Abstract
Polymeric micelles are being investigated as chemotherapy drug delivery carriers using ultrasound as a trigger mechanism. The aim of this paper is to measure the release of Doxorubicin (Dox) from the core of unstabilized Pluronic 105 micelles, Pluronic P105 micelles stabilized with an interpenetrating network of N,N-diethylacrylamide, and micelles of poly(ethylene oxide)-b-poly (N-isopropylacrylamide)-b-poly(oligolactylmethacrylate) with stabilized cores. An ultrasonic exposure chamber with fluorescence detection was used to measure the release of the antineoplastic agent from both stabilized and unstabilized micelles. The release of Dox at 37 °C from unstabilized Pluronic appears to be several times higher than release from the more stabilized and crosslinked copolymers at the same temperature. Although there is a difference in the amount of release between the different compounds, the onset of release occurs at about the same ultrasonic power density for all carriers investigated in this study. The threshold of drug release for all the compounds correlates to the emergence of subharmonic peaks detected in the acoustic spectra. We hypothesize that shearing events caused by cavitating bubbles play an important role in the acoustically activated release of chemotherapy agents delivered from various polymeric drug delivery vehicles.
Keywords: Micelles, Ultrasound, Cavitation Events, Doxorubicin, Drug Delivery, Micelle
1. INTRODUCTION
The ability to control the spatial and temporal delivery of chemotherapeutic agents is essential to the efficient treatment of disease as well as to the effective protection of the health and comfort of cancer patients. To this end, our research group is employing non-invasive ultrasound to activate the controlled release of drugs from nano-sized micellar carriers. Several challenges confront ultrasonic-activated drug delivery, including a careful balance in many aspects of the physical and chemical mechanism of the delivery vehicle. Not only must the vehicle be small enough to circulate freely in the blood and yet large enough to avoid renal excretion, but they must be stable enough to retain their content until activated by ultrasound, as well as to be eventually biodegradable so that the vehicle is eventually eliminated from the body. Most importantly, the vehicle must sequester the drug sufficiently to preclude premature release, and yet must release the drug upon a controlled nudge with ultrasound.
Our research group has found some success using Pluronic P105 micelles to deliver anthracycline agents or other drugs to cancerous cells and tissues. Previously we have shown adequate sequestration and effective release in vitro with leukemia cells (1). These HL-60 cells displayed no DNA damage when treated with Doxorubicin (Dox) sequestered in a solution of 10 wt% P105 without exposure to ultrasound. However, extensive DNA damage and cell death was observed in the same solution within 1 h of exposure to low-frequency ultrasound. Subsequent work showed that ultrasound caused release of Dox from the micelles,2–4 and suggested that this carrier system appears to work in vivo against solid tumors.5, 6
Although the P105 micellar system provides control of Dox sequestration and release, these vehicles are not stable upon dilution in blood. Thus we have devoted effort to synthesizing micellar nanoparticles with characteristics similar to P105, but which are transiently stabilized so they do not dissolve upon dilution, and yet eventually biodegrade into harmless products.7–10
In this article, we compare the ultrasonically activated release of Dox from unstabilized and stabilized micelles. Two different stabilization techniques were used to synthesize drug carriers capable of maintaining their micellar structure at concentrations lower than the critical micellar concentration (CMC). Additionally, we present drug release as a function of temperature. As part of this study, we investigated the mechanism of drug release using an improved ultrasonic exposure chamber. In examining the mechanism, we used acoustic spectroscopy to study the role of cavitation in releasing Dox from the novel nanosized carriers. The improvements introduced into the design of the new fluorescence detection ultrasonic exposure chamber enabled us to measure the fluorescence intensity and acquire acoustic frequency spectra simultaneously and at the same spatial position. The data were then analyzed to correlate release data to acoustically induced cavitation events.
Recently, we have shown that the onset of drug release from unstabilized micelles corresponds to the emergence of a subharmonic peak in the acoustic frequency spectra.11 These observations confirm an important role of acoustic cavitation in acoustically activated micellar drug delivery.
2. MATERIALS AND METHODS
2.1. Materials
Pluronic P105 was provided by BASF Corp. (Mount Olive, NJ); N ,N -diethylacrylamide (NNDEA) was obtained from Polyscience (Warrington, PA); N ,N -bis(acryloyl) cystamine (BAC) was obtained from Fluka (Milwaukee, WI); 2,2′-azobis(isobutyronitrile) (AIBN) was obtained from Aldrich (Milwaukee, WI); 2-hydroxyethyl methacrylate, 3,6-dimethyl-1,4-dioxane-2,5-dione (lactide or cyclic dilactate), tin (II) 2-ethylhexanoate, methoxy-poly(ethylene glycol), N -isopropylacrylamide, 4,4′-azobis (4-cyanopentanoic acid), 1,4-dioxane, and thionyl chloride were obtained from Sigma-Aldrich and used without further purification; 1,6-diphenyl-1,3,5-hexatriene was obtained from Molecular Probes (Eugene, Oregon).
Doxorubicin was obtained from the Primary Children’s Hospital (Salt Lake City, UT) in a 1 : 5 mixture with lactose in dosage form (Ben Venue Labs, Bedford, OH); it was dissolved in phosphate buffered saline (PBS) and sterilized by filtration through a 0.2 μm filter.
2.2. Drug Encapsulation in Pluronic Unstabilized/Stabilized Micelles
Stock solutions of Pluronic were prepared by dissolving P105 in a PBS solution to a final concentration of 10 wt%. Dox was dissolved into the P105 solutions at room temperature to produce a final Dox concentration of 10 μg/ml in 10 wt% Pluronic. The same drug concentration was also prepared in a suspension of stabilized micelles and in PBS as a control (without micellar carriers present).
The first type of stabilized micelle is the Pluronic micelle stabilized by polymerizing an interpenetrating network of poly(N ,N -diethyl acrylamide) (pNNDEA) within the core as described previously.2, 7 Briefly, 40 mL of double-distilled water containing 10 wt% P105 was placed in a round-bottomed flask. NNDEA monomer was added to give a final concentration of 0.05 wt% monomer. BAC was added as a cross-linking agent to produce a BAC : NNDEA mole ratio of 1 : 20. AIBN (at a mole ratio of 1 : 100 AIBN : NNDEA) was added as an initiator. The system was polymerized for 24 hours at 65 °C with magnetic stirring and a continuous nitrogen purge. Dox stock solution was added at room temperature to the resulting Plurogel to reach a final concentration of 10 μg/mL.
The second type of stabilized micelle system does not use P105, but still has a hydrophobic core with a shell of PEO. It is made from a novel block copolymer consisting of a block of PEO adjacent to a random copolymer of N -isopropylacrylamide (NIPAAm) and polylactate esters of hydroxyethyl methacrylate (HEMA-lactate).9 This novel copolymer forms micelles with a controlled lifetime before dissolving in an aqueous environment. Initially the NIPAAm-HEMA-lactate block is hydrophobic, and thus stabilizes the micelle and provides a hydrophobic environment to sequester drugs. Over time, the lactate esters hydrolyze and this block becomes sufficiently hydrophilic that the micelle dissolves. The lifetime can be controlled by the composition of NIPAAm, HEMA, and lactate in the polymer. For simplicity, this polymer is referred to a PNHL.
To synthesize this polymer, oligolactate esters of 2-hydroxyethyl methacrylate (HEMA-lactate5) were created by ring-opening oligomerization of lactide using HEMA as the initiator and stannous octoate as a catalyst as described by.12 Briefly, a mixture of lactide and HEMA was stirred at 110 °C under nitrogen purging until the lactide was molten. Then catalytic amounts of stannous octoate dissolved in toluene were added drop wise to the mixture and it was maintained at 110 °C for 1 hour.
To incorporate PEO into the copolymer, a PEO macroinitiator was first produced in two steps. In the first step, 4,4′-azobis(4-cyanopentanoic acid) (ABCPA) was reacted with SOCl2 at 100 °C for 20 minutes and converted to the corresponding acid chloride: 4,4′ azobis(4-cyanopentanoyl chloride) (ABCPC). In the second step, the PEO macroinitiator was created by a condensation reaction of ABCPC and PEO in dry dichloromethane in the presence of an excess amount of triethylamine for 24 hr as described by Virtanen et al.13
The PNHL was obtained by radical polymerization of NIPAAm with the HEMA-lactate5 using the PEO macroinitiator in 1,4-dioxane for 24 hours at 80 °C under a nitrogen atmosphere.12 The solution was cooled to room temperature and concentrated under reduced pressure. The formed solid was dissolved in distilled water at 100 mg/mL concentration and centrifuged at 40 °C to separate the unreacted PEO. The solid copolymer products were dried under reduced pressure.
2.3. Measuring Ultrasound-Triggered Release of Dox from Pluronic P105 Micelles
A modification of the previous apparatus.3 was used in these experiments (Fig. 1). The 488-nm beam of an argon ion laser (Ion Laser Technology, 5500 A) was directed through a dielectric-interface beam splitter; the intensity of the split portion of the beam was measured by a photodetector (Newport 818-SL with 835 display) and used to monitor the laser power throughout our experiments. The other portion of the beam was focused by a 20X microscope objective into one branch of a bifurcated fiber optic bundle (# DF13036M, Edmunds Optics, Barrington, NJ) that directed the light into an acoustically transparent plastic tube (cellulose butyrate, Tulox Plastics, Marion, Indiana), with a diameter of 2.54 cm, filled with the Dox solution. The laser light exited the common end of the bifurcated fiber optic bundle in a 0.09 steradian cone of light. Dox molecules within the cone of light absorb at 488 nm and isotropically emit fluorescent light within a spectrum of about 530 nm to 630 nm. In the same fiber optic bundle were fibers that collect and direct the fluorescence to a detector. Numerical integration of a mathematical model of isotropic fluorescence within the cone of excitation light showed that about 99% of the collected fluorescence originated from within 3 mm of the fiber optic tip. The collected fluorescence signal was directed through the second branch of the fiber optic bundle, through a dielectric bandpass filter (Omega Optical 535DF35), and to a silicon detector (EG&G 450-1). The filter rejected any emissions with a wavelength below about 517 nm, including any Rayleigh-scattered laser light. The photodetector signal was captured on an oscilloscope (Tektronix TDS 3012) and subsequently stored on a computer for further processing.
Fig. 1.
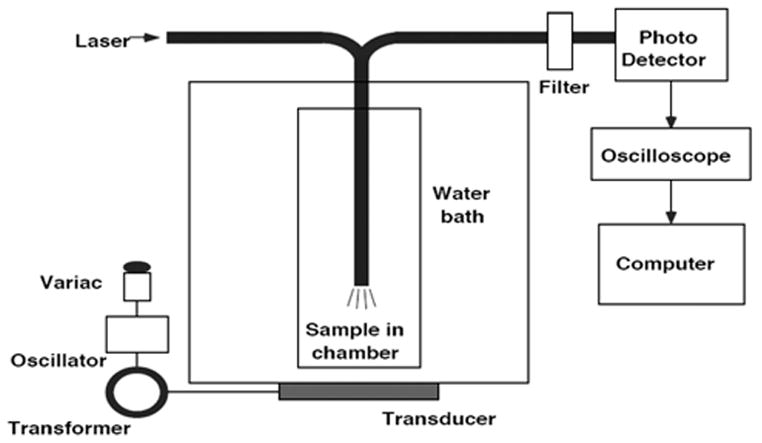
Experimental Setup for the detection of Doxorubicin Fluorescence during ultrasonic exposure.
This apparatus can measure the amount of acoustically-activated Dox release from micelles because Dox exhibits a decrease in fluorescence in contact with an aqueous solution. Such is the case when Dox is released from micelles, and it follows that the magnitude of decrease in fluorescence intensity upon application of ultrasound provides a quantifiable measure of drug release.
The decrease in fluorescence of the encapsulated drug solution is assumed proportional to the amount of drug released relative to a known baseline.11 To estimate the emission at 100% release, the fluorescence of Dox in PBS (in the absence of micelles) was measured in separate experiments just prior to experiments with micelles. Then the percent release was calculated using the following:
| (1) |
where IUS is the fluorescence intensity upon exposure of the sample to ultrasound, IPBS is the fluorescence intensity of an unsonicated solution of free Dox in PBS, and IP105 is the intensity recorded when the drug is encapsulated in Pluronic P105 (which corresponds to 0% release and 100% encapsulation).
In these experiments, the fluorescence intensity of the drug in PBS was first measured both with and without the application of ultrasound. Ultrasound was applied using a 70-kHz ultrasonicating bath (SC-40, Sonicor, Copiaque, NY) equipped with a single piezoceramic transducer that is driven at about 70 kHz. The best description of the waveform is that of a 70-kHz wave amplitude-modulated sinusoidally at about 0.12 kHz. The bath was powered by 60-Hz AC voltage from a variable AC transformer (variac). The voltage from the variac to the sonicating bath was adjusted to produce differing intensities of ultrasound. To execute the experiments, the end of the fiber optic was positioned at an acoustically intense position in the ultrasonicating bath, as determined by a hydrophone (described below). The bath was filled with degassed water and the tube surrounding the fiber optic probe tip was filled with the solution of Dox in PBS. Fluorescence emissions were collected for different voltages applied to the sonicating bath. Then, without changes in the experimental set-up, the Dox solution in PBS was carefully removed and replaced with a Dox solution of the same concentration in Pluronic micelles. During insonation, fluorescence dropped due to Dox coming in contact with the surrounding aqueous environment. Several fluorescence measurements were made at each intensity setting and averaged (n = 8 when I > 0.27 W/cm2 and n = 4 when I ≤ 0.27 W/cm2).
2.4. Acoustic Measurements
Ultrasonic power density measurements were obtained using a calibrated hydrophone (Bruel and Kjaer 8103, Decatur, GA) whose output voltage was monitored with an oscilloscope. After measurements of Dox fluorescence, the fiber optic probe was replaced with the tip of the hydrophone in the same location as the sample, and the hydrophone response was recorded at the same settings as used for the fluorescence measurements. The average acoustic intensity was calculated from where Q is the frequency-dependent calibration factor obtained from the manufacturer that relates pressure to voltage, Z is the acoustic impedance of water (1.5 × 106 kg/m2s), and Vrms is the root-mean-squared voltage of the hydrophone signal.
Acoustic spectroscopy was used to monitor the spectra of the vibrations of the cavitating bubbles in the ultrasonic field at the power settings used in the release measurements. The hydrophone signal was directed to a spectrum analyzer (Agilent E4401B), which displayed and recorded the acoustic spectrum.
3. RESULTS AND DISCUSSION
Figure 2 shows the calculated release of Doxorubicin from Pluronic, Plurogel, and PNHL. There are several noteworthy features of these data. First, the release of Dox at 37 °C from Pluronic is more than three times higher than release from the stabilized drug carriers at the same temperature. Secondly, there is a threshold in acoustic power above which drug release occurs, and even though the amount of release varies for stabilized and non-stabilized micelles, the threshold, or onset of release, occurs at about the same intensity of about 0.38 W/cm2 for all carriers. Furthermore, as shown in the insets in Figure 2, this threshold correlates to the onset of an f/2 subharmonic signal detected by the hydrophone.
Fig. 2.
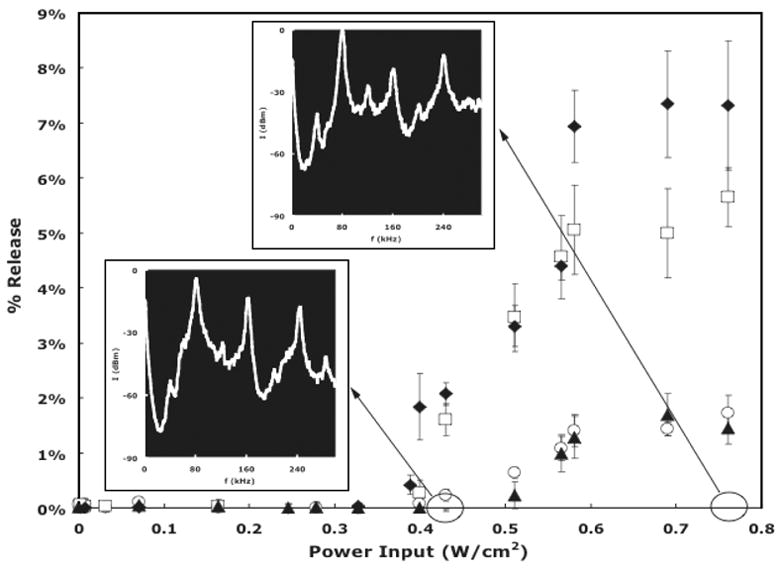
Percent Dox release from nonstabilized and stabilized micelles versus ultrasonic power density. □: 10% Pluronic P105 micelles in PBS at 37 °C; ◆: 10 wt% Pluronic P105 micelles in PBS at 58 °C ○: Stabilized micelles (PNHL); ▲: Stabilized Pluronic P105 micelles (Plurogel). Error bars represent standard deviations (n ≤ 3). The inserts are the acoustic spectra collected at 0.43 W/cm2 and 0.76 W/cm2.
There are two proposed sources for the f/2 subharmonic emissions. The first emission source is from bubbles undergoing “stable” cavitation at sufficiently high acoustic pressure that their non-linear dynamic oscillations produce an f/2 subharmonic, in addition to higher harmonics.14, 15 Such non-linear stable cavitation has been predicted by the solution of the Rayleigh-Plesset and similar equations,14, 16–18 which adequately models stable cavitation, but is inadequate to model collapse cavitation. Stable cavitation with a subharmonic component has been observed under carefully controlled experimental conditions.15, 19, 20
The second source of subharmonic emissions is proposed to arise from the prolonged expansion of the bubble immediately prior to the collapse event.21 Collapse cavitation is characterized acoustically by a shock wave that produces broad band white noise when the temporal pressure signal is Fourier transformed to the frequency domain.14, 15 An f/2 signal that is always accompanied by an increase in baseline or background noise is usually attributed to collapse cavitation, although it is theoretically possible that this f/2 signal arises from oscillating bubbles that are not of the correct size to collapse, but instead exhibit stable non-linear oscillations in the presence of other collapsing bubbles.
In our experiments, the appearance of the f/2 is always accompanied by a jump (an increase) in background noise. In fact, in the apparatus used in these experiments, we never could obtain the f/2 signal in the absence of increased background noise. Thus we propose that in these experiments, the f/2 is indicative of collapse cavitation. In addition, we have trapped free radicals at intensities as low as 0.68 W/cm2, just above the threshold for f/2 in these experiments of 0.38 W/cm2.22 Furthermore, the mechanical index (MI) corresponding to 0.38 W/cm2 is 0.4.23, 24 assuming a plane wave and symmetric pressure amplitude. The MI is defined as the peak negative pressure in MPa divided by the square root of the ultrasonic frequency in MHz. The MI for the onset of collapse cavitation is considered to be about 0.4.25 The previous data and observations support our assumption that the f/2 is indicative of, and correlated with, collapse cavitation in these experiments.
Apparently, both stabilized and non-stabilized micelles are perturbed by collapse cavitation sufficiently to release Dox, but the non-stabilized micelles appear be more susceptible to the disrupting forces associated with cavitation. If we assume that the amount of Dox release is a function of how many micelles are disrupted or how severely the micelles are disrupted by the shearing forces associated with cavitation, then we can conclude that much higher shearing forces are required to disrupt the stabilized micelles.
Another noteworthy feature of Figure 2 is that release from Pluronic at 58 °C appears to be slightly greater than release at 37 °C. However, a statistical analysis shows that these two data sets in Figure 1 have slopes that are not statistically different (0.24 vs. 0.25% release/W/cm2, p = 0.112). A linear extrapolation to determine the threshold of release indicates that release at 58 °C commences at a slightly lower intensity (0.355 W/cm2 vs. 0.379 W/cm2), but this difference is only marginally significant (p = 0.035). Differences in temperature influence the bubble dynamics by changing the viscosity of the surrounding media and the vapor pressure of water within the oscillating bubble.14 Qualitatively, decreasing the water viscosity (at higher temperatures) will decrease the damping of bubble oscillations and allow conditions leading to collapse cavitation to occur at a lower acoustic intensity. Thus our observation is qualitatively consistent with the bubble dynamics theory.
Figures 3 and 4 show the percent release versus the subharmonic peak for nonstabilized, crosslinked, and stabilized copolymers. The plots corroborate previous data published for the release of Dox from unstabilized Pluronic micelles at 24 °C where we reported that the onset of release corresponds to the emergence of a subharmonic peak in acoustic spectra. Figure 3 shows a fairly linear relationship between the logarithm of the subharmonic peak intensities and release at both temperatures. As mentioned, we posit that at the higher temperature the viscosity is lower which shifts the onset of collapse cavitation to a slightly lower energy level.
Fig. 3.
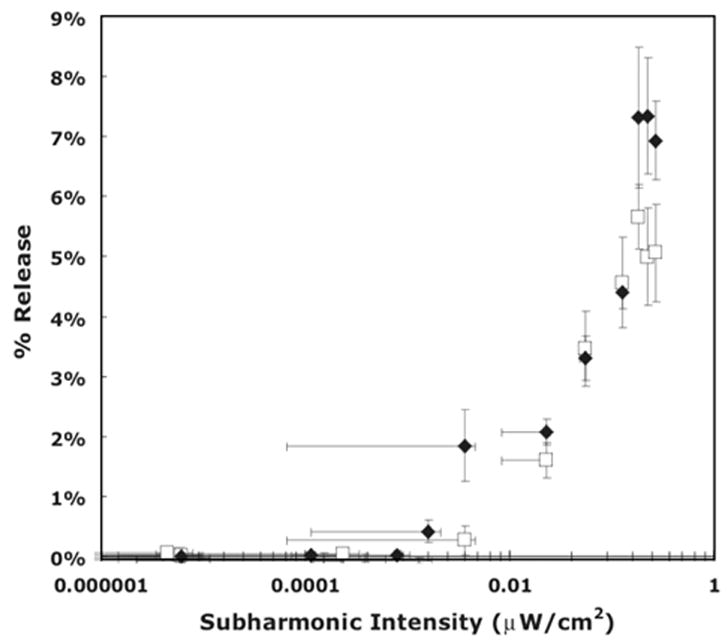
The amount of Doxorubicin release as a function of the f/2 subharmonic peak intensity of non-stabilized micelles at two temperatures. □: 37 °C; ◆: 58 °C.
Fig. 4.
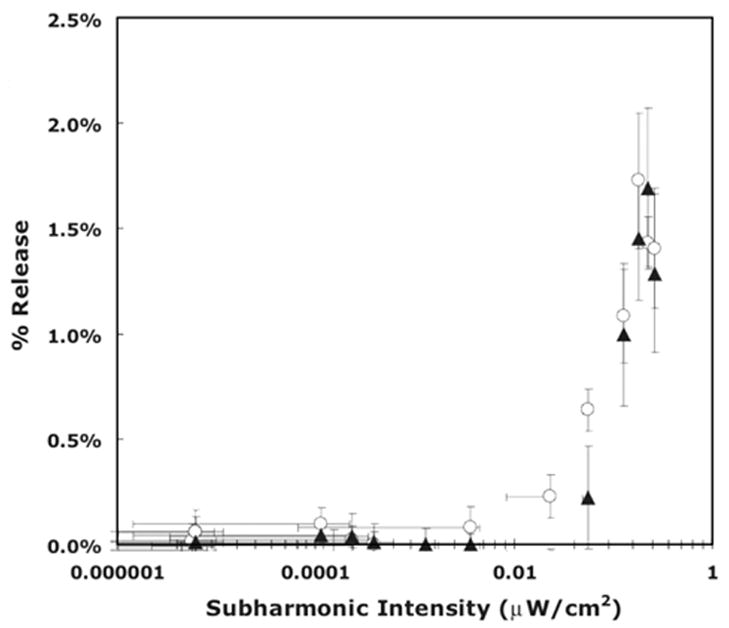
The amount of Doxorubicin release as a function of the f/2 subharmonic peak intensity of stabilized micelles at 37 °C. ○: Stabilized micelles (PNHL); ▲: Stabilized Pluronic P105 micelles (Plurogel).
Figure 4 shows a non-linear relationship between Dox release from stabilized and crosslinked P105 and the logarithm of the subharmonic peak intensities. The plot is non-linear for the Plurogel copolymer crosslinked by an interpenetrated network of N ,N -diethyl acrylamide, suggesting that shearing plays an important role in this release phenomena. During both stable and transient cavitation, oscillating bubbles are able to generate strong shearing forces near the surface of the bubble.26 These forces are capable of shearing open the micellar structure and allowing fluorescent drug molecules to interact with water, thus reducing its fluorescent. We hypothesize that the nonlinear behavior observed in Figure 4 as well as the higher threshold observed for the cross-linked and stabilized micelles when compared with the non-stabilized micelles (Fig. 3) may be attributed to a higher shear force needed to overcome stabilization forces (forces that keep the micellar structure intact, including hydrophobic-hydrophobic interactions and crosslinking).
It is important to keep in mind that although maximum release is observed at the highest power density attained experimentally, clinical use of ultrasonic power as a trigger mechanism may be limited by the tissue damage caused at high ultrasonic intensities. There may exist a window of therapy whereby chemotherapeutic agents are acoustically released from stabilized and unstabilized micelles without causing damage to the tissues exposed to ultrasonic power. More experiments are currently being conducted in our laboratory to determine ultrasonic power densities and frequencies corresponding to this therapeutic window.
Data presented in this paper give further evidence that micellar drug release under the influence of ultrasound is caused by the shearing phenomena associated with cavitation events. The question remains as to whether the f/2 subharmonic peak observed at the onset of measurable release is a signature of stable or collapse cavitation.
The mechanism of release appears to be independent of the drug carrier nature and of temperature. However, the amount of drug release is a function of the temperature and chemistry of the micellar carrier. Further experiments should be conducted at the different ultrasonic frequencies to test our hypothesis. It is also important to measure the release and re-encapsulation kinetics of this drug delivery system to determine the optimum duration of ultrasonic exposure in treatment.
Acknowledgments
The authors gratefully acknowledge funding from NIH grant R01 CA98138 that supported this research.
References and Notes
- 1.Husseini GA, El-Fayoumi RI, O’Neill KL, Rapoport NY, Pitt WG. Cancer Lett. 2000;154:211. doi: 10.1016/s0304-3835(00)00399-2. [DOI] [PubMed] [Google Scholar]
- 2.Husseini GA, Christensen DA, Rapoport NY, Pitt WG. J Control Rel. 2002;83:302. doi: 10.1016/s0168-3659(02)00203-1. [DOI] [PubMed] [Google Scholar]
- 3.Husseini GA, Myrup GD, Pitt WG, Christensen DA, Rapoport NY. J Control Rel. 2000;69:43. doi: 10.1016/s0168-3659(00)00278-9. [DOI] [PubMed] [Google Scholar]
- 4.Husseini GA, Rapoport NY, Christensen DA, Pruitt JD, Pitt WG. Coll Surf B: Biointerfaces. 2002;24:253. [Google Scholar]
- 5.Nelson JL, Roeder BL, Carmen JC, Roloff F, Pitt WG. Cancer Res. 2002;62:7280. [PubMed] [Google Scholar]
- 6.Rapoport N, Pitt WG, Sun H, Nelson JL. J Control Rel. 2003;91:85. doi: 10.1016/s0168-3659(03)00218-9. [DOI] [PubMed] [Google Scholar]
- 7.Pruitt JD, Husseini G, Rapoport N, Pitt WG. Macromolecules. 2000;33:9306. [Google Scholar]
- 8.Pruitt JD, Pitt WG. Drug Delivery. 2002;9:253. doi: 10.1080/10717540260397873. [DOI] [PubMed] [Google Scholar]
- 9.Zeng Y, Pitt WG. J Biomater Sci. 2005;17:591. doi: 10.1163/156856206776986297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Y, Pitt WG. J Biomat Sci Polym Ed. 2005;16:371. doi: 10.1163/1568562053654121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husseini GA, Diaz MA, Richardson ES, Christensen DA, Pitt WG. J Control Rel. 2005;107:253. doi: 10.1016/j.jconrel.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadee JA, De Kerf M, De Groot CJ, Den Otter W, Hennink WE. Polymer. 1999;40:6877. [Google Scholar]
- 13.Virtanen J, Holappa S, Lemmetyinen H, Tenhu H. Macromolecules. 2002;35:4763. [Google Scholar]
- 14.Brennen CE. Cavitation and Bubble Dynamics. Oxford University Press; New York: 1995. [Google Scholar]
- 15.Neppiras EA. J Acoust Soc Am. 1969;46:587. [Google Scholar]
- 16.Flynn HG, Church CC. J Acoust Soc Am. 1988;84:1863. doi: 10.1121/1.397253. [DOI] [PubMed] [Google Scholar]
- 17.Allen JS, Kruse DE, Dayton PA, Ferrara KW. J Acoustical Soc Amer. 2003;114:1678. doi: 10.1121/1.1600721. [DOI] [PubMed] [Google Scholar]
- 18.Eller A, Flynn HG. J Acoust Soc Am. 1969;46:722. [Google Scholar]
- 19.Mestas JL, Lenz P, Cathignol D. J Acoust Soc Am. 2003;113:1426. doi: 10.1121/1.1538198. [DOI] [PubMed] [Google Scholar]
- 20.Phelps AD, Leighton TG. Acustica. 1997;83:59. [Google Scholar]
- 21.Leighton TG. The Acoustic Bubble. Academic Press; London: 1994. [Google Scholar]
- 22.Rapoport N, Smirnov AI, Timoshin A, Pratt AM, Pitt WG. Archives Biochem Biophys. 1997;344:114. doi: 10.1006/abbi.1997.0176. [DOI] [PubMed] [Google Scholar]
- 23.Apfel RE, Holland CK. Ultrasound Med Biol. 1991;17:179. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- 24.Barnett S. Ultrasound Med Biol. 1998;24:S41. doi: 10.1016/s0301-5629(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 25.Shi WT, Forsberg F, Tornes A, Ostensen J, Goldberg BB. Ultrasound Med Biol. 2000;26:1009. doi: 10.1016/s0301-5629(00)00223-4. [DOI] [PubMed] [Google Scholar]
- 26.Nyborg WL. Br J Cancer. 1982;45:156. [PMC free article] [PubMed] [Google Scholar]


