Abstract
Background
The relationship between end-tidal sevoflurane concentration, BIS and the EEG bispectrum in children appears dependent on age. The aim of this study was to quantify the BIS values at 1 MAC for desflurane and halothane, and explore the relationship with age for these anaesthetic agents in children.
Methods
ECG, EEG and BIS were recorded continuously during the anaesthesia of ninety children aged 6–170 months requiring elective surgery. Fifty children were anaesthetised with desflurane, and forty children with halothane. Recordings were performed through to a steady state of 2 MAC, and thereafter at 1 MAC and 0.5 MAC respectively. The bispectrum of the EEG was estimated using MATLAB© software. For analysis, a multiple correspondence analysis (MCA) was used.
Results
At the steady state of 1 MAC, BIS values were significantly higher with halothane 62 (43–80) compared to desflurane 34 (18–64). BIS values were significantly correlated to age in both groups: DES (r2=0.57; p<0.01) and HALO (r2=0.48; p<0.01). Changes in position in the structured model of the MCA (dependent on the pattern of the EEG bispectrum) were different for the two volatile anaesthetic agents.
Conclusions
BIS values are linked to age of children irrespective of the volatile anaesthetic agent used. In children, the difference in BIS values for different agents at the same MAC can be explained by the specific effect on the EEG bispectrum induced by each anaesthetic agent, bringing into question the ability of the EEG bispectrum to accurately determine depth of anaesthesia in children.
Keywords: Adolescent; Age Factors; Anesthetics, Inhalation; pharmacology; Body Weight; physiology; Child; Child, Preschool; Electrocardiography; drug effects; Electroencephalography; drug effects; Female; Halothane; pharmacology; Humans; Infant; Isoflurane; analogs & derivatives; pharmacology; Male; Monitoring, Intraoperative; methods
Keywords: Depth of Anesthesia, EEG, Bispectrum, PCA, Factorial Analysis, Classification, Anesthesia, BIS, Monitoring
The BIS algorithm is derived from an adult EEG database obtained under various anaesthetic conditions1. In children the physiological pattern of the EEG is different, and evolves from infancy to adulthood. There is some evidence that the physiological differences between the EEG of adults and children may have clinical consequences on BIS monitoring. Aspect ™ suggests in its ‘Clinicians Guide to the Bispectral Index’ that BIS monitoring in children can yield benefits similar to those achieved in the adult population, despite many reservations already widely published. For instance, the relationship between end-tidal sevoflurane concentration, BIS and the EEG bispectrum in children has been found to be dependent on age2–4. There is also evidence that BIS values are agent dependent for the same level of MAC5,6. The relationship between other volatile anaesthetics agents and BIS, the EEG bispectrum and age has not yet been fully explored. The aim of this study was to explore the relationship between the age and the BIS values at one MAC for desflurane and halothane in children.
Methods
Following approval from the Human Studies Committee, ninety ASA I or II children aged 6–170 months requiring elective surgery were recruited into our observational study with parental consent. We excluded any children with central neurological disease and those taking medication acting on the central nervous system. No children were premedicated. EEG leads (3M Red Dot Silver/Silver Chloride model 2269T) were placed adjacent to the paediatric BIS lead (Aspect ™).
We induced anaesthesia with 8% sevoflurane in oxygen in the first fifty children. After obtaining intravenous access, all children were intubated without the use of muscle relaxants. Immediately post intubation, we switched the anaesthetic agent to desflurane. The children were then ventilated. Anaesthetic gas and carbon dioxide concentrations were measured continuously in order to ensure normocapnoea. In order to achieve both a washout, and a steady state at the effect site of 2 MAC desflurane (EEG signal stationary), the expired concentration of desflurane was maintained at 2 MAC age-corrected for 10 minutes with high fresh gas flows at 10 l.min−1 group DES). For the following forty children (group HALO), the conduct of anaesthesia was identical except that we used halothane for induction (3.5%) and subsequent maintenance at 2 MAC age-corrected until steady state at the effect site was obtained (EEG signal stationary).
After starting recording for analysis (T0), the end-tidal concentrations were decreased to 1 MAC corrected for age for both anaesthetic agents and recordings continued until steady state at 1 MAC was achieved (10 mins, T10). We then further decreased the end-tidal anaesthetic agent concentration to 0.5 MAC and continued recording until steady state at this concentration was achieved (T20). At the end of this period in the group HALO, anaesthetic agent was switched to sevoflurane at 1 MAC.
All recordings were obtained in the absence of any surgical stimulation and there no other drugs (anticholinergics or analgesics for example) administered during this period. ECG, raw EEG (PowerLab™) and Bispectral Index (BIS; Aspect XP™) were recorded continuously and mathematically processed as described previously2. Thus the EEG bispectrum for each child under desflurane or halothane anaesthesia was calculated and divided into 36 blocks of frequencies coupling every 20 sec throughout the study period.
For statistical analysis, we used a multiple correspondence analysis (MCA) derived from recordings of one hundred children anaesthetised with sevoflurane at 1 MAC age-corrected 2. In order to explore the effect of change in desflurane and halothane concentrations on the EEG bispectrum, the change in position in the structured model of the MCA during the decrease of anaesthetic agent concentration was analysed for each group of children. This change in position within the structured model of MCA was determined by changes in the MatBis (i.e. EEG bispectrum) during the decrease from 2 MAC to 0.5 MAC as explained in the appendix of our first article2. In addition, any correlation between age and BIS values obtained at 1 MAC for each anaesthetic agent were evaluated by means of a Spearman test. A Wilcoxon test was used to establish significant changes in parameters at various points during the decrease in sevoflurane. All results are described by median (range). A probability value <0.05 was considered significant. All statistical analyses were performed with the BI© LOGINSERM 1979/1987 software.
Results
We obtained complete recordings in all children except for two children in group HALO (because of protocol violations). Patient characteristics are represented in Table 1. No differences are seen between groups in terms of age and weight. The changes in BIS value with change in end-tidal desflurane or halothane concentration are represented in Figure 1. At steady state of 1 MAC age-corrected (T10), BIS values were significantly higher with halothane 62 (43–80) compared to desflurane 34 (18–64). BIS values were also significantly correlated to age in both groups at this MAC: DES (r2=0.57; p<0.01) and HALO (r2=0.48; p<0.01) (Figure 2).
Table 1.
Children’s characteristics and clinical parameters at 1 MAC desflurane (DES) and halothane (HALO)
| DBS (n=50) | HALO (n=38) | |
|---|---|---|
| Age (months) | 56 [6–161] | 60 [9–170] |
| Weight (kg) | 17.5 [4.5–48] | 17 [7.5–65] |
| Sex ratio M/F | 36/14 | 27/11 |
| End tidal volatile anaesthetic (%) | 8.4 [6.8–9.8] | 0.8 [0.7–1] |
| MBP (mmHg) | 54 [33–64] | 56 [44–76] |
MBP: Mean Blood pressure
Figure 1.
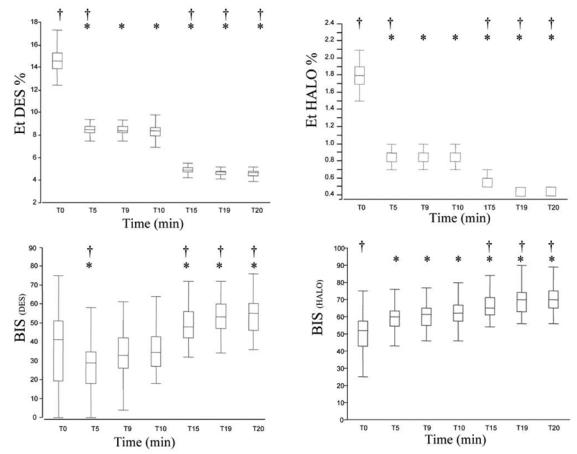
End-tidal anaesthetic agent concentration and BIS values obtained during recording (desflurane left, halothane right) T0 (2 MAC), T10 (1 MAC) and T20 (0.5 MAC). Box plot (range; 25–75th centile; median).
*vs T0: P<0.05; †vs T10 (1 MAC): P<0.05.
Figure 2.
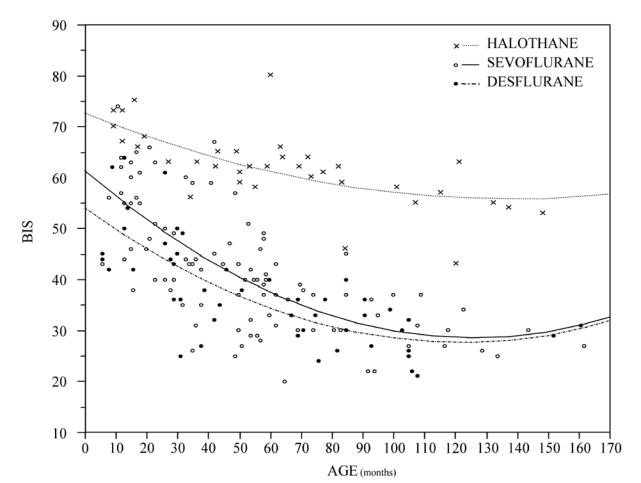
Relationship between BIS values at 1 MAC and age using three different volatile agents
In group DES, as end-tidal concentration of desflurane decreased from 2 MAC to 1 MAC and subsequently to 0.5 MAC, children’s position in the structured model of the MCA changed in a similar manner to those seen with sevoflurane anaesthesia (see Figure 3 and previous article2). In contrast, in group HALO, children’s position in the structured model at 2 MAC, and change in position during the concentration decrease was entirely different (Figure 3). When halothane 0.5 MAC was replaced by 1 MAC sevoflurane, children’s position in the MCA returned to the position that we found in children with sevoflurane anaesthesia alone.
Figure 3.
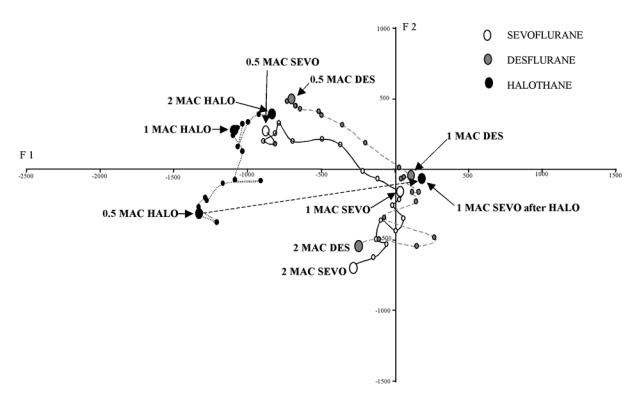
Change in children’s position in the structured model of the multiple correspondence analysis during decrease in concentration of the three volatile anaesthetic agents. Each point represents one minute. Larger dots represent steady state. The units of axes F1 and F2 are arbitrary.
An example of the shape of the EEG bispectrum obtained with sevoflurane, desflurane and halothane at 2 MAC, 1 MAC and 0.5 MAC respectively is represented in Figure 4.
Figure 4.
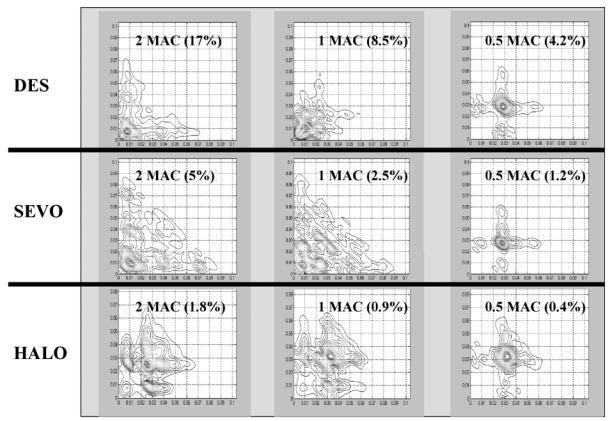
Examples of the different EEG bipsectrums obtained at the steady state of 2 and 1 and 0.5 MAC of Desflurane (DES), Halothane (HALO) and sevoflurane (SEVO) respectively.
Discussion
We have found, as previously reported with sevoflurane, that the BIS (Aspect XP™) measured at 1 MAC desflurane or halothane is strongly related to age of children. In addition, BIS values under 1 MAC halothane anaesthesia were found to be distinctly higher, 62 (43–80), than those under 1 MAC desflurane, 34 (18–64), or as previously found with 1 MAC sevoflurane anaesthesia2, 40 (20–60), irrespective of age of children (figure 2).
We have previously demonstrated, using a multiple correspondence analysis, that one of the main components used by Aspect™ to derive the BIS value, the EEG bispectrum, is itself dependent on age at 1 MAC sevoflurane in children above one year of age 2. This could explain the inverse correlation of BIS values with age above one year at 1 MAC sevoflurane 2,3. However, Davidson and colleagues have recently reported that in infants less than one year, pre-awakening BIS values show a tendency to be lower than those obtained in children above one year4. This correlates with the finding that target BIS values of 40–60 in infants less than six-months can be achieved with very low concentrations of sevoflurane7. These results taken together mean that in clinical practice, if sevoflurane is titrated against BIS in children above one year of age there will be a tendency to use levels greater than 1 MAC (but diminishing as age increases). In contrast in children less than one year sevoflurane concentration could be titrated down to a very low level against the BIS, resulting in levels of less than 1% (much less than 0.5 MAC) in this population, a practice than is generally not clinically acceptable, especially if a muscle relaxant is used7.
In this study, we have found that the same relationship exists for desflurane as for sevoflurane in children greater than one year. In contrast with halothane, although a relationship exists between age and BIS at 1 MAC, it appears different to that with the other two anaesthetic agents. In a study of forty children older than two years, Davidson and colleagues have demonstrated that BIS values obtained under halothane (56.5 +/− 8.1) were higher than those under isoflurane (35.9 +/− 8.5) at 1 MAC4. Similarly, results reported by Edwards and colleagues showed mean BIS values approximately 15 points higher with halothane than with sevoflurane in children6. Our results using the EEG bispectrum could help to explain this difference in BIS values. Constant and colleagues have reported that the effects of sevoflurane and halothane on the EEG in children show different patterns of depression8. In our study, sevoflurane and halothane show different patterns in the EEG bispectrum corresponding to their respective frequencies of coupling. For example, at 1 MAC the position of children under halothane anaesthesia is on the left side of the structured model of MCA, corresponding to high frequencies of coupling. In contrast with sevoflurane, children are positioned on the right side, reflecting the dominance of lower frequencies (figure 3) (see also figure 3B on the reference 2). Thus we suggest that the difference between BIS values found using sevoflurane and halothane are not dependent on the depth of anaesthesia, but due to the different pharmacological effect on EEG frequencies. Indeed, in the switch that we made from 0.5 MAC halothane to 1 MAC sevoflurane at the end of recording in group HALO, we were able to reproduce exactly the pattern of the EEG bispectrum that we obtained in children in group SEVO at 1 MAC. This further supports the notion that the EEG bispectrum is mainly agent dependent.
Some authors have suggested that the definition of MAC (movement to stimulus) may not truly reflect consciousness and depth of anaesthesia or EEG activity. Thus, the differences shown in the BIS values between halothane and sevoflurane at 1 MAC could be attributed to the fact that movements and MAC are determined more by spinal motor reflexes than ‘true’ hypnotic effect. BIS monitoring is based on the potential links between depth of anaesthesia and changes in the EEG, especially the EEG bispectrum. Using the up-and-down technique, Taylor found that the relationship between 1 MAC desflurane and age ranged from 9.92 (+/−0.44%) in infants 6–12 months to 7.98 (+/− 0.43%) in children 5–12 years9. Using a similar technique, 1 MAC sevoflurane was found to be 3.2% in infants 6–12 months and 2.5% in children 1–12 years10. Thus equipotent concentrations of desflurane are approximately three times those of sevoflurane in children. Despite the criticism of the ability of MAC to reflect true depth of anaesthesia, we found that the pattern of the EEG bispectrum for both sevoflurane and desflurane at three different equipotent concentrations, 2 MAC, 1 MAC and 0.5 MAC, were very similar in terms of their respective positions in the structured model of the MCA. These results therefore add evidence to the validity of the currently accepted MAC levels in determining equipotent effect on EEG with sevoflurane and desflurane. During this study, it was possible to intubate all children without muscle relaxant or opiate at 2 MAC halothane or sevoflurane without difficulty (no movement, haemodynamic response, cough or laryngospasm) indicating a similarly profound clinical depth of anaesthesia for both agents. However, it was noticable that the pattern of the EEG bispectrum with 2 MAC halothane did not correspond to the pattern with 2 MAC sevoflurane, but to the pattern with 0.5 MAC sevoflurane. At 0.5 MAC sevoflurane (and 0.5 MAC desflurane), the slightest stimulation of children resulted in signs of wakening, levels of anaesthesia at which it was certainly not possible to intubate. Thus, the ability of the EEG bispectrum to determine depth of anaesthesia in children may well be fundamentally flawed. Indeed, another recent study using sevoflurane anaesthesia also suggests similar problems with this modality11.
In our previous study using sevoflurane2, we suggested that it may be possible to improve the accuracy of BIS at deeper levels of anaesthesia in children, and make it independent of age, using an additional frequency of coupling. We found that changes in position of children along axis F2 of the MCA model appeared to be more related to depth of anaesthesia than changes along axis Fl (the axis to which BIS and age were related). This relationship may remain valid for desflurane but in light of our results with halothane this is by no means a universal reliable indicator of depth of anaesthesia. In fact, halothane shows an inverse relationship with axis F2 (figure 3). Hence, if depth of anaesthesia is to be assessed using frequencies of coupling of the EEG bispectrum, it is at least necessary to specify the agent used.
In conclusion, BIS values were linked to age of children irrespective of the volatile anaesthetic agent used. 1 MAC desflurane, together with 1 MAC sevoflurane, showed BIS values lower than 1 MAC halothane at all ages. These differences can be explained by the specific effect on the mechanisms underlying EEG generation induced by each anaesthetic agent12,13, bringing into question the ability of the EEG bispectrum to accurately determine depth of anaesthesia in children.
Acknowledgments
These works were partly financed by the French Ministry of Research and Technology (Grant: DT 03 B 107-108-109; CITH Rennes). The authors wish to thank paediatric surgeons, Olivier Azzis, MD and Edouard Habonimana, MD, for their kind patience during the recordings. Parts of this study were presented at the annual meeting of ASA, Las Vegas, 2004 and Atlanta 2005.
References
- 1.Sebel PS, Lang E, Rampil IJ, et al. A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effect. Anesth Analg. 1997;84:891–9. doi: 10.1097/00000539-199704000-00035. [DOI] [PubMed] [Google Scholar]
- 2.Wodey E, Tirel O, Bansard JY, et al. Impact of age on both BIS values and EEG bispectrum during anaesthesia with sevoflurane in children. Br J Anaesth. 2005;94:810–20. doi: 10.1093/bja/aei140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HS, Oh AY, Kim CS, Kim SD, Seo KS, Kim JH. Correlation of bispectral index with end-tidal sevoflurane concentration and age in infants and children. Br J Anaesth. 2005;95:362–6. doi: 10.1093/bja/aei196. [DOI] [PubMed] [Google Scholar]
- 4.Davidson AJ, Huang GH, Rebmann CS, Ellery C. Performance of entropy and Bispectral Index as measures of anaesthesia effect in children of different ages. Br J Anaesth. 2005;95:674–9. doi: 10.1093/bja/aei247. [DOI] [PubMed] [Google Scholar]
- 5.Davidson AJ, Czarnecki C. The Bispectral Index in children: comparing isoflurane and halothane. Br J Anaesth. 2004;92:14–7. doi: 10.1093/bja/aeh011. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JJ, Soto RG, Bedford RF. Bispectral Index values are higher during halothane vs. sevoflurane anesthesia in children, but not in infants. Acta Anaesthesiol Scand. 2005;49:1084–7. doi: 10.1111/j.1399-6576.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 7.Bannister CF, Brosius KK, Sigl JC, Meyer BJ, Sebel PS. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth Analg. 2001;92:877–81. doi: 10.1097/00000539-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Constant I, Dubois MC, Piat V, Moutard ML, McCue M, Murat I. Changes in electroencephalogram and autonomic cardiovascular activity during induction of anesthesia with sevoflurane compared with halothane in children. Anesthesiology. 1999;91:1604–15. doi: 10.1097/00000542-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RH, Lerman J. Minimum alveolar concentration of desflurane and hemodynamic responses in neonates, infants, and children. Anesthesiology. 1991;75:975–9. doi: 10.1097/00000542-199112000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Lerman J, Sikich N, Kleinman S, Yentis S. The pharmacology of sevoflurane in infants and children. Anesthesiology. 1994;80:814–24. doi: 10.1097/00000542-199404000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Davidson A. The correlation between bispectral index and airway reflexes with sevoflurane and halothane anaesthesia. Paediatr Anaesth. 2004;14:241–6. doi: 10.1046/j.1460-9592.2003.01181.x. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa K, MacIver MB. Agent-selective effects of volatile anesthetics on GABAA receptor-mediated synaptic inhibition in hippocampal interneurons. Anesthesiology. 2001;94:340–347. doi: 10.1097/00000542-200102000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]


