Abstract
The antemortem detection of a Parelaphostrongylus tenuis infection in a free-ranging wild elk (Cervus elaphus) in southern Ontario is documented. Postmortems on other free-ranging elk that died during 2000–2005 indicated that 59% (17/29) were infected with P. tenuis, based on presence of lesions in the brain.
Résumé
Preuves d’infections à Parelaphostrongylus tenuis chez le wapiti (Cervus elaphus) en élevage extensif dans le sud de l’Ontario. Cet article décrit la détection antémortem d’une infection à Parelaphostrongylus tenuis chez un wapiti en élevage extensif (Cervus elaphus) dans le sud de l’Ontario. Des examens post mortem réalisés sur d’autres wapitis en élevage extensif, morts entre 2000 et 2005, ont révélé que 59 % (17/29) présentaient des lésions au cerveau caractéristiques d’infection à P. tenuis.
(Traduit par Docteur André Blouin)
During 2000 and 2001, 120 elk (Cervus elaphus), also known as wapiti, were translocated from Elk Island National Park (EINP), Alberta, to the Bancroft, Ontario, area as part of a larger restoration project in which 443 elk (from EINP) were released in 4 areas of Ontario during 1998–2001 (1). All but 1 of the elk released near Bancroft were radio-collared and ear-tagged prior to release, as a means for monitoring the location of released elk, as well as their progeny born in Ontario. Mortality signals from the radio-collar alerted researchers that the animal had succumbed, which, in turn, allowed for rapid retrieval of animals, so that postmortems to determine the cause of death could be completed. Radio-telemetry also provided researchers with a means to locate animals for behavioral observation. The purpose of the study was to estimate the prevalence of P. tenuis in free-ranging elk in southern Ontario and to evaluate a recently developed ELISA for the detection of antibodies against P. tenuis.
Case 1
During 2003/2004, a group of about 30–40 elk, consisting of animals transported from EINP and their offspring born in Ontario, were observed frequenting the area of Hartsmere, Ontario (approximately 44°5′ N, 77°30′ W) (about 30 km east of Bancroft, Ontario). During October and November 2004, a sick yearling bull elk was observed in this group. By using radio-telemetry, it was determined that during December 2004, the animal left the group, became solitary (abnormal social behavior for a herd animal), and began to frequent a nearby barn. Eventually, the animal stayed at the barn continuously and was provided with feed, primarily alfalfa (Figure 1).
Figure 1.
The Parelaphostrongylus tenuis infected bull elk (Case 1) is pictured on February 1, 2005, with several white-tailed deer at a winter feeding site near Bancroft, Ontario (Photo by R. Rosatte).
Case description
Clinical signs exhibited by the yearling bull elk included a loss of fear of humans, ataxia, loss of balance, and a drooping head. Based on the observed clinical signs, a tentative diagnosis of P. tenuis infection was made.
On January 11, 2005, he (estimated weight 150–200 kg) was immobilized with an IM injection of 500 mg of tiletamine hydrochloride/zolazepam hydrochloride (Telazole; Fort Dodge Animal Health, Fort Dodge, Iowa, USA) and 300 mg of xylazine hydrochloride (Anased; Vet-A-Mix, Shenandoah, Iowa, USA) in the right upper hind limb area via a 5-mL sterile syringe and 22 g (4 cm) needle, attached to jab stick (an 80-cm section of copper tubing that contained a wooden dowel to apply pressure to the syringe plunger). After the animal was immobilized, 8 mL of blood was collected from the jugular vein into 2, 10-mL sterile blood collection tubes (Vacutainer; Becton Dickinson, Franklin Lakes, New Jersey, USA). The elk was also fitted with a VHF radio-collar and an ear-tag (Flex-Lok; Ketchum Manufacturing, Ottawa, Ontario) for future identification. When processing had been completed, the elk was given 12 mg of yohimbine hydrochloride (Yobine; Lloyd Laboratories, Shenandoah, Iowa, USA), IM, in the right hind limb, as an antagonist, in order to speed recovery from the effects of xylazine. The elk recovered fully from the procedure. The blood sample was centrifuged for 15 min, then a 4-mL plasma sample was collected and stored, for 1 wk, in 2, 2-mL sterile plastic microtubes at −12°C. The frozen sample was then shipped by courier to the Prairie Diagnostic Services laboratory in Regina, Saskatchewan.
The plasma sample was tested for the presence of antibodies against P. tenuis by using excretory-secretory (ES) products derived from infective 3rd-stage larvae of the parasite as coating antigen in an enzyme-linked immunosorbent assay (ELISA), as described by Ogunremi et al (2). The result (obtained on February 4, 2005) of the ELISA performed on the bull elk sample (S), expressed as the ratio of the optical density reading of the samples and of a reference positive (P), or S/P ratio, was 0.986, which translates to an ELISA index of 98.6 units, cut off = 45.0 units, indicating that the bull elk was exposed to P. tenuis.
Although field infected elk rarely pass enough P. tenuis first-stage larvae to make fecal examination a reliable diagnostic test (3), an attempt was made to recover larvae from the feces of the P. tenuis-suspect bull elk by collecting fecal samples at the location of the yearling bull elk on February 18, 2005, and having them screened for P. tenuis larvae at Trent University and the Regina laboratory of the Prairie Diagnostic Services, by using the Baermann beaker technique (4). No P. tenuis larva was detected; however, the samples were positive for the lung-worm Dictyocaulus. Historical observations indicate that larval shedding is inconsistent or undetectable in P. tenuis-infected elk (5,6). More recently, out of 4 elk inoculated with 6–20 stage 3 larvae, 2 larvae were recovered from only 1 animal that shed larvae and only on day 202 postexposure, despite the examination of feces twice a week (2).
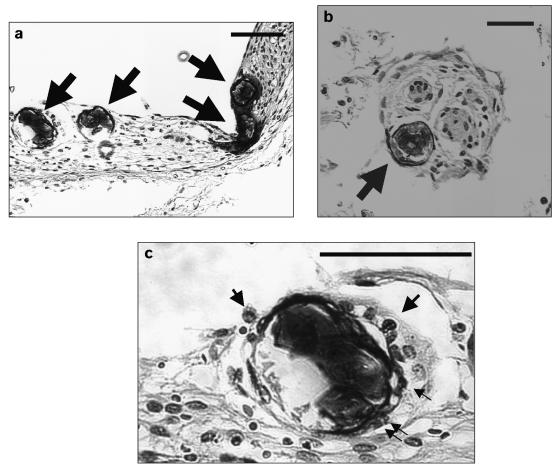
Between February and May 2005, the Bancroft bull elk intermittently demonstrated the same neurological signs, but by June 1, 2005, his condition had deteriorated and he was euthanized. At this time, a 2nd blood sample was collected; it again tested positive for P. tenuis. Findings on postmortem examination included meningoencephalomyelitis, characterized by moderate to severe perivascular cuffing of eosinophils, lymphocytes, plasma cells, and macrophages in the brain and spinal cord. Four sections of degenerate larvae (Figure 2a), and 1 of a normal-looking larva (Figure 2b) were present in the meninges of the brain. All larvae were surrounded by mononuclear cells and, in addition, 2 of the degenerate sections were closely associated with eosinophils (1 and 5 eosinophils; Figure 2c). The larvae had the same features as those reported previously in the histopathological sections of the brain of P. tenuis-infected elk (7). Areas of hemorrhages were observed in the white matter of the spinal cord and brain. Hemosiderin granules were present inside macrophages and extracellularly in sections of the brain and spinal cord. In the lungs, an adult female worm with eggs (85 × 45 μm) was recovered and identified as a Dictyocaulus sp. There was severe pulmonary congestion and infiltration of inflammatory cells, particularly eosinophils, macrophages, and lymphocytes. The central nervous system (CNS) and pulmonary lesions were attributable to P. tenuis and Dictyocaulus, respectively.
Figure 2.
Parelaphostrongylus tenuis larvating eggs in the brain meninges of the bull elk (Case 1): (a) degenerate larvae, (b) normal looking larva, (c) larva surrounded by eosinophils. Arrows indicate larvae (a, b) or eosinophils (c). Bar = 20μm.
Case 2
During the first 2 wk of September 2005, a yearling (1 1/2 y) cow elk, located near the area utilized by Case 1, demonstrated mobility problems (slow, stumbling, unstable movements), had no fear of humans, and remained in an approximately 10-m × 40-m area.
Case description
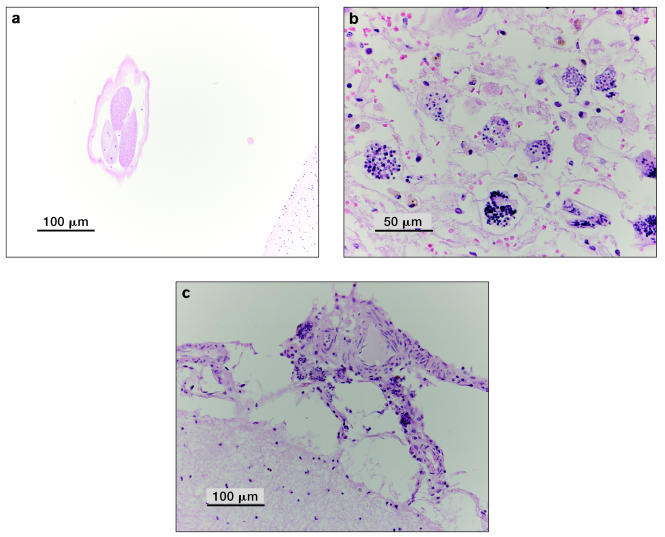
On September 14, 2005, the cow elk was observed in the same area and on September 15, 2005, she was found dead. On postmortem examination at the Canadian Cooperative Wildlife Health Centre (CCWHC), Guelph, Ontario the cause of death was confirmed as predation by wild canids. Histologic examination revealed an adult nematode, compatible with P. tenuis, in a cerebral sulcus and larvating nematode eggs (compatible with P. tenuis) in the meninges (in tissue as well as blood vessels) (Figure 3). The adult nematode was oriented in the section in such a way that it did not allow for an evaluation of the features to classify its genus and species; however, based on visible features, it was likely a metastrongyle. The larvae had evoked an inflammatory response characterized by eosinophilic infiltration. Blood serum samples collected 1 d post mortem revealed a positive P. tenuis ELISA S/P ratio of 1.04 and 0.72 (104 and 72 ELISA units). This animal was negative (immunohistochemical staining) for chronic wasting disease (CWD).
Figure 3.
Photos of cross sections of brain from the cow elk (Case 2) showing (a) a submature or adult nematode (compatible with P. tenuis) in a cerebral sulcus bar = 100 μm; (b) (c) larvating nematode eggs (compatible with P. tenuis) in the meninges (photos by D. Campbell); (b) bar = 50 μm, (c) bar = 100 μm.
Case 3
During 2000–2005, elk (including elk that originated at EINP, as well as their progeny born in Ontario) that died in southern Ontario were collected opportunistically to determine their state of health, body condition, and cause of death.
Case description
Postmortem examinations on 42 elk yielded 3 classes of animals at their time of death: 1) elk that may have been exposed to P. tenuis and developed inflammatory lesions in the brain but were in good condition; 2) elk with clinical disease due to P. tenuis infection, which included neurological signs, and were in an emaciated state; 3) elk without evidence of P. tenuis infection. For some animals, infection was not assessed, due to unsuitability or unavailability of brain tissue for examination. Of the 42 elk that had died due to a variety of causes (found dead, illegally shot, collisions with vehicles, drowning, hit by a train), 13 had died too soon following release (1–5 mo) to have become clinically ill with P. tenuis (the elk originated in Alberta where apparently there is no P. tenuis infection in wild deer or elk). Of the 29 that survived longer than 6 mo post release, 17 (59%) had lesions in the brain compatible with P. tenuis infection. The lesions consisted of mild to moderate perivascular cuffing, primarily with mononuclear cells (lymphocytes and macrophages) with occasional eosinophils present. Hemosiderin was present, typically within macrophages in perivascular locations, usually in the meninges. There was mild, multifocal gliosis and, rarely, small foci of malacia. Greater numbers of females (n = 12) had lesions than males (n = 5) (P = 0.016; chi square = 5.85); however, no difference with respect to the age of elk and occurrence of lesions was found (9 elk were ≤ 2 y of age and 8 elk were ≥ 3 y of age) (P = 0.88, chi square = 0.02). The sex/age composition of the 17 elk that had lesions was as follows: 35% (6/17) cows ≥ 3 y; 12% (2/17) 2-y-old cows; 24% (4/17) yearling cows; 12% (2/17) bulls ≤ 3 y; 6% (1/17) 2-y-old bulls; and 12% (2/17) yearling bulls. Of the 12 elk that were not suspect for P. tenuis, 3 were adult cows, 3 were adult bulls, 5 were yearling bulls, and 1 was a male calf. None of the 13 elk that died 1–5 mo post release had any evidence of P. tenuis infection.
Discussion
Parelaphostrongylus tenuis, also known as meningeal worm or brain worm, rarely causes serious disease in white-tailed deer, the definitive host in Ontario, as deer and P. tenuis have coevolved (8). It is, however, one of the most pathogenic nematodes of cervids such as elk, moose (Alces alces), and caribou (Rangifer tarandus caribou), and it can cause severe neurological disease and death in species such as elk. Parelaphostrongylus tenuis has probably limited the success of previous elk restorations in eastern North America (3,8,9), although some reintroduced elk herds have persisted on the same range as P. tenuis-infected white-tailed deer (10).
It was postulated during 2003–2004 that elk restored to the Bancroft, Ontario, area may have acquired P. tenuis infections from deer in the Hartsmere area (Figure 1), as brain lesions, possibly due to parasite migration, were found in elk during postmortem examinations at the CCWHC in Guelph. Similar findings have been reported in other areas where P. tenuis is enzootic and elk have been reintroduced. Woolf et al (11) found lesions present in the brain of 37 animals infected with P. tenuis in Pennsylvania, although only 11 clinical cases were observed among the 87 elk examined (12). As well, Carpenter et al (7) detected lesions and P. tenuis larvae in the meninges of elk sampled in Oklahoma. Since the reintroduction of elk to Ontario, larval or adult meningeal worms have not been found in necropsied elk until now, and despite the strong suspicion of elk acquiring and dying of P. tenuis, a serological test (P. tenuis ELISA) has not previously been available to assist with the diagnosis (2). Now that a P. tenuis ELISA (2) is commercially available, serological testing can be carried out to provide supportive evidence for the role of P. tenuis in elk mortality in Ontario.
The orientation of the adult nematode worm that was found in a histological section of the brain of the cow elk did not allow for classification beyond family, but anatomical features, such as the multinucleated cells in the intestine, place it in the metastrongyles. However, the possible species of this group that are likely to be found in the brain of an elk in eastern Ontario is somewhat limited to P. tenuis. Thus, the logical conclusion was that the adult nematode was “compatible with P. tenuis.” This phrase is commonly used by pathologists when a definite diagnosis is not possible but is suspected, based on lesions, physical appearances, geographic location, and other etiologies. In addition, P. tenuis infections in elk, based on clinical signs, lesions, and the presence of adult nematodes and larvae in neural tissue, have been documented in numerous references (8,12).
It seems reasonable to conclude that the source of the P. tenuis infection for the elk was via ingestion of infected gastropods that had acquired their infections via consumption of, or penetration by, first stage P. tenuis larvae shed in the feces of white-tailed deer. The bull elk is known to have wintered during 2003–2004 in the yarding areas east of Bancroft (13), where a high density of deer is found and most of their fecal samples, (82%) have dorsal-spined larvae, presumably P. tenuis (14). Furthermore, the infected bull elk likely spent the summer/fall of 2004 in the same general area as the other members of its social group (as determined by radio-telemetry) and probably acquired the infection in the vicinity of the deer yarding areas near Hartsmere. In a previous study in Minnesota, P. tenuis larvae found in gastropods had reached the infective 3rd stage by July–October (15). However, only a small proportion of gastropods will be infected; 1% of gastropods were found to be infected with P. tenuis in Algonquin Park, Ontario, north of the Hartsmere area (16).
The severity and outcome of the P. tenuis infection in cervids such as elk is correlated with the infective dose of larvae. Significant doses (>125, 3rd stage larvae) can result in neurological symptoms and death. Elk receiving moderate numbers (25–75 larvae) developed neurological signs, some died, and some shed larvae. However, elk that were exposed to small numbers (15 larvae) did not develop clinical signs or shed larvae (10). Thus, the ingestion of low doses of larvae may partially explain the survival of some eastern elk populations. The impact of P. tenuis on the Bancroft elk population will most likely be related to many factors, including 1) the prevalence of P. tenuis in resident deer populations, 2) the range overlap between deer and elk, 3) the abundance and type of gastropods found on deer and elk range, 4) the number of P. tenuis-infected gastropods that elk ingest, 5) the age of elk at the time of infection, 6) the amount of damage caused by worms within the CNS, 7) immunity to infection, and 8) the ability of elk to survive low level infections of P. tenuis.
Other studies have noted clinical signs in elk due to P. tenuis infection similar to those in this study; however, those diagnoses were made postmortem. Meningeal worms were found postmortem in the brains of elk in Oklahoma that exhibited signs prior to death such as ataxia and circling (7). In Pennsylvania, Woolf et al (11) observed clinical signs such as ataxia, circling, tameness, and head/neck tilt in captive elk that were confirmed on postmortem examination to be infected with P. tenuis. Neurological signs in elk in Kentucky and Michigan were also attributed to P. tenuis infection (17,18). It should also be noted that Woolf et al (11) found that although there was a high number of P. tenuis-infected elk on a preserve in Pennsylvania, many did not exhibit any clinical signs. Thus, a diagnosis based only on clinical signs may provide an underestimate of the true prevalence of P. tenuis in a wild elk population.
Olsen and Woolf (12) and Woolf et al (11) found a higher prevalence of P. tenuis infections in yearling and 2.5-y-old elk (60%) than in calves and elk > 3.5 y (25%) in a preserve in Pennsylvania. More recently, Larkin et al (17) showed that 73% of elk dying of P. tenuis in Kentucky were < 3 y. In this study, only 53% of wild elk sampled in the Bancroft area and suspected of being infected with P. tenuis were ≤ 2.5 y (of those, 35% were yearlings). As noted by Woolf et al (11), a high prevalence of P. tenuis in younger-aged elk could affect the productivity of the herd and limit population growth over the long term. The true impact of P. tenuis in Ontario remains to be seen, as elk were only recently (2000/2001) introduced to the Bancroft area. However, the impact could be minimal in Ontario, as, in Michigan, P. tenuis accounted for only 3% of elk mortalities, much lower than mortality due to harvesting (58%), illegal kills (22%), other diseases (7%), and malnutrition (4%) (18).
During a preliminary validation exercise of the ELISA, all 12 elk experimentally infected with P. tenuis obtained from the CNS tested positive for antibodies to P. tenuis (Ogunremi et al, unpublished observations). As a confirmation of infection, adult worms were recovered at necropsy from the CNS of the animals, starting 4 mo after inoculation (10), but all were serologically positive starting as early as 1 mo postinoculation and lasting until the termination of the experiment. While serological cross-reactivity has been observed between sera from Dictyocaulus-infected elk and white-tailed deer and the somatic antigens of P. tenuis (2,19), no such cross-reactivity was observed against the ES products of P. tenuis stage 3 larvae (2,20). Specificity of the P. tenuis ELISA was found to be 97.2% (95% confidence interval, = 95.8–98.6%) among 579 elk sourced from P. tenuis-free areas where the prevalence of Dictyocaulus was estimated at about 12% (21,22). Based on the above estimate of specificity and an apparently high sensitivity, albeit on a small sample size (12 out of 12 animals; unpublished observations), it is reasonable to conclude that the use of larval ES products in the ELISA may provide strong evidence that an elk may have been exposed to P. tenuis.
One may question whether diseases, parasites, or deficiencies, other than P. tenuis, may have been responsible for the signs and clinical condition in the bull elk. Since neurological signs were evident in the bull elk, the brain was tested for rabies virus by the fluorescent antibody test and found to be negative. Chronic wasting disease is also a cause of neurological signs in elk. As in P. tenuis infections, elk affected with CWD show abnormal head posture and loss of fear of humans. Excessive salivation is commonly seen in animals terminally ill, but it is not a common finding in P. tenuis-infected elk (23–25), and was not observed in this bull elk. Importantly, none of the pathognomonic signs of CWD, namely spongiform degeneration of grey matter neuropil, intraneuronal vacuolation, and astrocytic hypertrophy and hyperplasia (24,26) were observed in the brain of this animal. Both meningitis and encephalitis, which are absent in CWD infections (24), were observed in this elk. Trace mineral deficiency, particularly of copper, resulting in ataxia has been reported in a conspecific species (red deer Cervus elaphus subsp.). However, the effects of copper deficiency are different from those of P. tenuis infection: there are accompanying skeletal deformities (27,28); many animals in the same social group are usually affected (28); and the main CNS lesion is neural demyelination (29). In a recent report, behavioral observations in Michigan over a period of 14 y of 100 radio-collared elk belonging to different age and gender classes failed to reveal any neurological signs other than those due to P. tenuis infection (18). Given the above evidence, we feel confident the bull elk was infected with P. tenuis.
In the past, the only technique available for the detection of P. tenuis in live hosts was the Baermann funnel technique, which relies on the recovery of 1st stage larvae in the feces of infected animals. The more efficacious modified technique (4) may lead to the recovery of up to 13% of P. tenuis larvae actually present in feces. The probability of recovery of larvae from infected elk using these techniques is low, as elk intermittently shed only low numbers of 1st stage larvae in feces (2,6,8). This may be related to the fact that field-infected elk may harbor only a few (1–3) adult worms (7). As well, unisexual infections may be present in infected animals, making larval shedding impossible (30). Furthermore, worms may die, or the elk may die, before infections become patent (8). As demonstrated for moose in which infected animals also harbor a few parasites (31), the newly developed P. tenuis ELISA that uses ES antigen may be useful for detecting meningeal worm infections. However, a presumptive diagnosis based on serological methods could have limitations and an adequate field of validation of data based on a range of elk populations harboring closely related parasites is required. The availability of more field samples, especially those obtained antemortem from animals showing clinical symptoms suggestive of a P. tenuis infection, should provide further opportunity to assess the dependability of the P. tenuis ELISA as a diagnostic tool and its utility in wildlife management and for monitoring the prevalence of P. tenuis in elk.
There are documented antemortem cases based on clinical signs. To our knowledge, this is the 1st documented report of antemortem diagnosis of P. tenuis infection in which a serological method was used in an individual free ranging elk in the wild. The potential impact of P. tenuis on restored eastern elk populations (32) is of concern, as failures of previous introduction attempts have been attributed to P. tenuis infections (33). This is supported by other observations indicating that P. tenuis was responsible for reduced growth and mortalities in elk populations in Pennsylvania and Kentucky, respectively (17,34). Given the observations reported in this study, it would not be prudent to transport elk from eastern North America to the west, where P. tenuis is currently absent [see Lankester (8) for a map of the current range of P. tenuis]. In addition, managers should be aware that moving elk from areas free of P. tenuis to eastern North America, where P. tenuis is present, could result in significant mortalities as elk will not have had sufficient time to co-evolve with this parasite, as white-tailed deer have done in the east.
Acknowledgments
The Ontario Elk Restoration program is supported by the Provincial Elk Technical Team and the Ontario Ministry of Natural Resources (OMNR), Wildlife Section (Deb Stetson, manager) and the Wildlife Research and Development Section (Dr. J. Chris Davies, manager). We thank Tom Simpson, Suzy Scott, and Mike Scafie, OMNR, Bancroft District, Arthur Dupuis, Trent University, Joe Neuhold, Elwood Snider, and John O’Donnell for assistance in the field and for the provision of observational data. Thanks to Dr. I. Barker (Canadian Cooperative Wildlife Health Centre, Guelph, Ontario) for providing helpful comments to improve the manuscript, and R. Velarde (CCWHC, Guelph) for elk postmortem data. Nicole Warmington, CCWHC, Guelph, assisted with the postmortem on the bull elk. The rabies test was performed by staff at the Canadian Food Inspection Agency, Ottawa Laboratory Fallowfield, Nepean, Ontario. Thanks to Gail Krohn, technical supervisor for Prairie Diagnostic Services. Dr. C. Davies also reviewed the manuscript and provided constructive comments. CVJ
References
- 1.Rosatte RC, Hamr J, Ranta B, Young J, Cool N. Elk restoration in Ontario, Canada: Infectious disease management strategy, 1998–2001. Ann NY Acad Sci. 2002;969:358–363. doi: 10.1111/j.1749-6632.2002.tb04405.x. [DOI] [PubMed] [Google Scholar]
- 2.Ogunremi O, Lankester M, Gajadhar A. Immunuodiagnosis of experimental Parelaphostrongylus tenuis infection in elk. Can J Vet Res. 2002;66:1–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Pybus M, Samuel W, Crichton V. Identification of dorsal spined larvae from free-ranging wapiti (Cervus elaphus) in southwestern Manitoba. J Wildl Dis. 1989;25:291–293. doi: 10.7589/0090-3558-25.2.291. [DOI] [PubMed] [Google Scholar]
- 4.Forrester S, Lankester M. Extracting protostrongylid nematode larvae from ungulate feces. J Wildl Dis. 1997;33:511–516. doi: 10.7589/0090-3558-33.3.511. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RC, Lankester MW, Strelive UR. Further experimental studies of Pneumostrongylus tenuis in cervids. Can J Zool. 1966;41:851–861. doi: 10.1139/z66-086. [DOI] [PubMed] [Google Scholar]
- 6.Welch DA, Pybus MJ, Samuel WM, Wilke CJ. Reliability of fecal examination for detecting infections of meningeal worm in elk. Wildl Soc Bull. 1991;19:326–331. [Google Scholar]
- 7.Carpenter JW, Jordan HE, Ward BC. Neurologic disease in wapiti naturally infected with meningeal worms. J Wildl Dis. 1973;9:148–153. doi: 10.7589/0090-3558-9.2.148. [DOI] [PubMed] [Google Scholar]
- 8.Lankester M. Extrapulmonary lungworms of cervids. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic Diseases of Wild Mammals. Ames, Iowa: Iowa State Univ Pr; 2001. pp. 228–278. [Google Scholar]
- 9.Raskevitz R, Kocan A, Shaw J. Gastropod availability and habitat utilization by wapiti and white-tailed deer sympatric on range enzootic for meningeal worm. J Wildl Dis. 1991;27:92–101. doi: 10.7589/0090-3558-27.1.92. [DOI] [PubMed] [Google Scholar]
- 10.Samuel W, Pybus M, Welch D, Wilke C. Elk as a potential for meningeal worm: Implications for translocation. J Wildl Manag. 1992;56:629–639. [Google Scholar]
- 11.Woolf A, Mason C, Kradel D. Prevalence and effects of Parelaphostrongylus tenuis in a captive wapiti population. J Wildl Dis. 1977;13:149–154. doi: 10.7589/0090-3558-13.2.149. [DOI] [PubMed] [Google Scholar]
- 12.Olsen A, Woolf A. A summary of the prevalence of Parelaphostrongylus tenuis in a captive wapiti population. J Wildl Dis. 1979;15:33–35. doi: 10.7589/0090-3558-15.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Bellhouse T, Rosatte RC. Assessment of the potential for negative interaction between re-introduced elk (Cervus elaphus) and resident white-tailed deer (Odocoileus virginianus) in their wintering areas in Ontario, Canada. Mammalia. 2005;69(1):35–56. [Google Scholar]
- 14.McIntosh T. Movements, survival and habitat use by elk (Cervus elaphus) reintroduced to northwestern Ontario [MSc thesis] Thunder Bay, Ontario: Lakehead University; 2003. [Google Scholar]
- 15.Lankester M, Peterson W. The possible importance of wintering yards in the transmission of Parelaphostrongylus tenuis to white-tailed deer and moose. J Wildl Dis. 1996;32:31–38. doi: 10.7589/0090-3558-32.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Lankester M. Gastropods as intermediate hosts of Pneumostrongylus tenuis Dougherty, of white-tailed deer [PhD thesis] Guelph, Ontario: University of Guelph; 1967. [Google Scholar]
- 17.Larkin JL, Alexy KJ, Bolin DC, et al. Meningeal worm in a reintroduced elk population in Kentucky. J Wildl Dis. 2003;39:588–592. doi: 10.7589/0090-3558-39.3.588. [DOI] [PubMed] [Google Scholar]
- 18.Bender L, Schmitt S, Carlson E, Haufler JB, Beyer DE., Jr Mortality of Rocky Mountain elk in Michigan due to meningeal worm. J Wildl Dis. 2005;41:134–140. doi: 10.7589/0090-3558-41.1.134. [DOI] [PubMed] [Google Scholar]
- 19.Bienek DR, Neumann NF, Samuel WM, Belosevic M. Meningeal worm evokes a heterogenous immune response in elk. J Wildl Dis. 1998;34:334–341. doi: 10.7589/0090-3558-34.2.334. [DOI] [PubMed] [Google Scholar]
- 20.Ogunremi O, Lankester M, Loran S, Gajadhar A. Evaluation of excretory- secretory products and somatic worm antigens for the serodiagnosis of experimental Parelaphostrongylus tenuis infection in white-tailed deer. J Vet Diagn Invest. 1999;11:515–521. doi: 10.1177/104063879901100605. [DOI] [PubMed] [Google Scholar]
- 21.Kingscote BF, Yates WD, Tiffin GB. Diseases of wapiti utilizing cattle range in southwestern Alberta. J Wildl Dis. 1987;23:86–91. doi: 10.7589/0090-3558-23.1.86. [DOI] [PubMed] [Google Scholar]
- 22.Pybus MJ. Survey of hepatic and pulmonary helminths of wild cervids in Alberta, Canada. J Wildl Dis. 1990;26:453–459. doi: 10.7589/0090-3558-26.4.453. [DOI] [PubMed] [Google Scholar]
- 23.O’Rourke KI, Besser TE, Miller MW, et al. PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol. 1999;80:2765–2769. doi: 10.1099/0022-1317-80-10-2765. [DOI] [PubMed] [Google Scholar]
- 24.Williams ES, Young S. Spongiform encephalopathies in cervidae. Rev Sci Tech. 1992;11:551–567. doi: 10.20506/rst.11.2.611. [DOI] [PubMed] [Google Scholar]
- 25.Thorne T, Williams E, Samuel W, Kistner T. Diseases and parasites. In: Toweill D, Thomas JW, editors. North American Elk, Ecology and Management. Washington DC: Smithsonian Inst Pr; 2002. pp. 351–387. [Google Scholar]
- 26.Spraker TR, Zink RR, Cummings BA, Sigurdson CJ, Miller MW, O’Rourke KI. Distribution of protease-resistant prion protein and spongiform encephalopathy in free ranging mule deer (Odocoileus hemionus) with chronic wasting disease. Vet Pathol. 2002;39:546–556. doi: 10.1354/vp.39-5-546. [DOI] [PubMed] [Google Scholar]
- 27.Gogan PJ, Jessup DA, Barrett RH. Antler anomalies in tule elk. J Wildl Dis. 1988;24:656–662. doi: 10.7589/0090-3558-24.4.656. [DOI] [PubMed] [Google Scholar]
- 28.Audigé L, Wilson PR, Morris RS, Davidson GW. Osteochondrosis, skeletal abnormalities and enzootic ataxia associated with copper deficiency in a farmed red deer (Cervus elaphus) herd. NZ Vet J. 1995;43:70–76. doi: 10.1080/00480169.1995.35852. [DOI] [PubMed] [Google Scholar]
- 29.Terlecki S, Done JT, Clegg FG. Enzootic ataxia of red deer. Br Vet J. 1964;120:311–321. [Google Scholar]
- 30.Slomke A, Lankester M, Peterson W. Infrapopulation dynamics of Parelaphostrongylus tenuis in white-tailed deer. J Wildl Dis. 1995;31:125–135. doi: 10.7589/0090-3558-31.2.125. [DOI] [PubMed] [Google Scholar]
- 31.Ogunremi OA, Lankester MW, Dergousoff SJ, Gajadhar A. Detection of anti-Parelaphostrongylus tenuis antibodies in experimentally infected and free-ranging moose (Alces alces) J Wildl Dis. 2002;38:796–803. doi: 10.7589/0090-3558-38.4.796. [DOI] [PubMed] [Google Scholar]
- 32.Rosatte RC, Hamr J, Young J, Filion I, Smith H. The Restoration of Elk (Cervus elaphus) in Ontario, Canada: 1998–2005. Restoration Ecol. 2007;15:34–43. [Google Scholar]
- 33.Severinghaus C, Darrow R. Failure of elk to survive in the Adirondacks. NY Fish Game J. 1976;23:98–99. [Google Scholar]
- 34.Eveland J, George J, Hunter N, Forney D, Harrison R. A preliminary evaluation of the ecology of the elk in Pennsylvania. In: Boyce M, Hayden L, editors. North American Elk: Ecology Behavior and Management. Laramie, Wyoming: Univ Wyoming Pr; 1979. pp. 145–151. [Google Scholar]