Abstract
A number of studies have demonstrated that non-neuronal acetylcholine can play a role in the regulation of T cell function. Recently, we reported that CD8+ T cells, from mice with a targeted deletion of the M1 muscarinic receptor, had a defect in differentiating into cytolytic T lymphocytes when stimulated in vitro. In the current report, we analyze the in vivo function of CD8+ T cells from mice with targeted deletions of either M1 or M5 muscarinic receptors. M1 or M5 knockout mice were infected with either lymphocytic choriomeningitis virus or vesicular stomatitis virus. Expansion of anti-viral CD8+ T cells was monitored by staining with tetramer reagents specific for the immunodominant peptides of the viruses. No defect in expansion of CD8+ T cells was observed in either M1 or M5 knockout mice. The extent to which one can draw a generalized conclusion that M1 and M5 are not involved in anti-viral immunity depends upon issues of antigen strength, genetic background, induction of redundant receptors, and the potential for qualitative defects in the expanded CD8+ T cells.
Introduction
Over the past several decades, considerable evidence has accumulated to suggest that the function of T lymphocytes can be regulated by acetylcholine (ACh) (Kawashima and Fujii, 2003b; Kawashima and Fujii, 2004). It is possible that such regulation represents mechanisms by which the nervous system can influence immune function. However, ACh of neuronal origin is not a requirement, as it is becoming increasingly appreciated that ACh can be synthesized and secreted from non-neuronal sources (Kawashima and Fujii, 2003b; Kawashima and Fujii, 2004). Indeed, T lymphocytes have all the required machinery to synthesize, secrete, and respond to ACh, including choline acetyltransferase (Fujii et al., 1996; Fujii et al., 1998; Rinner et al., 1998), high affinity choline transporter (Fujii et al., 2003a), and both muscarinic and nicotinic receptors (Kawashima and Fujii, 2000; Kawashima and Fujii, 2003a). In addition, T cells express acetylcholinesterase (Szelenyi et al., 1982; Tayebati et al., 2002). Thus, all the necessary components of a fully functional non-neuronal cholinergic system are found in lymphocytes.
An in-depth analysis of T cell lines derived from humans has demonstrated that muscarinic cholinergic receptors on T cells actively signal when stimulated with agonist (Kawashima and Fujii, 2000; Kawashima and Fujii, 2003a). Moreover, activation of muscarinic receptors has a functional outcome, as T cell receptor signaling in the presence of muscarinic agonists results in increased calcium flux, c-fos expression, IL-2 secretion, IL-2R expression, and proliferation (Kawashima and Fujii, 2000; Kawashima and Fujii, 2003a; Nomura et al., 2003; Rinner et al., 1995).
The five known muscarinic cholinergic receptors (M1–M5) can be subdivided into two general groups based upon signaling pathways. M1, M3 and M5 each signal through Gq proteins leading to activation of phospholipase Cβ, followed by generation of inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) (Felder, 1995). In contrast, both M2 and M4 signal through Gi proteins leading to inhibition of adenylyl cyclase with a subsequent decrease in cyclic AMP (Felder, 1995). It has been demonstrated that these signaling pathways are intact in response to cholinergic stimulation of murine CD8+ T lymphocytes (Genaro et al., 1993).
The above observations were a major step forward in our understanding of regulation of T cells by ACh. However, they are mostly restricted to analysis of long-term immortalized cell lines. To extend these studies to the function of primary T cells, we have utilized mice with targeted deletions of each of the known muscarinic receptors (M1–M5). We have previously reported that CD8+ T cells from the M1 knockout mouse had a defect in the differentiation into cytolytic T lymphocytes (Zimring et al., 2005). No defect was observed in mice with targeted deletions of either M3, M5, or a double deletion of M2/M4 (Zimring et al., 2005).
Although our previous study demonstrated a functional defect in primary CD8+ T cells, it relied upon in vitro methodologies, carrying the advantage of an easily controlled reductionist system, but lacking the physiological relevancy of in vivo studies. In the current report, to extend our investigations into the in vivo setting, we infected muscarinic knockout mice with two different viruses, lymphocytic choriomeningitis virus (LCMV) or vesicular stomatitis virus (VSV), and examined in vivo expansion of anti-viral CD8+ T cells.
Materials and Methods
Mice
Mice with targeted deletions of the M1 or M5 receptors (Fisahn et al., 2002; Yamada et al., 2001) were backcrossed onto C57BL/6 mice at Taconic labs, for at least 10 generations. Wild-type C57BL/6 mice were purchased from Taconic labs. Mice were maintained on standard laboratory chow and water ad libitum in a temperature- and light-controlled environment. All procedures were approved by the Emory IACUC committee.
Infection of Mice and Preparation of Cells
Injection of 2 × 105 pfu LCMV strain Armstrong (i.p. injection) or 1 × 105 pfu vesicular stomatitis virus (VSV) strain Indiana (i.v. injection) were given to mice of at least 8 weeks of age. As the peak of CD8+ T cell response to infection with LCMV and VSV occurs at approximately 7–10 days post-infection using the above inoculum, animals were sacrificed and analyzed at day 9 post infection. Spleens were harvested and a single cell suspension was prepared after RBC lysis. Lymphocytes were counted using Trypan blue to exclude dead cells.
Analysis of anti-viral CD8+ T cells by flow cytometry
1 × 106 splenocytes were stained with both anti-CD8 and MHC class I tetramers, consisting of the immunodominant epitopes of LCMV or VSV for the H-2b haplotype. Cells were incubated with tetramers specific for one of two different LCMV peptides presented by Db (Db-gp33–41; Db-gp276–286), or tetramer specific for the immunodominant VSV peptide (Kb-VSV –N 52–59) for 30 min at 4°C. Antibodies against CD8 α (BD Pharmingen, San Diego, CA) were used according to manufacturer’s directions. All data was acquired on a FACSCalibur (BD Biosciences, San Diego, CA) and analyzed with FlowJo software (Ashland, OR).
Results
Analysis of CD8+ T cell responses to LCMV and VSV in M1 or M5 knockout mice
Our previous findings demonstrated that naïve CD8+ T cells from C57BL/6 mice express mRNA for both the M1 and M5 muscarinic receptors, whereas there was no detectable mRNA for M3 (Zimring et al., 2005). Accordingly, we focused our studies on mice with targeted deletions of either M1 or M5, on a C57BL/6 background. To test the role of M1 or M5 receptors in the ability of CD8+ T cells to expand in response to a viral infection, M1 or M5 knockout mice were infected with either LCMV or VSV. At 9 days post-infection, leukocytes were isolated from the spleens of infected animals. CD8+ T cells were visualized by staining cells with anti-CD8 and analyzing by flow cytometry. Within the CD8+ population, T cells specific for LCMV or VSV were visualized by staining with tetramer reagents consisting of peptide/MHC I complexes known to represent the immunodominant antigens for LCMV or VSV, respectively.
Robust expansion of anti-viral CD8+ T cells, specific for LCMV or VSV, was observed in all infected animals. This was visualized as a CD8+, tetramer + population (figure 1). This population was specifically induced by viral infection, as no tetramer positive population was observed in uninfected control mice (data not shown). The flow cytometry plots shown in figure 1 are representative of the typical results. Combined results of the absolute numbers of anti-viral CD8+ T cells from multiple animals demonstrated no significant difference between M1 knockout, M5 knockout, or wild-type mice (Figure 2). Thus, neither M1 nor M5 receptors are required for the expansion of antigen-specific CD8+ T cells in response to viral infection.
Figure 1. Visualization of anti-viral CD8+ T cells in mice with deletions of the M1 or M5 muscarinic receptors.
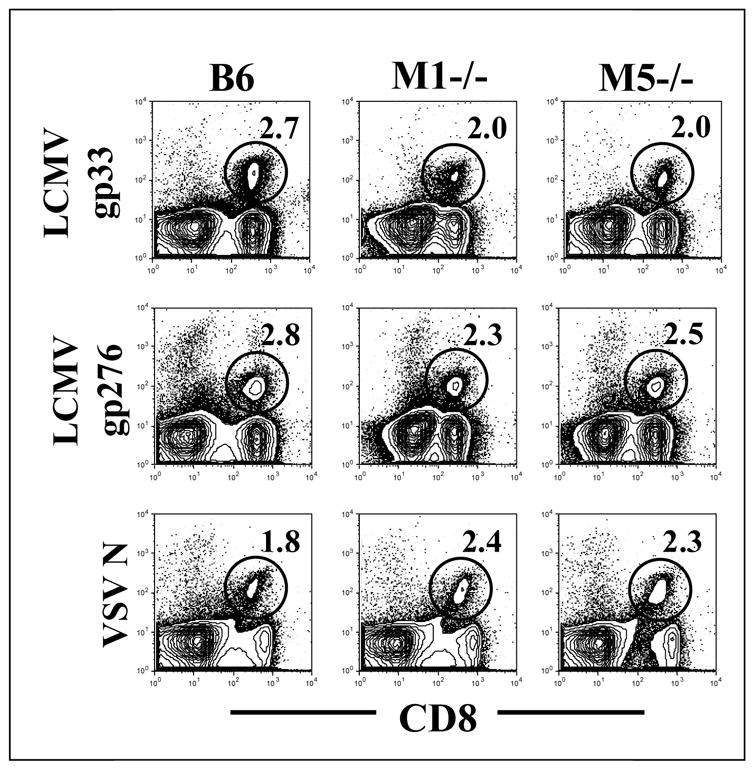
Mice were infected either with LCMV or VSV. Nine days later, splenocytes were stained with anti-CD8 antibody and tetramer specific for LCMV (gp33 or gp276) or VSV. Flow plots are gated on total lymphocytes and CD8+ tetramer + cells were visualized (shown in circles) The number within the plots reflects the percent of lymphocytes which bind the indicated tetramer.
Figure 2. Expansion of anti-viral CD8+T cells in mice with deletions of the M1 or M5 muscarinic receptors.
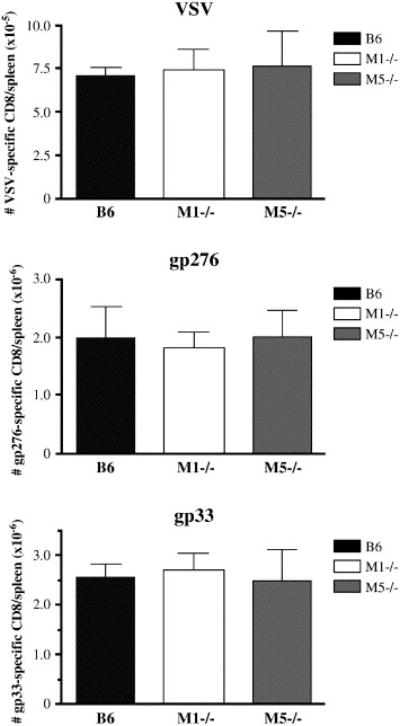
The absolute numbers of CD8+ tetramer+ cells were averaged from multiple animals. Numbers in bar graphs are calculated by multiplying the total number of splenocytes by the percent of lymphocytes binding a particular tetramer. For the experiment shown, sample size was n=3 for B6 infected with LCMV or VSV, n=3 for M1−/− infected with LCMV, n=5 for M5−/− infected with LCMV, n=4 M1−/− infected with VSV and n=4 M5−/− infected with VSV. This experiment has been reproduced with similar results.
Discussion
The data presented in this report indicate that CD8+ T cells undergo normal expansion in mice with targeted deletions of either the M1 or M5 cholinergic receptors. Although these data assess the quantity of anti-viral CD8+ T cells that expand in infected mice, the current approach does not assess effector function of the anti-viral cells. Ongoing studies to assess cytolytic T lymphocyte (CTL) activity and viral loads will help to address this issue.
We have previously reported that mRNA for both M1 and M5 are expressed in naïve CD8+ T cells from C57BL/6 mice (Zimring et al., 2005). Along these lines, one must also consider the possibility of redundancy between M1 and M5 such that they compensate for the absence of each other. We have previously reported a defect in CD8+ T cells from mice lacking the M1 receptor (Zimring et al., 2005), indicating that M5 did not rescue the defect in M1 knockout mice. However, it is important to note that these studies were performed in vitro and on a different strain background from the current studies. It is possible that M5 can compensate for the absence of M1 in vivo and/or on a C57BL/6 background. Thus, a double M1/M5 knockout may uncover a functional defect in CD8+ T cells. Moreover, although no mRNA for M3 was detected in naïve CD8+ T cells (Zimring et al., 2005), as muscarinic receptor expression can change upon T cell activation (Fujii et al., 2003b), it is also possible that CD8+ T cells express M3 upon activation. To definitively address this issue, a triple knockout of M1, M3 and M5 will be required. As such an animal would not likely be viable, conditional knockouts in the CD8+ T cell compartment would be most useful for these purposes.
The studies in this report were carried out using LCMV and VSV, as they are well characterized pathogens with sufficient immunological tools to perform in vivo analysis of CD8+ T cells. However, it is important to note that our previous analysis indicated that the defect in CD8+ T cells from mice missing M1 was not absolute (Zimring et al., 2005). CD8+ T cells from M1 knockout mice showed a clear defect when given a weak to moderate in vitro stimulation. However, a strong stimulation of the TCR could overcome the defect (Zimring et al., 2005), resulting in normal CTL function. As both LCMV and VSV represent a strong stimulus to the immune system, it is possible that like our previous in vitro studies, defects in muscarinic receptor knockout mice were overcome due to the strength of the stimulus. Ongoing studies are addressing this hypothesis by challenging mice with weaker stimuli, such as immunization to minor histocompatibility antigens.
As is often the case with characterizations of knockout mice, there is some concern regarding the genetic background of the M1 and M5 knockout mice. Our previous report that demonstrated a defect in CD8+ T cells from M1 knockout mice utilized animals on an outbred background (Zimring et al., 2005). As many well-characterized tetramer reagents containing immunodominant epitopes have been described for the H-2b haplotype, for the current studies, it was necessary to have the knockout mice on a genetic background that was H-2b. Accordingly, the current studies utilized M1 and M5 knockout mice backcrossed onto a C57BL/6 background. It has been reported that the pattern of expression of muscarinic receptor sub-types on human leukocytes varies from individual to individual (Kawashima and Fujii, 2003b; Sato et al., 1999). Thus, it seems likely that patterns of muscarinic receptor expression varies between different inbred murine strains. As the M1, M3 and M5 receptors each signal by redundant pathways, knockout of a single receptor may result in a phenotype in some but not other strains of mice. This possibility is further complicated by the observations that expression of muscarinic receptors is altered during T cell activation (Fujii et al., 2003b), which may also differ between strains.
In summary, the data presented herein demonstrate that neither M1 nor M5 are required for expansion of antigen-specific CD8+ T cells, in response to viral infection in C57BL/6 mice. As explained above, the extent to which one can draw a generalized conclusion that M1 and M5 are not involved in anti-viral immunity mediated by CD8+ T cells, depends upon issues of antigen strength, genetic background, and receptor redundancy.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (NIAID R03 AI060017)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB Journal. 1995;9:619–625. [PubMed] [Google Scholar]
- Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron. 2002;33:615–624. doi: 10.1016/s0896-6273(02)00587-1. [DOI] [PubMed] [Google Scholar]
- Fujii T, Okuda T, Haga T, Kawashima K. Detection of the high-affinity choline transporter in the MOLT-3 human leukemic T-cell line. Life Sciences. 2003a;72:2131–2134. doi: 10.1016/s0024-3205(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Fujii T, Tsuchiya T, Yamada S, Fujimoto K, Suzuki T, Kasahara T, Kawashima K. Localization and synthesis of acetylcholine in human leukemic T cell lines. Journal of Neuroscience Research. 1996;44:66–72. doi: 10.1002/(SICI)1097-4547(19960401)44:1<66::AID-JNR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Fujii T, Watanabe Y, Inoue T, Kawashima K. Upregulation of mRNA encoding the M5 muscarinic acetylcholine receptor in human T- and B-lymphocytes during immunological responses. Neurochemical Research. 2003b;28:423–429. doi: 10.1023/a:1022840416292. [DOI] [PubMed] [Google Scholar]
- Fujii T, Yamada S, Watanabe Y, Misawa H, Tajima S, Fujimoto K, Kasahara T, Kawashima K. Induction of choline acetyltransferase mRNA in human mononuclear leukocytes stimulated by phytohemagglutinin, a T-cell activator. Journal of Neuroimmunology. 1998;82:101–107. doi: 10.1016/S0165-5728(97)00195-1. [DOI] [PubMed] [Google Scholar]
- Genaro AM, Cremaschi GA, Borda ES. Muscarinic cholinergic receptors on murine lymphocyte subpopulations. Selective interactions with second messenger response system upon pharmacological stimulation. Immunopharmacology. 1993;26:21–29. doi: 10.1016/0162-3109(93)90063-v. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacology & Therapeutics. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. The lymphocytic cholinergic system and its biological function. Life Sciences. 2003a;72:2101–2109. doi: 10.1016/s0024-3205(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sciences. 2003b;74:675–696. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Frontiers in Bioscience. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- Nomura J, Hosoi T, Okuma Y, Nomura Y. The presence and functions of muscarinic receptors in human T cells: the involvement in IL-2 and IL-2 receptor system. Life Sciences. 2003;72:2121–2126. doi: 10.1016/s0024-3205(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Rinner I, Felsner P, Falus A, Skreiner E, Kukulansky T, Globerson A, Hirokawa K, Schauenstein K. Cholinergic signals to and from the immune system. Immunology Letters. 1995;44:217–220. doi: 10.1016/0165-2478(94)00220-l. [DOI] [PubMed] [Google Scholar]
- Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. Journal of Neuroimmunology. 1998;81:31–37. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]
- Sato KZ, Fujii T, Watanabe Y, Yamada S, Ando T, Kazuko F, Kawashima K. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neuroscience Letters. 1999;266:17–20. doi: 10.1016/s0304-3940(99)00259-1. [DOI] [PubMed] [Google Scholar]
- Szelenyi JG, Bartha E, Hollan SR. Acetylcholinesterase activity of lymphocytes: an enzyme characteristic of T-cells. British Journal of Haematology. 1982;50:241–245. doi: 10.1111/j.1365-2141.1982.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Tayebati SK, El-Assouad D, Ricci A, Amenta F. Immunochemical and immunocytochemical characterization of cholinergic markers in human peripheral blood lymphocytes. Journal of Neuroimmunology. 2002;132:147–155. doi: 10.1016/s0165-5728(02)00325-9. [DOI] [PubMed] [Google Scholar]
- Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimring JC, Kapp LM, Yamada M, Wess J, Kapp JA. Regulation of CD8+ cytolytic T lymphocyte differentiation by a cholinergic pathway. Journal of Neuroimmunology. 2005;164:66–75. doi: 10.1016/j.jneuroim.2005.03.018. [DOI] [PubMed] [Google Scholar]


