Abstract
Methylmercury (MeHg) is a well known neurotoxicant, responsible for neurological and cognitive alterations. However, there is very little information available on the effects of MeHg administration on activation of murine neuronal pathways involved in the stress response, and whether this is altered as a function of repeated exposure to MeHg. Moreover, interactions between MeHg and other psychogenic and inflammatory stressors have yet to be fully determined. Acute intraperitoneal (IP) exposure of male C57BL/6J mice to MeHg (2−8 mg/Kg) dose-dependently attenuated exploratory behavior in the open field in the presence and absence of a novel object. In addition, increased numbers of c-Fos immunoreactive cells appeared in response to acute IP and ICV MeHg within thalamic (PVA/PV), hypothalamic (PVN), central amygdaloid (CeC), septal and hippocampal (dentate gyrus) nuclei, medial bed nucleus (BSTm) and the locus coeruleus (Lc). The increase in c-Fos positive cells in response to acute IP and ICV MeHg did not appear to be influenced further by open field exposure. Repeated administration of MeHg led to an attenuation of most parameters of open field behavior altered by acute MeHg. However, increased c-Fos was significant in the CeC, Dg, supracapsular bed nucleus (BSTs), and Lc. Moreover, open field exposure after repeated treatments resulted in significant c-Fos responses in similar areas. Interestingly, 3 days after the final repeated MeHg dose (2 or 4 mg/kg) c-Fos increases to an immunogenic stressor (LPS) were not affected by MeHg pretreatment. These results demonstrate that systemic exposure to acute and repeated MeHg serves to activate the brain's stress circuitry, and furthermore appears to engage normal neuronal habituation processes.
1. Introduction
Methylmercury is an environmental neurotoxicant derived from combustion of fossil fuels, volcanic activity, weathering of marine sediments, and industrial waste. Recognition of methylmercury as an environmental hazard has been noted for many decades, most notably in response to reports of pathological sequelae to human ingestion of contaminated food, as in the Minamata Bay fish and Iraq grain incidents (Takeuchi, 1968; Bakir et al., 1973). The direct cellular effects of methylmercury include inhibition of protein synthesis (Yosino et al., 1966; Verity et al., 1977), attenuation of energy production through mitochondrial dysfunction (Verity et al., 1975), and disassembly of mitotic spindles and induction of mitotic arrest (Miura et al., 1978). The central nervous system (CNS) effects of methylmercury have been well documented and include faulty neuronal migration and targeting leading to brain size reductions, cerebral and cerebellar heterotopias, and abnormal alignment and organization of cortical neurons (Choi et al., 1978). Additional neurophysiological changes include perturbations in Ca 2+ homeostasis and neuronal death (Limke et al., 2003), alterations in mitochondrial membrane potential (Insug et al., 1997), disruption of reactive oxygen species balance (Usuki et al., 2001), and impairment of glutamate uptake (Aschner, 1996).
While these direct neuronal effects indicate that methylmercury can disrupt neural function, there is a paucity of evidence to show how methylmercury engages the stress circuitry of the brain. The neuroanatomical basis of the stress response involves recruitment of discrete brain regions that in the face of stressor exposure serve to direct adaptive physiological and behavioral responses (Senba and Ueyama, 1997). Many of these brain regions are located in the forebrain, and include hypothalamic, amygdaloid, and septo-hippocampal nuclei (Herrera and Robertson, 1996). The most commonly used tool for neuroanatomical mapping of the stress response is immediate early gene (IEG) detection, and in particular, the c-fos gene and its protein product c-Fos (Morgan et al., 1987; Herrera and Robertson, 1996). Of particular importance is that neuronal c-fos expression is elevated by social stressors and fearful and/or novel stimuli (Matsuda et al., 1996 and Miczek et al., 1999), which allows for determination of important regions of stressor-related information processing during stressor exposure.
In recent years, a distinction has been made between psychogenic stressors, that engage neural circuitry by way of initial information processing (hence, processive stressors), and systemic stressors, which impact the brain as a function of internal physiological changes (e.g., metabolic challenges and immune responses) (Herman & Cullinan, 1997; Rossi-George et al, 2005). Sustained, chronic exposure to psychogenic stressors has been the basis of theories of adaptational breakdown that may lead to psychiatric conditions, such as anxiety and depression (McEwen, 2004). Behavioral experimentation has also demonstrated that modulation of T-cell immune responses occurs in response to neuroendocrine activation following chronic stress (Silberman et al., 2003, Reichlin 2004, Kusnecov & Goldfarb, 2005). Psychological stressors have also been shown to deleteriously affect the immune response to bacterial infection and progression of autoimmune neurodegenerative diseases such as multiple sclerosis (Schwartz et al., 1999 and Kiank et al., 2006).
Much current MeHg research is focused on neurodevelopmental influences as a function of chronic exposure in utero or during the early postnatal period (Davidson et al., 2004, Tamm et al., 2006). Recently, Cheng et al. (2006) have shown that IP injection of MeHg results in MeHg accumulation in the brain and significant induction of c-Fos protein levels that may potentially predict MeHg-induced neurotoxicity in the rat. Further it has been demonstrated that route of exposure (whether IP or oral) to MeHg does not alter the toxicokinetic whole-body retention or distribution of MeHg in mice (Nielsen, 1992). In light of this, the question of whether functionally active doses of MeHg can temporarily or even permanently alter neuronal function in the adult brain, such that responses to subsequent exposure to heterotypic neurogenic stimuli other than MeHg are altered, has not been widely explored. Given the research on the sensitizing effects of various stressors for reactivity to other heterotypic stressors (Bell et al., 1999, Hayley et al., 2003, Dunn et al., 2004), it would be of interest to examine the effects of MeHg pre-exposure on the activity of stress-associated neural circuits and the production of c-Fos protein.
Bacterial endotoxins such as lipopolysaccharide (LPS) are potent stimulators of the mammalian innate immune system. Lipopolysaccharide is found within the cell membrane of pathogenic Gram-negative bacteria and has been shown experimentally to induce production of proinflammatory cytokines and stress hormones, and activation of the hypothalamic-pituitary-adrenal axis (Verma et al., 2006; Johnson et al., 2004; Neveu & Liege, 2000). Lipopolysaccharide exposure has also been demonstrated to increase permeability of the blood-brain barrier, increase lymphocyte proliferation, and activation of stress-associated brain circuits (Jaworowicz et al., 1998; Ulmer et al., 2000; Lenczowski et al., 1998). Further, LPS is also a well known activator of cFos production within the murine brain (Akasaka et al., 2006; Brochu et al., 1999, Rossi-George et al., 2005). To date however there is little information addressing how repeated treatment of MeHg affects cFos expression within the brain's stress circuitry, and in addition, influences subsequent responses to bacterial endotoxin.
There is little question that exposure to environmental toxicants, such as methylmercury, represents a homeostatic threat, and may be viewed as a systemic stressor. This may impact the brain and recruit similar pathways in the brain as other systemic stressors. However, no information is presently available on the extent to which acute or chronic methylmercury exposure engages recognized neuronal pathways involved in the stress response. In the present report, this question was addressed using either an acute peripheral (intraparitoneal-IP), acute central (intracerebroventricular-ICV) dose of methylmercury or repeated IP exposure to lower doses of methylmercury. In addition, the degree to which the neurobiological impact of acute and repeated IP exposures to methylmercury interacted with a psychogenic stressor was also determined. Finally, the impact of repeated MeHg exposure on the subsequent recruitment of stress circuits by LPS was determined.
2. Materials and Methods
2.1 Animals
Six-week-old C57BL/6J male mice (Jackson Labs, Bar Harbor, ME) were housed (4/cage) on a 12h light/12h dark cycle (lights on 0700h), with ad libitum access to food and water. Upon arrival mice were acclimated for at least one week prior to experimentation. Six week old mice were chosen on the basis of previous research that found this age to show sufficient sexual and neurological maturity for studying the effects of methylmercury exposure in mice (Doi and Kobayashi, 1982; Yasutake et al., 1990; Nielsen, 1992; Adachi et al., 1994). All procedures were approved by the Rutgers Institutional Animal Care and Use Committee.
2.2.1 Acute Methylmercury – Experimental Procedure
Mice were administered 2, 4, 8mg/kg methylmercuric chloride (MeHg) (Sigma, St. Louis, MO) or 0.9% saline in a volume of 0.2 ml by intraparitoneal (IP) injection and subsequently exposed to either an open field (with and without a novel object) or allowed to remain in the home cage (HC). Therefore, there were four main groups: (i) MeHg/Home Cage, (ii) MeHg/Open Field, (iii) Saline/Home Cage, (iv) Saline/Open Field. Exposure to the open field (OF) occurred 1 hr after injection and lasted 15 minutes as described below (section 2.3). Following exposure to the OF, mice were returned to the home cage for 45 minutes, at the end of which they were anesthetized for perfusion (see section 2.4).
2.2.2 Acute Methylmercury - Open Field/Novel Object Test
The open field/novel object test was conducted as previously described by Kawashima & Kusnecov (2002). The open field apparatus was a 63×57×28 cm (L×W×H) Plexiglas box with opaque walls and floor. A smooth white curtain enclosed the area and an overhead video camera connected to a VHS tape player recorded all animal movements. Animals were allowed to freely explore the field for 10 minutes, at the end of which a novel object (metal cylinder) was placed in the center of the field for the remaining 5 minutes of the test. Therefore, total exposure time to the open field was 15 minutes.
Spontaneous Motor Activity Recording and Tracking (SMART) software (San Diego Instruments, San Diego, CA) was used as a video-tracking system to monitor location of the animal within the open field. For the purpose of analysis by SMART, the open field was divided into two zones (outer perimeter and inner zones) for the initial 10-minute period and divided into three zones (outer, inner, and centered novel object zones) during the 5-minute novel object period. The outer/peripheral zone was a 9.5cm wide area bordering the outer sidewall; the inner zone was the remainder of the central zone during the initial 10 minute period. During the period of exposure to the novel object the outer zone remained the same, while for purposes of analysis within the inner zone a third central zone was introduced. This central zone was larger than the novel object itself was introduced to register movements involving the area immediately surrounding the novel object. Percent time spent in each zone was measured for the 10 and 5-minute time periods as was the total distance traveled within each time period.
2.3 Repeated Methylmercury – Experimental Procedure & Open Field/ Novel Object Test
Methylmercury (2 or 4mg/kg) or saline were administered IP every third day over a15 day period beginning on day zero, for a total of six injections. All injection volumes were 0.2ml. One hour after the final injection mice were either examined in the open field (OF) for 15 minutes or allowed to remain in the home cage (HC), as described in Methods section 2.2.1 and 2.2.2. All mice were transcardially perfused 2h after the injection. Overall there were six treatment groups: i) Saline/OF, ii) 2mg/kg MeHg/ OF, iii) 4mg/kg MeHg/ OF, iv) Saline/ HC, v) 2mg/kg MeHg/ HC, vi) 4mg/kg MeHg/ HC.
2.4 Chronic Methylmercury and LPS – Experimental Procedure
Six injections of saline, 2, or 4mg/ kg methylmercury was administered IP every third day for 15 days beginning on day zero, as in Methods section 2.3. On day 18 mice were treated with either saline or 10μg LPS (Sigma Aldrich, St. Louis, MO) and transcardially perfused 2h after the injection. All injection volumes were 0.2ml. Overall there were six treatment groups compromising those that received saline or one of the two doses of MeHg (2 or 4mg/ kg) followed by subsequent administration of saline or LPS.
2.5 Intracerebroventricular MeHg – Experimental Procedure
The procedure for intracerebroventricular (ICV) administration of MeHg was conducted according to methods previously described (Kanteta and Kusnecov, 2005). Briefly, three days prior to experimentation mice underwent stereotaxic surgery to place an indwelling cannula into the right lateral ventricle. Mice were anesthetized with ∼0.20− 0.25ml of ketamine/ xylazine (80/12 mg/ml) from Sigma (St. Louis, MO), placed in to the stereotaxic apparatus (David Kopf Instuments, Tujunga, CA), and fitted with a 26-gauge stainless steel guide cannula (Plastics One, Roanoke, VA) unilaterally implanted in the right lateral ventricle using the following coordinates: 0.25mm anterior to bregma, 1.0mm lateral from midsagittal suture, and 2.5mm ventral. A dummy cannula was then inserted and the entire assembly secured with cyanoacrlyate glue (Plastics One, Roanoke, VA). Mice also received 50ug (in 0.1ml of saline) dose of gentamicin sulfate (ICN Biomedicals, Aurora, OH) to prevent potential infection. One and two days after surgery mice underwent sham infusion as habituation and on the third day mice were administered saline, 19ng MeHg, or 0.5ng LPS ICV with a Harvard Apparatus infusion pump (Holliston, MA). All injection volumes were 2.5μl. Sham mice received ICV surgery but were not fitted with an indwelling cannula. Two hours after infusion, animals were perfused as described below in Section 2.6.
The ICV MeHg dose for this experiment was determined to be 19ng, which is equivalent to the amount that would be present in the brain after an IP dose of 8mg/kg, as determined by Cheng et al (2006). Accurate cannula placement was validated for each animal after tissue was mounted on slides. Animals failing to show proper placement were excluded from analysis. All animals were naïve to ICV treatments.
2.6 Tissue Preparation and cFos Immunohistochemistry
Animals were anesthetized IP with 0.4ml of 7.5mg/ml of pentobarbital (Sigma Aldrich, St. Louis, MO) and then transcardially perfused with saline and 4% paraformaldehyde solutions. Brains were post-fixed in paraformaldehyde for 7 days and in a 30% sucrose solution for at least 7 days. Brains were sectioned at 30μm on a Leica SM 2000R freezing microtome and stored in cryoprotectant until assayed. Immunohistochemical detection of cFos was conducted using a free-floating assay as described previously (Rossi-George et al., 2005). Cryopreserved sections were washed with KPBS, incubated with rabbit anti-mouse, anti-Fos primary antibody (1:15,000) (CalBiochem, La Jolla, CA) for 2h at room temperature, and then at 4°C for 48h. After the primary incubation, sections were washed, and incubated in a secondary anti-rabbit antibody (1:500) for 1.5h. Tissue was then washed and incubated for 1h with the VectaStain ABC streptavidin horseradish peroxidase reagent (Vector Labs, Burlingame, CA), and subsequently reacted with NiSO4-diaminobenzidine (DAB) substrate solution (Sigma Chemical, St. Louis, MO) for 3−5 minutes. The reaction was stopped with a 0.175M sodium acetate solution. Sections were then mounted onto Fisher Superfrost slides, allowed to dry overnight, counterstained in neutral red for 4.5 minutes and coverslipped.
2.7 Image analysis and cell quantification
Regions analyzed for Fos-positive cells included the anterior paraventricular nucleus of the thalamus (PVA), posterior paraventricular nucleus of the thalamus (PV), paraventricular nucleus of the hypothalamus (PVN), central amygdaloid nucleus (CeC), dentate gyrus (Dg), locus coeruleus (LC), bed nucleus of the stria terminalis (medial and supracapsular), and lateral septum (LS). Images were taken on a Nikon Eclipse E400 microscope with 10−20× objectives. Fos-positive cells were counted with NIH Scion Image software as previously described (Rossi-George et al, 2005). The quantification of c-Fos positive cells was conducted on coded slides thereby rendering the experimenter blind to all treatments.
2.8 Data Analysis
For behavioral tests, two way ANOVAs were conducted, with repeated measures incorporated where comparisons of behavior before and after novel object exposure were assessed. For the c-Fos experiments, data were subjected to ANOVAs consistent with the factorial nature of the experimental design. Hence, a two-way ANOVA was conducted for the acute MeHg study involving open field exposure or LPS challenge. Further for the repeated and intracerebroventricular MeHg experiments, one way ANOVAs were conducted. In follow up analyses, post hoc comparisons were restricted to comparisons between groups according to the significance of main treatment effects. For this purpose, the Fisher's LSD test was used. Finally, where a priori expectations were present based on previously published literature on the effects of open field on c-Fos immunoreactivity in the brain (eg., Rossi-George et al, 2005), planned Student's t tests were conducted. In all statistical tests, differences were considered significant at p < 0.05.
3. Results
3.1 Acute and Repeated MeHg - Open Field Behavioral Testing
Analysis of the open field/novel object behavioral data in response to acute or repeated 2, 4, 8 mg/kg doses of MeHg or saline treatment revealed several significant findings as detailed below and shown in Figure 1.
Figure 1.

Impact of acute and repeated exposure to MeHg on behavior in the open field with and without a novel object. Top graphs (panel A) represent percent time spent in the outer and inner zones of the enclosed field in the absence of the novel object. Bottom graphs (panel B) represent the same paramaters as panel A, but in the presence of the novel object. Each bar represents the mean value (+/− standard error) of N = 3−8/group. * and ** represent p < 0.05 and 0.01 respectively when compared to corresponding saline injected controls.
3.1.1 Outer Zone
Analysis of the percentage of time spent in the outer zone, with and without the novel object, revealed that irrespective of acute treatment the time spent in the outer zone increased significantly after the novel object was introduced (F(3,17)=12.29, p<0.001)(Figures 1A and 1B). Acute open field data without the novel object showed a dose-dependent increase in time spent in the outer zone following MeHg treatment (F(3,17)=12.81, p<0.001), with the 4 and 8 mg/kg groups showing the longest duration of time in the outer zone when compared to saline treatments (p<0.001 and p<0.0001, respectively). Provocation by a psychogenic stressor with the novel object, resulted in acute saline and 2 mg/kg animals increasing the time spent in the outer zone, and the 4 and 8mg/kg MeHg being significantly different from saline (p=0.03 and p=0.04).
In contrast to acute MeHg treatment, the percentage of time spent in the outer zone of the open field was not significant in response to repeated MeHg treatment (Figures 1A and 1B), which differed markedly from the acute MeHg outer zone open field data. This was the case whether the novel object was present or not.
3.1.2 Inner and Novel Object Zones
Exploration of the inner zone after acute treatments was regulated by the presence of the novel object, as expected from previous findings (Kawashima & Kusnecov, 2002). In the absence of the novel object, mice spent a significantly greater percentage of time in the inner zone (F(3,17)=14.06, p<0.0001) (Figure 1A). There was a reciprocal reduction in the percentage of time spent in the inner zone as a function dose of MeHg treatment, with the most significant reduction occurring for the acute 4 and 8mg/kg MeHg doses. Introduction of the novel object produced a significant attenuation of inner zone exploration for all acute groups, with the 4 (p=0.03) and 8mg/kg MeHg (p=0.04) doses producing the greatest reductions (Figure 1B). Analysis of the percentage of time spent in the novel object zone revealed no significant statistical differences between treatment groups.
Repeated exposure to MeHg did not result in statistically significant differences in time spent exploring the inner or novel object zones when compared to saline controls (Figures 1A and 1B). This trend differed from the acute OF data that demonstrated significant decreases in percent time spent in inner and novel object zones in response to increasing doses of MeHg.
3.1.3 Total Distance Traveled
Distance traveled in the open field without the novel object present exhibited a dose dependent decrease in total distance traveled following acute MeHg treatment (F(3,17)=23.35, p<0.0001), with the same dose dependent decrease in distance traveled noted in the presence of the novel object (F(3,17)=7.75, p=0.0018) (Figure 2A and 2B, respectively). Without the novel object present, only comparisons of acute saline-2mg/kg MeHg (p=0.055) treatments were found to not be significant. In the presence of the novel object there was less overall statistical significance between acute groups, as saline-2mg/kg MeHg (p=0.273) and 4−8mg/kg (p=0.883) MeHg were not found to be significantly different.
Figure 2.

Impact of acute and repeated exposure to MeHg on total distance traveled in the open field in the absence (A) and presence (B) of the novel object. Each bar represents the mean value (+/− standard error) of N = 3−8/group. * and ** represent p < 0.05 and 0.01 respectively when compared to corresponding saline injected controls.
With respect to repeated MeHg treatment, total distance traveled in the open field without the novel object found that mice repeatedly given 4mg/kg MeHg traveled a greater distance than those given vehicle (p<0.05). All other distance comparisons between treatments were not found to be significant, including those in the presence of the novel object. Also, total distance traveled after repeated administration of MeHg did not result in dose dependent effects similar to those present in the acute study.
3.2 Acute and Repeated MeHg - c-Fos Immunoreactivity
Stressor, dosing regime, and dose-dependent changes in neuronal c-Fos immunoreactivity were noted within stress-associated brain regions. Analysis of selected brain regions was guided by previous evidence that exposure to psychogenic stressors activates brain nuclei located in the hypothalamus, thalamus, hippocampus, amygdala, and lateral septum (Senba and Ueyama, 1997). In addition, attention was directed to the locus coeruleus, a critical region responsive to stress, and implicated in anxiety-like behaviors (Carrasco and Van de Kar, 2003).
3.2.1 Paraventricular thalamic nucleus
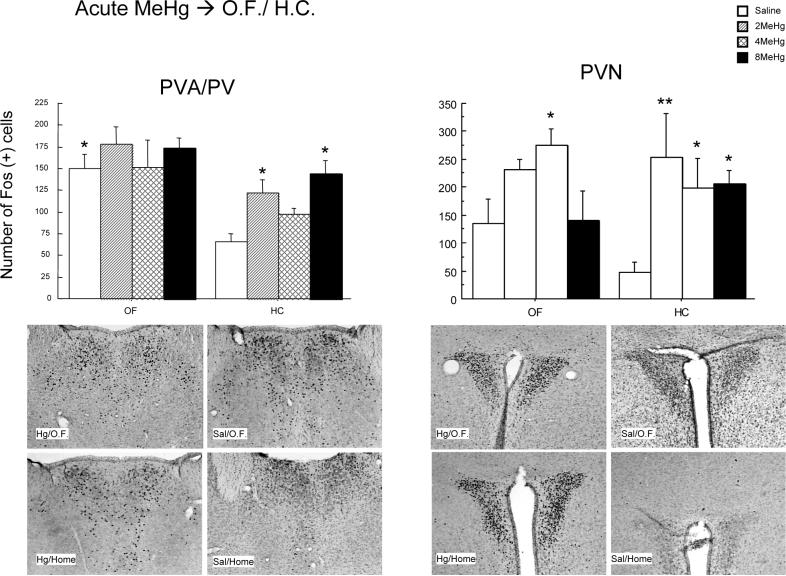
In the thalamus, the paraventricular nucleus (PVA/PV) showed marked increases in the number of c-Fos positive immunoreactive cells after acute MeHg exposure in response to both open field (F(1,34)=20.07, p<0.0001) and treatment with MeHg (F(3,34)=3.98, p<0.05) when compared to the home cage and saline controls (Figure 3). The most statistically significant differences were between acutely treated saline mice and the 2 and 8mg/kg MeHg treatments (both p<0.01). Interestingly, there was a trend toward increased c-Fos cell counts when 4mg/kg was compared to 8mg/kg MeHg (p=0.07). There was no significant interaction between open field exposure and MeHg treatment.
Figure 3.
Effect of open field stress and/or acute MeHg exposure on c-Fos immunoreactivity in the PVA of the thalamus and PVN of the hypothalamus. Each bar represents the mean number of c-Fos immunoreactive (ir) cells of N = 4−10/group. Representative photomicrographs indicate c-Fos immunoreactivity for the various treatment groups as described in materials and methods. * and ** represent p < 0.05 and 0.01 respectively when compared to corresponding saline injected controls.
The response of the PVA/PV to repeated MeHg was not significantly different than control levels, while c-Fos immunoreactivity in response to the open field did increase after repeated MeHg treatment (F(1,23)=21.39, p<0.0001) (Figure 7). Specifically, saline and 2mg/kg MeHg-OF mice had more robust c-Fos activation when compared to the respective HC treatments.
Figure 7.

Effect of open field stress and/or repeated MeHg exposure on c-Fos immunoreactivity within the paraventricular thalamic (PVA/PV) and hypothalamic (PVN) nuclei, central amygdaloid nucleus (CeC) and dentate gyrus of the hippocampus (Dg). Each bar represents the mean number of c-Fos ir cells of N = 4−10/group. * and ** represent p < 0.05 and 0.01 respectively when compared to corresponding saline injected controls or where indicated as combined group comparisons.
3.2.2 Paraventricular hypothalamic nucleus
Increased numbers of c-Fos positive cells were noted in the paraventricular hypothalamic nucleus (PVN) following acute MeHg treatment (F(3,34)=7.11, p<0.001). Significant differences were noted between saline-HC and the 2, 4, and 8mg/kg MeHg-HC treated mice (p<0.001, p<0.01 and p<0.01, respectively). The number of c-Fos positive cells also increased in response to exposure to greater concentrations of MeHg (Figure 3). In the open field treatments, the 4mg/kg MeHg had significantly greater c-Fos immunoreactivity when compared to saline controls (p<0.05). No MeHg-open field interaction effects were noted.
An interaction effect was noted in the PVN's c-Fos response to repeated MeHg exposure and the open field (F(2,23)=4.68, p<0.05) (Figure 7). It follows that this response stems from the MeHg dose-dependent increase in c-Fos (+) cells among home cage groups, while OF groups remained similar to one another.
Due to data previously published from our lab noting that open field stress produces statistically significant increases in the number of c-Fos positive cells within the PVN (Rossi-George et al, 2005), an a priori Student's T-test was conducted comparing saline-HC to saline-OF mice (t(18)=2.21, p<0.05), confirming that open field exposure increased the number of c-Fos immunoreactive cells in the PVN.
3.2.3 Central amygdaloid nucleus
The central nucleus of the amygdala (CeC) showed increased numbers of c-Fos immunoreactive cells in response to all doses of acutely administered MeHg (F(7,34)=10.24, p<0.0001) (Figure 4). Specifically acute treatment with MeHg resulted in increased c-Fos counts in 2, 4, 8mg/kg MeHg-HC animals (all p<0.001) when compared to the saline-HC control. Also open field groups given 2 or 8mg/kg MeHg had a greater c-Fos response when compared to saline (both p<0.05). There were no significant differences in c-Fos positive cell counts noted in response to open field or open field-acute MeHg exposure.
Figure 4.
Effect of open field stress and/or acute MeHg exposure on c-Fos immunoreactivity in the central amygdaloid nucleus (CeC) and the dentate gyrus (DG) of the hippocampus. * and ** represent p < 0.05 and 0.01 respectively when compared to corresponding saline injected controls or where indicated as combined group comparisons. See Figure 3 for further details.
Similarly, repeated exposure to MeHg led to the final MeHg injection still strongly activating the CeC (F(2,23)=8.21, p<0.01). However the CeC was not activated in response to open field alone or in combination with MeHg (Figure 7). The 4mg/kg MeHg-HC treatment created a greater c-Fos response when compared to either the saline or 2mg/kg MeHg-HC treatments.
3.2.4 Dentate gyrus of the hippocampus
The dentate gyrus of the hippocampus (Dg) expressed significant increases in the number of c-Fos positive cells with respect to both open field (F(3,34)=25.32, p<0.0001) and acute MeHg (F(1,34)=3.16, p<0.05) exposure (Figure 4). Saline, 2, and 4mg/kg acute MeHg-OF groups had higher c-Fos immunoreactivity when compared to all home cage treatments (all p<0.05). No open field-MeHg interaction effects were found to be significant.
The dentate gyrus also responded with a reduction of c-Fos cell counts in response to repeated MeHg treatment (F(2,23)=4.75, p<0.05) and increased cells after OF stimulation (F(71,23)=77.489, p<0.0001) (Figure 7). Saline, 2 and 4mg/kg MeHg-OF treatments were found to have significantly higher c-Fos concentrations than the home cage group, while in response to MeHg the 2mg/kg treatment had significantly lower c-Fos levels than the saline-HC control.
3.2.5 Locus coeruleus
The response of the locus coeruleus (Lc) to acute MeHg exposure resulted in significantly increased numbers of c-Fos positive cells after acute MeHg treatment (F(3,34)=11.62, p<0.0001). Specifically, home cage mice given acute dosed of 2mg/kg MeHg (p<0.01), 4, and 8mg/kg MeHg (both p<0.0001) had significantly higher c-Fos cell counts than saline controls (Figure 5). Within the open field exposed groups, a greater number of c-Fos cells was associated with acute 4 and 8mg/kg MeHg (both p<0.05) when compared to saline-OF. The number of c-Fos positive cells was not significantly affected by open field stress or interaction effects with MeHg and the open field.
Figure 5.
Effect of open field stress and/or acute MeHg exposure on c-Fos immunoreactivity in the locus coeruleus (Lc) and lateral septum (LS). See Figure 4 for further details.
The c-Fos response to repeated MeHg exposure increased significantly (F(2,23)=4.27, p<0.05) and after open field stimulation (F(1,23)=5.54, p<0.05) (Figure 8). Specifically, in response to MeHg saline-HC had lower c-Fos immunoreactivity than the 4mg/kg MeHg-HC group (p<0.01) and the 2mg/kg MeHg-OF group had higher c-Fos levels than saline-OF controls.
Figure 8.

Effect of open field stress and/or repeated MeHg exposure on c-Fos immunoreactivity in the locus coeruleus (Lc), lateral septum (LS), and medial and supracapsular BST. See Figure 7 for further details.
3.2.6 Lateral septum
Fos positive cell counts in the lateral septum (LS) were increased significantly following exposure to the open field (F(1,34)=30.51, p<0.0001) and acute MeHg exposure (F(3,34)=6.45, p<0.01) (Figure 5). In response to open field stimulation, saline, 2 and 4mg/kg MeHg-O.F. treatments had greater c-Fos positive cell counts than any home cage treatment. Methylmercury exposure led the 4 and 8mg/kg MeHg-O.F. groups to have statistically different c-Fos responses when compared to the saline-O.F. control (both p<0.05). Interestingly, a trend toward OF-MeHg interaction was of borderline significance (p=0.0568).
Repeated exposure to MeHg and open field stimulation resulted in an interaction effect (F(2,23)=5.42, p<0.05). The interaction effect noted in the LS likely resulted from the MeHg-induced dose dependent decrease in c-Fos levels within the open field groups, while the home cage treatments showed lower c-Fos activity (Figure8).
3.2.7 Bed nucleus of the stria terminalis
Counts for c-Fos positive cells in the bed nucleus were divided into medial (BSTm) and supracapsular (BSTs) areas. The BSTm response to the open field and acute MeHg dosing resulted in c-Fos counts that varied significantly with respect to saline and/or home cage controls (Open Field main effect: F(1,34)=6.35, p<0.05; MeHg main effect F(3,34)=8.10, p<0.001) (Figure 5). All acute doses of MeHg increased cFos counts relative to saline treated mice (p<0.0001). There were no interaction effects between open field and MeHg exposures for the BSTm. In the BSTs exposure to the open field stressor yielded increased cFos positive cell counts when compared to home cage controls (F(1,24)=22.34, p<0.0001). However, neither MeHg treatment nor combined open field-MeHg treatment significantly altered cFos immunoreactivity in the BSTs.
After repeated administration of MeHg and exposure to the OF, the resulting c-Fos stimulation in the BSTm and BSTs differed. Within the BSTm there was a c-Fos interaction effect in response to the repeated MeHg and OF stimuli (F(2,23)=4.08, p<0.05). The repeated MeHg-OF interaction in the BSTm could be due to the MeHg-induced, dose dependent, increase in c-Fos counts within HC animals while OF treatments remained similar to one another (Figure 8). Significant c-Fos activation in the BSTs resulted from both repeated exposure to MeHg (F(1,23)=36.83, p<0.0001) and open field (F(2,23)=6.04, p<0.01). Specifically, repeated MeHg reduced c-Fos cell counts within the 2 and 4mg/kg MeHg-OF groups when compared to saline-OF (p<0.05 and p<0.01, respectively) (Figure8).
Summary of Acute vs Repeated MeHg Results
In summary, repeated exposure of MeHg led to very different effects on exploratory behavior than observed after a single exposure, although similar activation of stress-associated brain regions was observed. Acute MeHg exposure resulted in a dose-dependent disruption of exploratory behavior as reported above. In contrast, after repeated preexposure to MeHg, such disrupted behavior was significantly attenuated. Increased numbers of c-Fos positive cells were observed in response to acute MeHg in the PVA/PV, PVN, CeC, Dg, Lc, LS, and BSTm, while repeated MeHg increased activation in the CeC and Lc, but decreased activation in the Dg and BSTs.
3.3 Repeated MeHg and LPS – c-Fos Immunoreactivity
The repeated MeHg experiment revealed that even by the sixth injection of MeHg, there was still a significant recruitment of stress-associated brain regions in terms of c-Fos immunoreactivity. The present experiment tested whether 3 days after the final MeHg injection there was a significant response to handling and injection of saline or a neuroactive dose of the proinflammatory stimulus, LPS.
Repeated exposure to MeHg followed by LPS administration 72 hours later did not attenuate the c-Fos response to LPS, while activation of these stress nuclei to LPS was found to be significant when compared to controls. Treatment with LPS led to a dramatic increase in the number of cFos positive cells present in the PVA, PVN/PV, CeC, Lc, and BSTm (all p≤0.01) when compared to repeatedly treated MeHg or saline groups not given LPS (Figures 10 & 11). Also, repeated administration of varying doses of MeHg did not produce significant c-Fos positive cell count differences in any of the brain regions analyzed after the final injection. Differences in cFos immunoreactivity between initial MeHg/saline treatments were not significant.
Figure 10.

Effect of LPS treatment on c-Fos immunoreactivity in the locus coeruleus (Lc), lateral septum (LS), and medial and supracapsular BST in mice previously exposed to repeated injections of MeHg. See Figure 9 for further details.
Figure 11.

Effect of intracerebroventricular (ICV) MeHg exposure on c-Fos immunoreactivity in the PVA of thalamus, PVN of hypothalamus, central amygdaloid nucleus (CeC) and dentate gyrus (Dg). Each bar represents the mean number of c-Fos immunoreactive (ir) cells of N = 4−9/group. * p < 0.05 relative to saline infused animals.
3.4 Intracerebroventricular MeHg and LPS – c-Fos Immunoreactivity
The previous experiments tested the neural effects of systemic MeHg, which may be mediated by mechanisms that are independent of MeHg accumulation in the brain. Therefore, the present experiment determined the impact of direct ICV administration of MeHg into the brain. Infusion of MeHg led to a significant increase of c-Fos positive cell numbers, and activation of several stress-associated brain nuclei while LPS infusion was typically less active. Analysis of selected brain regions was consistent with both the acute and repeated MeHg studies noted in Methods section 3.2.
Activation of stress associated pathways after ICV administration of MeHg and LPS gave results that varied with region when compared to saline and sham controls. Activation of the paraventricular thalamic and hypothalamic nuclei were highest response in the MeHg group when compared to all groups (p<0.01), while no effect was noted in response to the 0.5ng LPS dose (Figure 11). Responses of the central amygdala and dentate gyrus to MeHg and LPS treatments were not significant when compared to saline controls. Similar to both the acute and repeated IP MeHg studies, the locus coeruleus responded very strongly to MeHg when administered ICV after comparison with LPS and control groups (p<0.01) (Figure 12). Response of the Lc to ICV LPS was similar to that of saline. The lateral septum had significantly higher c-Fos activity in response to MeHg when compared to either LPS or saline (p<0.01 and p<0.05, respectively), which themselves were not significantly different from one another. The medial bed nucleus of the stria terminalis (BSTm) was activated after administration of MeHg when compared with vehicle control (p<0.05) with no effect in response to LPS. The supracapsular BST did not respond significantly to ICV MeHg or LPS administration. It should also be reported that sham animals had very low levels of c-Fos.
Figure 12.

Effect of intracerebroventricular (ICV) MeHg exposure on c-Fos immunoreactivity in the locus coeruleus (Lc), lateral septum (LS), and medial and supracapsular BST. Each bar represents the mean number of c-Fos immunoreactive (ir) cells of N = 4−9/group. * and ** p < 0.05 and p < 0.01 relative to saline infused animals.
4. Discussion
This study has demonstrated that centrally and peripherally administered acute doses of methylmercuric chloride (MeHg), repeated peripheral treatment with MeHg, and exposure to a psychogenic stressor, open-field (OF), differentially affect c-Fos protein production across a number of murine limbic brain regions commonly associated with mediating stress responses in the brain. While activation of these brain regions by open field exposure is not inconsistent with previously published reports (Rossi-George et al, 2005), the observation that exposure of mice to MeHg similarly recruits these very same brain regions has not previously been reported. The pattern and extent of methylmercury-induced stress circuit activation within the murine brain was found to depend on the route, dose, and frequency of administration. Repeated exposure to similar doses of MeHg engaged these stress circuits differently than noted in response to acute MeHg exposure. While the c-Fos response to repeated MeHg exposure 72 hours after the last dose demonstrated low c-Fos induction, this drop in c-Fos was shown not to be due to a failure of c-fos induction, since the c-Fos response in these brain regions was strongly induced by subsequent administration of LPS. Finally, acute intracerebroventricular (ICV) MeHg administration strongly activated these same stress nuclei with some notable differences.
Acute and repeated administration of MeHg yielded dramatically different behaviors in the open field. Behavioral testing showed that acute treatment across several MeHg doses was capable of significantly and uniquely affecting the exploratory response of mice in the open field. The open field test consisted of two phases. In the first phase, animals were introduced to an empty open field for the purpose of observing their exploratory behavior within the outer (perimeter) and inner regions of the field. This is the traditional test of open field behavior, and is generally believed to provide an index of emotion and/or arousal (Crawley, 1985; Weiss et al., 2000). In the second phase, a novel object was introduced into the open field, a procedure that is designed to provoke an orienting response, which may be interpreted as providing a stimulus-specific measure of arousal and/or defensive behavior, and typically induces a change in ongoing exploratory behavior. Indeed, as shown in the results, saline treated mice displayed a reduction in ongoing locomotor behavior upon introduction of the novel object, with spatial location being confined more to the outer zone of the open field.
In general, acute treatment with MeHg altered the initial exploratory response upon introduction to the open field, and subsequently, also differentiated mice from saline controls once the novel object was introduced. That is, relative to saline treated controls, MeHg treatment increased the percentage time spent in the peripheral zone (during the first 10 min of the open field test without the novel object) and decreased the percentage of time spent in exploring the inner zone when the novel object was introduced. This effect was dose-dependent, with animals treated with the highest dose (8 mg/kg) showing the greatest reduction in exploratory behavior. It is difficult to determine from these observations whether the treatment with MeHg retarded movement as a function of extreme illness or malaise. However given that administration of methylmercury to rodents results in immediate appetite suppression (Berthoud et al., 1976; Magos, 1982), it is possible that a sickness-associated behavior may be affecting exploratory movement in the open field. Also, noting that acutely treated MeHg animals reacted to the presentation of the novel object by reducing entry into the inner zone where the object was placed, it is likely that in spite of MeHg induced toxicity, attention to environmental modifications was not impaired. At the very least, behavioral testing in the open field confirmed that at least for the two highest doses (4 and 8 mg/kg), acute administration of MeHg was behaviorally active. Of interest is the observation (see below) that the lowest acute dose (2 mg/Kg) was not generally observed to be strongly disruptive of exploratory behavior, but nevertheless, induced increased c-Fos protein production in the brain.
While acute intraparitoneal administration of MeHg had a general dose-dependent effect on OF behavior, performance in response to the final injection of a repeated MeHg dosing regimen resulted in behavior that was similar to saline controls. The only significant difference in OF behavior noted in the chronic MeHg experiment was that saline controls traveled a greater distance than MeHg treated mice without the novel object present. The discrepancies in open field behavior in response to acute and repeated MeHg exposure demonstrates that repeated administration of MeHg results in an attenuated behavioral response in the open field. It is therefore possible that the mechanism through which MeHg is eliciting its effect on open field behavior is no longer recruited after repeated MeHg treatment. Finally, the most sensitive of the behavioral measures collected in this experiment was total distance traveled as it produced a strong, dose-dependent response to acute MeHg and was the only significant behavioral parameter in response to repeated MeHg administration. Indeed, these findings are consistent with Goulet et al., 2003 that showed repeated perinatal exposure to MeHg results in decreased locomotion in mice when compared to saline controls. Other studies on the effects of chronic exposure to MeHg on behavior have been conducted and these have shown lifetime exposure to low levels (1 and 3 parts per million) of methylmercury additionally alters hindlimb splay (a test of motor function) and delayed spatial alternation and inhibition of exploratory behavior in the open field measured by hind limb rearings (Weiss et al., 2005; Morganti et al., 1976).
Analysis of c-Fos positive cell counts in response to administration of MeHg revealed varying intensities of c-Fos activation within stress-associated nuclei of the mouse brain. The paraventricular thalamic nucleus (PVA/PV) is responsible for processing afferent sensory input to the cortex, mediating influence on both motor and cognitive functions (Spencer et al., 2004). An increase in PVA/PV neuronal activity was observed in response to both IP and ICV acute MeHg treatment and following stimulation by the open field after acute IP exposure. However, repeated MeHg exposure did not significantly induce c-Fos production in the PVA/PV, while open field stimulation did. Similarly, the PVN, which regulates autonomic and behavioral responses to various psychogenic and systemic stressors, also showed substantial c-Fos immunoreactivity in response to both IP and ICV acute doses of MeHg and open field. Repeated administration of MeHg resulted in a MeHg-open field interaction effect within in the PVN. This likely resulted from a MeHg dose-dependent increase in c-Fos production in non-open field exposed animals while c-Fos levels for open field mice were higher but not different from one another. It is likely therefore that the two major efferent indices of PVN activation, namely, increased HPA axis and sympathetic nervous system function, were likely evident following MeHg treatment. However, measures of catecholamines and corticosterone (or ACTH) were not conducted in the present study to confirm whether this was the case. Future studies should verify this possibility.
The central amygdaloid nucleus (CeC) is known to influence the activity of the hypothalamus following input from cortical areas responding to environmental information (Koob & Heinrichs, 1999). In general, it has been well demonstrated that changes in neuroendocrine and autonomic functions, such as heart rate, respiration rate, and blood pressure, following exposure to fearful and/or stressful stimuli are mediated by neurons in the CeC (Shekhar et al., 2003). Increased activity in the CeC was noted in response to acute and repeated IP MeHg treatment but was not appreciably influenced by open field exposure or ICV administration of MeHg. While open field exposure per se likely involves induction of a fear-like state due to the unfamiliar circumstances, the lack of a significant c-Fos increase may be due to insufficient intensity of this psychogenic stressor. Indeed, in other studies it has been shown that c-Fos is reliably increased in the CeC in response to a variety of psychological stressors (Pacak et al., 1995, Senba and Ueyama, 1997, Martinez et al., 2002, Rossi-George et al., 2005). Nonetheless, CeC activation by MeHg suggests engagement of neural substrates necessary for adaptational physiological and behavioral responses, and is consistent with the significant reduction in exploratory behavior observed in MeHg treated mice.
Exposure of hippocampal neurons to methylmercury has been demonstrated to induce permanent changes in learning and memory with specific sensitivity in neurons of the dentate gyrus (Annau and Cuomo, 1988, Kakita et al., 2000, Andersson et al., 1997). In the current study, it was noted that the dentate gyrus of the hippocampus (Dg), showed a significant increase in the number of c-Fos positive cells in response to open field exposure after both acute and repeated IP MeHg treatments. The Dg also had a significantly greater c-Fos response to acute IP administration of MeHg alone, while response of this nucleus to centrally administered (ICV) MeHg was not found to be significant. In response to stressful stimuli, the Dg takes efferent neuroendocrine signals from the limbic system (entorhinal cortex), processes, and relays them to the fornix, which is consistent with data from this study showing that acute MeHg engages the brain's hippocampal stress circuitry. Robust increases in c-Fos immunoreactive cells were observed in response within the locus coeruleus to acute and repeated IP MeHg exposure, open field stimulation after acute exposure and ICV administered MeHg. The Lc functions as a hindbrain region rich in forward-projecting noradrenergic neurons innervating much of the forebrain limbic brain regions (Saper, 1987). Moreover, the Lc has long been considered an important regulator of arousal and anxiety (Sullivan et al., 1999, Berridge and Waterhouse, 2003). Similarly, forebrain regions known to receive noradrenergic input, such as the bed nucleus of the stria terminalis (BST) and lateral septum (LS) (Mulders et al., 1997, Forray 2004), and known to be critical to stress and anxiety (Morilak et al., 2005), were also activated by the open field and MeHg exposure. Given that the BST, LC and LS have all been shown to be critical to anxiety-like behavior, this suggests that toxic doses of MeHg generate neural responses that likely induce an anxiogenic state. The reduced exploratory behavior in the open field and novel object test supports this conclusion, although how much of the immobility is due to impaired locomotor function as a result of somatic discomfort and malaise is not known.
In a separate experiment, it was determined whether repeated exposure to 2 and 4 mg/Kg MeHg influenced the number of c-Fos immunoreactive neurons in response to systemic LPS, which was administered 3 days after the final saline or MeHg injection. At this time, there was no residual increase in c-Fos immunoreacivity or sensitized responsiveness to the stress of a saline injection. Moreover, it appeared that for most brain regions that were examined, there was no major alteration in the increased number of c-Fos positive cells that is seen in response to LPS challenge of C57BL/6 mice as a function of prior MeHg treatment (eg., Rossi-George et al, 2005). Indeed, LPS exposure caused a significant increase of c-Fos positive cells in the PVA/PV, PVN, CeC, Lc, and BSTm. Interestingly, no major effect of LPS was observed in the dentate gyrus, although increased c-Fos expression occurred in response to acute MeHg treatment. Whether this reflects passage of MeHg into the brain and a more direct impact on Dg cells remains to be determined.
In conclusion, the present study showed that acute ICV and IP MeHg administration, repeated IP MeHg dosing, in addition to open field exposures were capable of strongly and distinctly activating stress-associated neural circuitry in the murine brain, while repeated exposure to MeHg attenuated the dose-dependent effects on exploratory behavior that resulted from acute MeHg exposure. As noted above, repeated administration of MeHg does not attenuate the c-Fos response within these regions after a final dose of MeHg. However, 3 days after the last repeated MeHg dose the c-Fos response is back down to control levels with LPS stimulation of these circuits demonstrating that repeated MeHg does not permanently alter the c-Fos response within these stress-associated nuclei. While this study showed that MeHg activates the underlying neuroanatomical circuitry involved in the response of an organism to stress, examination of how similar centrally and peripherally administered doses of MeHg are capable of affecting brain inflammatory cytokine levels would also be an important question for future investigations.
Figure 6.
Effect of open field stress and/or acute MeHg exposure on c-Fos immunoreactivity in the bed nucleus of the stria terminalis (medial and supracapsular regions). See Figure 4 for further details.
Figure 9.

Effect of LPS treatment on c-Fos immunoreactivity in the PVA of thalamus, PVN of hypothalamus, central amygdaloid nucleus (CeC) and dentate gyrus (Dg) in mice previously exposed to repeated injections of MeHg. Each bar represents the mean (+/− SE) of N=5/group. * and ** represent p < 0.05 and 0.01 respectively when compared to corresponding saline or MeHg pretreated groups injected with Saline on the day of sacrifice.
Acknowledgments
Supported by PHS Grants MH60706, DA141186, NIEHS P30 ES022, NIH-ES07148
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adachi T, Yasutake A, Hirayama K. Influence of dietary levels of protein and sulfur amino acids on the fate of methylmercury in mice. Toxicology. 1994;93:225–34. doi: 10.1016/0300-483x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Akasaka S, Nomura M, Nishii H, Fujimoto N, Ueta Y, Tsutsui M, Shimokawa H, Yanagihara N, Matsumoto T. The hypothalamo-pituitary axis responses to lipopolysaccharide-induce endotoxemia in mice lacking inducible nitric oxide synthase. Brain Res. 2006;1089(1):1–9. doi: 10.1016/j.brainres.2006.02.112. [DOI] [PubMed] [Google Scholar]
- Andersson H, Lindqvist E, Olson L. Downregulation of brain-derived neurotrophic factor mRNA in adult rat brain after acute administration of methylmercury. Mol Chem Neuropath. 1997;31(3):225–33. doi: 10.1007/BF02815126. [DOI] [PubMed] [Google Scholar]
- Annau Z, Cuomo V. Mechanisms of neurotoxicity and their relationship to behavioral changes. Toxicology. 1988;49(2−3):219–225. doi: 10.1016/0300-483x(88)90002-9. [DOI] [PubMed] [Google Scholar]
- Aschner M. Astrocytes as modulators of methylmercury-induced neurotoxicity. Neurotoxicol. 1996;17:663–670. [PubMed] [Google Scholar]
- Bakir F, Damiuji SF, Amin-Zaki L, Murthadha M, Khaldidi A, Al-Rawi NY, Tikriti S, Dhahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Santolo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci. 2005;119(6):1459–1466. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell IR, Baldwin CM, Fernandez M, Schwartz GE. Neural sensitization model for multiple chemical sensitivity. Toxicol Indust Health. 1999;15(3−4):295–304. doi: 10.1177/074823379901500303. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-norandrenergic system. Brain Res Brain Res Rev. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Garman RH, Weiss B. Food intake, body weight, and brain histopathology in mice followed by chronic methylmercury treatment. Tox Appl Pharmacol. 1976;36:19–30. doi: 10.1016/0041-008x(76)90023-5. [DOI] [PubMed] [Google Scholar]
- Brochu S, Olivier M, Rivest S. Neuronal activity and transcription of proinflammatory cytokines, IkappaBalpha, and iNOS in the mouse brain during acute endotoxemia and chronic infection with Trypanosoma brucei brucei. J Neurosci Res. 1999;57(6):801–816. [PubMed] [Google Scholar]
- Burton GV, Meikle AW. Acute and chronic methyl mercury poisoning impairs rat adrenal and testicular function. J Toxicol Environ Health. 1980;6(3):597–606. doi: 10.1080/15287398009529877. [DOI] [PubMed] [Google Scholar]
- Cheng JP, Wang WH, Jia JP, Zheng M, Shi W, Lin XY. Expression of c-fos in the rat brain as a prelude marker of central nervous system injury in response to methylmercury-stimulation. Biomed Environ Sci. 2006;19(1):67–72. [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9(1):37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmocol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW, Amin-Zaki L, Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization and diffuse white matter astrogliosis of human fetal brain: A major effect of methylmercury exposure in utero. J Neuropath Exp Neurol. 1978;37:719–733. doi: 10.1097/00005072-197811000-00001. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Weiss B. Mercury exposure and child development outcomes. Pediatrics. 2004;113(4):1023–1029. [PubMed] [Google Scholar]
- Doi R, Kobayashi T. Organ distribution and biological half-time of methylmercury in four strains of mice. J Exp Med. 1982;52(6):307–14. [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Palamarchouk V. Brain ciruits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann NY Acad Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- Forray MI, Gysling K. Role of norandrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Goulet S, Dore FY, Mirault ME. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and postnatal development. Neurotox Teratol. 2003;25(3):335–347. doi: 10.1016/s0892-0362(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Hayley S, Merali Z, Anisman H. Stress and cytokine-elicited neuroendocrine and neurotransmitter sesitization. Stress. 2003;6(1):19–32. doi: 10.1080/1025389031000091167. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Insug O, Datar S, Koch CJ, Shapiro IM, Shenker BJ. Mercury compounds inhibit human monocytes function by inducing apoptosis: evidence for formation of reactive oxygen species, development of mitochondrial membrane permeability transition and loss of reductive reserve. Toxicology. 1997;124:211–224. doi: 10.1016/s0300-483x(97)00153-4. [DOI] [PubMed] [Google Scholar]
- Jaworowicz DJ, Korytko PJ, Singh Lakhman S, Boje KM. Nitric oxide and prostaglandin E2 formation parallels blood-brain barrier disruption in an experimental rat model of bacterial meningitis. Brain Res Bull. 1998;46(6):541–546. doi: 10.1016/s0361-9230(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127(3):569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kakita A, Wakabayashi K, Su M, Yoneoka Y, Sakamoto M, Ikuta F, Takahashi H. Intrauterine methylmercury intoxication. Brain Res. 2000;877(2):322–30. doi: 10.1016/s0006-8993(00)02717-7. [DOI] [PubMed] [Google Scholar]
- Kaneta T, Kusnecov AW. The role of central corticotrophin-releasing hormone in the anorexic and endocrine of the bacterial T-cell superantigen, Staphylococcal enterotoxin A. Brain Behav Immun. 2005;19:138–146. doi: 10.1016/j.bbi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Kusnecov AW. Effects of staphylococcal enterotoxin A on pituitary-adrenal activation and neophobic behavior in the C57BL/6 mouse. J Neuroimmunology. 2002;123:41–49. doi: 10.1016/s0165-5728(01)00486-6. [DOI] [PubMed] [Google Scholar]
- Kiank C, Holtfreter B, Starke A, Mundt A, Wilke C, Schutt C. Stress susceptibility predicts the severity of immune depression and the failure to combat bacterial infections in chronically stressed mice. Brain, Behav, Immun. 2006;20:359–368. doi: 10.1016/j.bbi.2005.10.151. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Kelley KW, Dantzer R. Temporal and spatial relationship between lipopolysaccharide-induced expression of Fos, interleukin-1β and inducible nitric oxide syntase in rat brain. Neurosci. 1999;89:535–548. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848(1−2):141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Kusnecov AW, Golfarb Y. Neural and behavioral responses to systemic immunologic stimuli: a consideration of bacterial T cell superanitgens. Cur Pharm Des. 2005;11(8):1039–1046. doi: 10.2174/1381612053381602. [DOI] [PubMed] [Google Scholar]
- Lee HY, Whiteside MB, Herkenham M. Area postrema removal abolishes stimulatory effects of intravenous interleukin-1β on hypothalamic-pituitary-adrenal axis activity and c-fos mRNA in the hypothalamic paraventricular nucleus. Brain Res Bull. 1998;46:495–503. doi: 10.1016/s0361-9230(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Schmidt ED, Van Dam AM, Gaykema RP, Tilders FJ. Individual variation in hypothalamus-pituitary-adrenal responsiveness of rats to endotoxin and interleukin-1 beta. Ann NY Acad Sci. 1998;856:139–147. doi: 10.1111/j.1749-6632.1998.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Limke TL, Otero-Montanez JK, Atchison WD. Evidence for interactions between intracellular calcium stores during methylmercury-induced calcium dysregulation in rat cerebellar granular neurons. J Pharmacol Exp Ther. 2003;304:949–958. doi: 10.1124/jpet.102.042457. [DOI] [PubMed] [Google Scholar]
- Magos L. Neurotoxicity, anorexia and the preferential choice of antidote in methylmercury intoxicated rats. Neurobehav Toxicol Teratol. 1982;4(6):643–646. [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping Brain Responses to Social Stress in Rodents with c-Fos Expression: A Review. Stress. 2002;5(1):3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–170. [PubMed] [Google Scholar]
- Miczek KA, Nikulina E, Kream RM, Carter G, Espejo EF. Behavioral sensitization to cocaine after a brief social defeat stress: c-fos expression in the PAG. Psychopharmacology. 1999;141:225–234. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Morganti JB, Lown BA, Salvaterra P, Massaro EJ. Effects on open-field behavior of mice exposed to multiple doses of methyl mercury. Gen Pharmacol. 1976;7:41–44. doi: 10.1016/0306-3623(76)90030-6. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neurophsycopharmacol Biol Psychiatry. 2005;29(8):1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Muira K, Suzuki K, Imura N. Effects of methyl mercury on mitotic mouse glioma cells. Environmental Research. 1978;17:453. doi: 10.1016/0013-9351(78)90048-8. [DOI] [PubMed] [Google Scholar]
- Mulders WHAM, Meek J, Hafmans TGM, Cools AR. Plasticity in the stress-regulating circuit. Eur J Neurosci. 1997;9:2462–2471. doi: 10.1111/j.1460-9568.1997.tb01663.x. [DOI] [PubMed] [Google Scholar]
- Murphy E. Social origins of depression in old age. Brit J of Psychiatry. 1982;141:135–142. doi: 10.1192/bjp.141.2.135. [DOI] [PubMed] [Google Scholar]
- Nielsen JB. Toxicokinetics of mercuric chloride and methylmercuric chloride in mice. J Toxicol Environ Health. 1992;37(1):85–122. doi: 10.1080/15287399209531659. [DOI] [PubMed] [Google Scholar]
- Neveu PJ, Leige S. Mechanisms of behavioral and neuroendocrine effects of interleukin-1 in mice. Ann NY Acad Sci. 2000;917:175–185. doi: 10.1111/j.1749-6632.2000.tb05382.x. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic pituitary nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol. 1995;16(2):89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Perisco AM, Schindler CW, O'Hara BF, Brannock MT, Uhl GR. Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Brain Res. 1993;20(1−2):91–100. doi: 10.1016/0169-328x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov A. Neuronal, endocrine, and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: dependence on tumor necrosis factor-alpha. J Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. Function of the locus coeruleus. Trends Neurosci. 1987;10:343–344. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Foley FW, Rao SM, Bernardin LJ, Lee H, Genderson MW. Stress and course of disease in multiple sclerosis. Behav Med. 1999;25(3):110–116. doi: 10.1080/08964289909596740. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascurlar responses. Ann NY Acad Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Silberman DM, Ayelli-Edgar V, Zorilla-Zubilete M, Zieher LM, Genaro AM. Impaired T-cell dependent humoral response and its relationship with T lymphocyte sensitivity to stress hormones in a chronic mild stress model of depression. Brain, Behav, Immun. 2004;18:81–90. doi: 10.1016/s0889-1591(03)00109-0. [DOI] [PubMed] [Google Scholar]
- Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Fox JC, Day TA. Thalamic paraventricular nucleus lesions facilitate central amygdala neuronal responses to acute psychological stress. Brain Res. 2004;997(2):234–237. doi: 10.1016/j.brainres.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety. Biological Psychiatry. 1999;46(9):1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi T. Minamata Disease. Study group of Minamata disease, Kumamoto University; Japan: 1968. pp. 141–228. [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity. J Neurochem. 2006;97(1):69–78. doi: 10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- Ulmer AJ, Flad H, Rietschel T, Mattern T. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS). Toxicology. 2000;152:37–45. doi: 10.1016/s0300-483x(00)00290-0. [DOI] [PubMed] [Google Scholar]
- Usuki F, Yasutake A, Umehara F, Tokunaga H, Matsumoto M, Eto K, Isiura S, Higuchi I. In vivo protection of a water-soluble derivative of vitamin E, Trolox, against methylmercury-intoxication in the rat. Neurosci Let. 2001;304:199–203. doi: 10.1016/s0304-3940(01)01764-5. [DOI] [PubMed] [Google Scholar]
- Verity MA, Brown WJ, Cheung M. Organic mercurial encephalopathy: in vivo and in vitro effects of methyl mercury on synaptosomal respiration. J. Neurochem. 1975;25:759–766. doi: 10.1111/j.1471-4159.1975.tb04405.x. [DOI] [PubMed] [Google Scholar]
- Verity MA, Brown WJ, Cheung M, Czer G. Methylmercury inhibition of synaptosome and brain slice protein synthesis: in vivo and in vitro studies. J Neurochem. 1977;29:673–679. doi: 10.1111/j.1471-4159.1977.tb07785.x. [DOI] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain epithelial cells: A polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Weiss B, Stern S, Cox C, Balys M. Perinatal and lifetime exposure to methylmercury in the mouse: behavioral effects. Neurotox. 2005;26:675–690. doi: 10.1016/j.neuro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Lightowler S, Stanhope KJ, Kennett GA, Dourish CT. Measurement of anxiety in transgenic mice. Rev Neurosci. 2000;11(1):59–74. doi: 10.1515/revneuro.2000.11.1.59. [DOI] [PubMed] [Google Scholar]
- Yasutake A, Hirayama K, Inouye M. Sex difference in acute renal dysfunction induced by methylmercury in mice. Renal Failure. 1990;12(4):233–40. doi: 10.3109/08860229009060730. [DOI] [PubMed] [Google Scholar]
- Yoshino Y, Mozai T, Nakao K. Biochemical changes in the brain in rats poisoned with an alkylmercury compound, with special reference to the inhibition of protein synthesis in brain cortex slices. J Neurochem. 1966;13:1223–1230. doi: 10.1111/j.1471-4159.1966.tb04281.x. [DOI] [PubMed] [Google Scholar]