]
- 32.Marder BA, Morgan WF. Delayed chromosomal instability induced by DNA damage. Mol Cell Biol. 1993;13:6667–6677. doi: 10.1128/mcb.13.11.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIlrath J, Lorimore SA, Coates PJ, Wright EG. Radiation-induced genomic instability in immortalized haemopoietic stem cells. Int J Radiat Biol. 2003;79:27–34. [PubMed] [Google Scholar]
- 34.Miller AC, Brooks K, Stewart M, et al. Genomic instability in human osteoblast cells after exposure to depleted uranium: delayed lethality and micronuclei formation. J Environ Radioact. 2003;64:247–259. doi: 10.1016/s0265-931x(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 35.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Muller-Sievers K, Ertan E, Kober B. Dosimetry of rotational partial-skin electron irradiation. Radiother Oncol. 2001;58:187–192. doi: 10.1016/s0167-8140(00)00329-7. [DOI] [PubMed] [Google Scholar]
- 38.Nagar S, Smith LE, Morgan WF. Characterization of a novel epigenetic effect of ionizing radiation: the death-inducing effect. Cancer Res. 2003;63:324–328. [PubMed] [Google Scholar]
- 39.O’Driscoll MC, Scott D, Orton CJ, et al. Radiation-induced micronuclei in human fibroblasts in relation to clonogenic radiosensitivity. Br J Cancer. 1998;78:1559–1563. doi: 10.1038/bjc.1998.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 41.Pathak MA, Zarebska Z, Mihm MC, Jr, et al. Detection of DNA-psoralen photoadducts in mammalian skin. J Invest Dermatol. 1986;86:308–315. doi: 10.1111/1523-1747.ep12285506. [DOI] [PubMed] [Google Scholar]
- 42.Peacock J, Ashton A, Bliss J, et al. Cellular radiosensitivity and complication risk after curative radiotherapy. Radiother Oncol. 2000;55:173–178. doi: 10.1016/s0167-8140(00)00173-0. [DOI] [PubMed] [Google Scholar]
- 43.Pelevina II, Gotlib V, Kudriashova OV, Antoshchina MM, Serebrianyi AM. Properties of progeny of irradiated cells. Tsitologiia. 1998;40:467–477. [PubMed] [Google Scholar]
- 44.Russell NS, Begg AC. Editorial radiotherapy and oncology 2002: predictive assays for normal tissue damage. Radiother Oncol. 2002;64:125–129. doi: 10.1016/s0167-8140(02)00189-5. [DOI] [PubMed] [Google Scholar]
- 45.Russell NS, Grummels A, Hart AA, et al. Low predictive value of intrinsic fibroblast radiosensitivity for fibrosis development following radiotherapy for breast cancer. Int J Radiat Biol. 1998;73:661–670. doi: 10.1080/095530098141915. [DOI] [PubMed] [Google Scholar]
- 46.Savage JR. A comment on the quantitative relationship between micronuclei and chromosomal aberrations. Mutat Res. 1988;207:33–36. doi: 10.1016/0165-7992(88)90008-5. [DOI] [PubMed] [Google Scholar]
- 47.Seymour CB, Mothersill C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander-killing environment. Radiat Oncol Investig. 1997;5:106–110. doi: 10.1002/(SICI)1520-6823(1997)5:3<106::AID-ROI4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13:1209–1215. doi: 10.1245/s10434-006-9028-6. [DOI] [PubMed] [Google Scholar]
- 49.Ullrich RL, Davis CM. Radiation-induced cytogenetic instability in vivo. Radiat Res. 1999;152:170–173. [PubMed] [Google Scholar]
- 50.Weissenborn U, Streffer C. Analysis of structural and numerical chromosomal aberrations at the first and second mitosis after X irradiation of two-cell mouse embryos. Radiat Res. 1989;117:214–220. [PubMed] [Google Scholar]
- 51.Werier J, Ferguson P, Bell R, et al. Model of radiation-impaired healing of a deep excisional wound. Wound Repair Regen. 2006;14:498–505. doi: 10.1111/j.1743-6109.2006.00145.x. [DOI] [PubMed] [Google Scholar]