Abstract
Firing patterns of subthalamic nucleus (STN) neurons influence normal and abnormal movements. The STN expresses multiple 5-hydroxytryptamine (5HT) receptor subtypes that may regulate neuronal excitability. We used whole-cell patch-clamp recordings to characterize 5HT receptor-mediated effects on membrane currents in STN neurons in rat brain slices. In 80 STN neurons under voltage-clamp (-70 mV), 5HT (30 μM) evoked inward currents in 64%, outward currents in 17%, and biphasic currents in 19%. 5HT-induced outward current was caused by an increased K+ conductance (1.4 ± 0.2 nS) and was blocked by the 5HT1A antagonist WAY 100135. The 5HT-evoked inward current, which was blocked by antagonists at 5HT2C and/or 5HT4 receptors, had two types of current-voltage (I-V) relations. Currents associated with the type 1 I-V relation showed negative slope conductance at potentials < -110 mV and were occluded by Ba2+. In contrast, the type 2 I-V relation appeared linear and had positive slope conductance (0.64 ± 0.11 nS). Type 2 inward currents were Ba2+-insensitive, and the reversal potential of -19 mV suggests a mixed cation conductance. In STN neurons in which 5HT evoked inward currents, 5HT potentiated burst firing induced by N-methyl-D-aspartate (NMDA). But in neurons in which 5HT evoked outward current, 5HT slowed NMDA-dependent burst firing. We conclude that 5HT receptor subtypes can differentially regulate firing pattern by modulating multiple conductances in STN neurons.
Keywords: 5-hydroxytryptamine, serotonin, subthalamic nucleus, brain slice, patch clamp, burst firing
The subthalamic nucleus (STN) is composed of glutamate-containing neurons that project excitatory pathways to the two major output nuclei of the basal ganglia: the substantia nigra pars reticulata and the globus pallidus interna (Parent and Hazrati, 1995). By regulating output from the basal ganglia, the STN exerts a powerful influence on normal and abnormal movements. According to a widely used model of basal ganglia physiology, the rigidity, tremor and bradykinesia of Parkinson’s disease is worsened by excessive output from the STN (DeLong, 1990). This association has been strengthened by studies that have demonstrated beneficial effects from surgical lesions of the STN in animal models of Parkinson’s disease (Bergman et al., 1990; Guridi et al., 1996) and from high frequency electrical stimulation of the STN in patients with Parkinson’s disease (Limousin et al., 1998). Although symptoms of Parkinson’s disease are associated with increased firing rates of STN neurons (Bergman et al., 1994; Hutchison et al., 1998), several studies have shown that the burst firing pattern of STN neuronal discharge is a better indicator of parkinsonism (Benedetti et al., 2004; Ni et al., 2001).
Firing patterns of STN neurons are regulated by the combined influences of intrinsic membrane properties and the actions of excitatory and inhibitory neurotransmitters (Beurrier et al., 1999; Hallworth and Bevan, 2005; Plenz and Kitai, 1999). Of the many transmitters that may influence STN neuronal activity, serotonin (5HT) may be one of the more important transmitters that have not yet been fully investigated. The dorsal raphe nucleus sends a rich serotonergic input to the STN in rodents and primates (Mori et al., 1985; Canteras et al., 1990). Immunohistochemical and in situ hybridization studies show that STN neurons express high levels of the 5HT2C receptor (Pompeiano et al., 1994), but they also express 5HT1A, 5HT1B, and 5HT4 receptor subtypes (Wright et al., 1995; Bruinvels et al., 1993; Compan et al., 1996). Behavioral studies in rats have demonstrated that microinjections of 5HT1 or 5HT2 receptor agonists into the STN cause hyperlocomotion in rats (Martinez-Price and Geyer, 2002; Belforte and Pazo, 2004), whereas injection of a 5HT2C agonist induces orofacial dyskinesia (Eberle-Wang et al., 1996). Moreover, STN microinjection with a 5HT2 antagonist has been reported to block apomorphine-induced orofacial stereotypy in rats (Barwick et al., 2000). These reports show that activation of 5HT receptors in the STN can produce significant changes in motor behavior.
Previous neurophysiological studies have reported significant effects of 5HT on firing rate of STN neurons. Using extracellular recording techniques, Flores et al. (1995) showed that 5HT increased the spontaneous firing rate in 84% of STN neurons in the rat brain slice. Xiang et al. (2005) also found that 5HT excited STN neurons, and that this action was mediated by 5HT2C and 5HT4 receptors that triggered a reduction in a K+ conductance. In contrast, Stanford et al. (2005) found both excitatory and inhibitory effects of 5HT on spontaneous firing rates of STN neurons recorded with extracellular electrodes in slices of mouse brain. These reports suggest that 5HT may have multiple actions in the STN that have not been fully characterized. Therefore, in the present study, we used whole-cell patch-clamp recordings to identify 5HT receptor subtypes and characterize effects of 5HT on membrane currents in STN neurons in rat brain slices.
EXPERIMENTAL PROCEDURES
Tissue preparation
Horizontal slices containing diencephalon and rostral midbrain (300 μm thick) were prepared from adult (120 - 180 g) male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) as described previously (Shen and Johnson, 2000). Rats were euthanized under isoflurane anesthesia in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The brain was removed rapidly and slices were cut in cold physiological saline with a vibratome. A slice containing the STN was then placed on a supporting net and submerged in a continuously flowing artificial cerebrospinal fluid (2 ml/min) of the following composition (in mM): NaCl (126), KCl (2.5), CaCl2 (2.4), MgCl2 (1.2), NaH2PO4 (1.2), NaHCO3 (19), and glucose (11). Superfusate was gassed with 95% O2 and 5% CO2 (pH 7.4) and heated to 36°C. Using a dissection microscope for visual guidance, the STN was located as grey matter approximately 2.7 mm lateral to the midline and 2 mm rostral to the center of the substantia nigra pars reticulata.
Electrophysiological recordings
Whole-cell recordings were made with pipettes containing (in mM): potassium gluconate (130), MgCl2 (2), CaCl2 (1), EGTA (11), HEPES (10), ATP (1.5), and GTP (0.3). Pipette solutions were adjusted to pH 7.3. Membrane currents were recorded under voltage clamp (-70 mV holding potential), filtered at 5 kHz, and amplified 5-fold with an Axopatch-1D amplifier (Molecular Devices, Foster City, CA, USA). Data were sampled at a rate of 10 kHz and acquired using a personal computer with a Digidata 1320A analog/digital interface and analyzed using pCLAMP 9.0 software (Molecular Devices). Holding currents were recorded continuously using a MacLab analog/digital interface, Chart software (AD Instruments, Castle Hill, Australia) and a Macintosh computer. Membrane potentials have been corrected for the liquid junction potential (10 mV).
Current-voltage studies
Current-voltage relationships were studied by recording currents during hyperpolarizing voltage steps (10 mV increments, 400 ms duration) from a holding potential of -70 mV. In general, currents were measured immediately after capacitive transients in order to avoid the influence of hyperpolarization-activated time-dependent cation currents (Ih). For current-voltage plots that appeared nearly linear, chord conductance was calculated as the slope of a straight line over all test potentials (-70 to -140 mV). Chord conductances were measured over smaller ranges of voltage when current-voltage relations appeared to be non-linear. In some experiments, we report the “net” voltage-dependent current evoked by 5HT; in these cases, “net” currents were calculated by subtracting currents recorded before (control) 5HT from those evoked during 5HT application.
Drugs and chemicals
All drugs were dissolved as stock solutions in water or dimethyl sulfoxide. Stock solutions of drugs were diluted at least 1:1000 to the desired concentration in superfusate immediately prior to use. Dimethyl sulfoxide, diluted 1:1000 in artificial cerebrospinal fluid, had no effect on holding current. Approximately 30 s were required for the drug solution to enter the recording chamber; this delay was due to passage of the perfusate through a heat exchanger. WAY 100135, SB 216641, RS 102221, GR 113808, and RS 23597-190 were obtained from Tocris Cookson Inc. (Ellisville, MO, USA). Tetrodotoxin (TTX), 5-hydroxytryptamine HCl (5HT), N-methyl-D-aspartate (NMDA) and barium chloride (BaCl2) were obtained from Sigma-Aldrich Corp. (St Louis, MO, USA).
Data analysis
Numerical data in text and error bars in figures are expressed as mean ± S.E.M. In current-voltage plots, chord conductance (slope of the current-voltage curve) was determined by linear regression for each cell, and the mean value was calculated by averaging the results from all cells. Unless stated otherwise, differences in data were evaluated for statistical significance using two-way analysis of variance (ANOVA) with repeated measures, followed by a Holm-Sidak pairwise multiple comparison test (SigmaStat, Jandel Scientific, San Rafael, CA, USA).
RESULTS
5HT-dependent currents
As seen in Fig. 1, three types of response to 5HT were observed in 80 STN neurons when recording under voltage-clamp at -70 mV. 5HT (30 μM) produced an outward current (26.9 ± 8.1 pA) in 14 cells, an inward current (16.8 ± 1.6 pA) in 51 cells, and a biphasic response consisting of mixed inward and outward currents was observed in 15 cells. Actions of 5HT began within 1 min of perfusion and reached peak effect within 2 to 3 min. The membrane current returned to baseline 10 - 15 min after removing 5HT from the perfusate. When 5HT evoked an inward current, a decline from peak current was frequently observed during 5HT application. However, the same amount of inward current could be evoked 20 min later by a repeat application of 5HT. TTX (0.5 μM) did not reduce peak 5HT-induced currents (n = 8), which suggests that effects of 5HT are not mediated by action potential-dependent transmitter release.
Fig. 1.
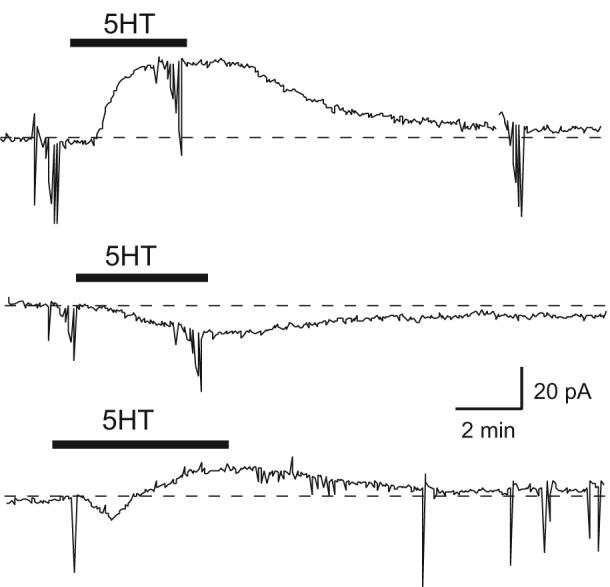
Three types of response to 5HT in STN neurons. Current traces show that 5HT (30 μM) causes an outward current (top), an inward current (middle), or an inward followed by an outward current (bottom) in three different STN neurons (holding potential, -70 mV). Vertical lines in these and subsequent current traces are caused by passing current to measure series resistance and to construct current-voltage curves. Dashed line indicates zero current.
5HT receptor pharmacology
We proceeded to use selective antagonists to characterize receptors that mediate inward and outward currents evoked by 5HT (Fig. 2). 5HT-induced outward current was completely blocked in all neurons (n = 11) by the selective 5HT1A antagonist WAY 100135 (10 μM). However, Fig. 2A illustrates that the 5HT-dependent outward current was frequently replaced by an inward current in the presence of WAY 100135. On average, WAY 100135 converted the 5HT-induced outward current of 19.8 ± 5.0 pA to an inward current of 13.9 ± 4.7 pA (n = 11). This finding suggests that more than one ionic mechanism may be active during 5HT-evoked outward current. We next investigated the receptor pharmacology in those neurons in which 5HT evoked an inward current at -70 mV. In 11 neurons, the selective 5HT2C antagonist RS 102221 (10 μM) reduced the 5HT-evoked inward current from a control value of 16.4 ± 2.4 pA to 5.8 ± 3.5 pA. However, as seen in Fig. 2B, blocking the inward current with RS 102221 sometimes uncovered a 5HT-induced outward current that could be blocked by the 5HT1A antagonist WAY 100135. Furthermore, we found that a 5HT2C antagonist could not block 5HT-evoked inward currents in all cells. For example, Fig. 2C shows current traces from a neuron in which the 5HT-induced inward current was unaffected by RS 102221, and yet this inward current could be completely blocked by adding the selective 5HT4 antagonist RS 23597-190 (10 μM) to the perfusate. The fact that both 5HT4 and 5HT2C receptors may mediate inward currents in the same cell is illustrated in Fig. 2D, which shows that the combination of the 5HT4 antagonist GR113808 (10 μM) and the 5HT2C antagonist RS 102221 (10 μM) was required to completely block this 5HT-induced inward current. On average, perfusing the slice with a selective 5HT4 antagonist (either RS 23597-190 or GR 113808) reduced the 5HT-evoked inward current from a control value of 17.8 ± 4.1 pA to 4.8 ± 1.8 pA (n = 7). These results, which are summarized in the bar graphs in Fig. 2E, show that 5HT-induced inward currents can be mediated by either 5HT2C or 5HT4 receptors, whereas outward current is mediated by activation of 5HT1A receptors. Our data also demonstrate that a single STN neuron can express multiple 5HT receptor subtypes that may have opposing influences on membrane properties.
Fig. 2.

Actions of 5HT (30 μM) are mediated by multiple receptor subtypes. (A) Outward current evoked by 5HT is completely blocked by the 5HT1A receptor agonist WAY 100135 (10 μM). Note that block of outward current reveals a 5HT-dependent inward current. (B) In a different neuron, inward current evoked by 5HT is blocked by the 5HT2C antagonist RS 102221 (10 μM). Note that 5HT2C receptor block allows 5HT to evoke an outward current that is sensitive to WAY 100135. (C) 5HT-evoked inward current can also be mediated by 5HT4 receptors because it can be blocked by RS 23597-190, a 5HT4 receptor antagonist (10 μM), but not by RS 102221. (D) In this neuron, the 5HT-evoked inward current is mediated by 5HT2C and 4 receptors because its block required the combination of GR 113808, a 5HT4 receptor antagonist (10 μM), and RS 102221. Each recording in “A”, “B”, “C” & “D” is from a different STN neuron. (E) Summary data showing block of outward currents by a 5HT1A antagonist (WAY 100135), block of inward currents by a 5HT2C antagonist (RS102221), or block of inward currents by a 5HT4 antagonist (GR 113808 or RS 23597-190). Graphic bars represent paired data from 7 - 11 cells. All recordings were made at -70 mV.
Ionic basis of 5HT-evoked outward current
In many central neurons, 5HT1A receptor-dependent outward current is caused by an increase in a K+ conductance (Penington et al., 1993; Bobker and Williams, 1995; Sodickson and Bean, 1998). In support of a role for K+, we found that the K+ channel blocker Ba2+ (BaCl2, 300 μM) completely blocked 5HT (30 μM)-dependent outward current (measured at -70 mV) in STN neurons (n = 8). But similar to our results with WAY 100135 (see Fig. 2A), we found that 5HT also evoked small inward currents in most neurons when outward currents were blocked by Ba2+ (n = 5). To investigate voltage-dependent effects of 5HT-induced outward current, we proceeded to measure currents during a series of hyperpolarizing voltage steps in the presence and absence of 5HT (30 μM). Current traces in Fig. 3A show that outward current evoked by 5HT are associated with an increase in membrane conductance. Results of similar experiments are summarized in Fig. 3B; this current-voltage (I-V) plot shows net (subtracted) currents evoked by 5HT that were calculated by subtracting voltage-dependent currents recorded before 5HT (control) from those recorded during 5HT perfusion. On average, the 5HT-evoked outward currents were associated with an increase in chord conductance of 1.4 ± 0.2 nS (n = 30). Fig. 3B also shows that outward current evoked by 5HT reversed direction at -95.5 ± 1.9 mV (n = 30), which is 12 mV more positive than the expected K+ reversal potential of -107.5 mV as calculated from the Nernst equation. To further explore a role for K+ in 5HT-induced outward current, we studied the voltage-dependence of 5HT-evoked currents in different concentrations of external K+. Results, which are shown in Fig. 3C, demonstrate that the reversal potential for 5HT-evoked outward current is shifted to less hyperpolarized values with increasing concentrations of extracellular K+ (2.5, 7.5, and 12.5 mM). Although these data are consistent with a 5HT-dependent increase in K+ conductance, the semi-log plot of these data shown in Fig. 3D indicates that each reversal potential obtained in different concentrations of external K+ is about 15 mV more depolarized than would be expected based upon the Nernst equation for K+. Therefore, these data suggest that more than one ionic mechanism may be activated when 5HT evokes a K+-dependent outward current.
Fig. 3.
Potassium dependence of 5HT-induced outward current. (A) Currents recorded during a series of hyperpolarizing voltage steps (from -70 to -140 mV) show that 5HT (30 μM) evokes an outward current that is associated with a conductance increase. Dashed line indicates zero current. (B) I-V plot showing that net (subtracted) currents evoked by 5HT (30 μM) reverse near -90 mV. Net 5HT currents were calculated by subtracting currents recorded before 5HT from those recorded in 5HT at each test potential for each cell (n = 30). (C) Net 5HT currents in a single STN neuron. The reversal potential of 5HT current is shifted to less negative potentials by higher concentrations of extracellular K+ (from 2.5 to 12.5 mM indicated beside each trace). (D) Although reversal potentials are linearly related to log[K+]o, each is more positive than that predicted from the Nernst equation. Data in “D” are from the same cell as in “C”.
Voltage-dependence of 5HT-induced inward currents
To characterize the voltage-dependence of inward currents evoked by 5HT, we proceeded to study I-V relations in 33 neurons in which 5HT evoked only inward currents at -70 mV. We also studied 5HT-induced inward currents in 6 STN neurons in which outward currents were blocked by the 5HT1A receptor antagonist WAY 100135 (10 μM). Because we found no significant difference between 5HT-induced inward currents in these two types of cell, these data were pooled. Fig. 4A shows that inward currents evoked by 5HT (30 μM) followed two different types of I-V relation. The type 1 I-V relation was relatively flat, indicating that these 5HT-dependent currents exhibited little voltage-dependence at these test potentials. Thus, type 1 5HT inward currents were associated with a conductance decrease (0.70 ± 0.20 nS) when measured between -130 and -140 mV, whereas currents had no significant conductance (0.11 ± 0.16 nS) when measured between -70 and -90 mV (Fig. 4A, filled squares; n = 22). In contrast, the type 2 I-V relation for 5HT currents was relatively linear and was associated with a conductance increase of 0.64 ± 0.11 nS (Fig. 4A, open squares; n = 17). Extrapolation of data for the type 2 I-V relation yielded an estimated reversal potential of -38.4 ± 5.0 mV (n = 17). These data further support the hypothesis that 5HT evokes inward currents by more than one ionic mechanism.
Fig. 4.

5HT evokes inward currents with two types of current-voltage relations. Data are from STN neurons in which 5HT (30 μM) evoked inward current (at -70 mV) either in the absence (n = 33) or presence (n = 6) of the 5HT1A receptor antagonist WAY 100135. (A) I-V plots showing net (subtracted) currents evoked by 5HT (n = 39). Neurons were divided into two groups based upon I-V relation: the type 1 I-V relation (closed squares; n = 22 cells) has a relatively flat I-V relation, whereas the type 2 I-V relation (open squares, n = 17 cells) is more linear with positive slope conductance. (B) I-V plots for net 5HT-evoked currents recorded in type 1 neurons in the presence and absence of Ba2+ (300 μM). Ba2+ converts the relatively flat type 1 I-V relation for 5HT-induced currents to one with positive slope that is more typical of the type 2 I-V relation (n = 3).
5HT has been shown to evoke inward currents by closing K+ channels in many central neurons including the STN (North and Uchimura, 1989; Fagni et al., 1992; Xiang et al., 2005). Because 5HT currents with the type 1 I-V relation showed a region of negative slope conductance (Fig. 4A), this is consistent with the possibility that part of the 5HT-induced inward current might be mediated by reducing a K+ conductance. If this were the case, then we would expect that application of Ba2+ would occlude this component of 5HT-induced inward current. Indeed, as seen in Fig. 4B, we found that perfusing brain slices with Ba2+ (300 μM) converted the relatively flat type 1 I-V relation for 5HT inward current into one that was more linear with positive slope conductance (P < 0.05, n = 3Fig. 4B). Measured at -140 mV, the 5HT-dependent inward current increased from a control value of 17.0 ± 13.7 pA to 69.6 ± 18.8 pA in the presence of Ba2+ (P = 0.065; n = 3). This result is consistent with the hypothesis that Ba2+ occludes a 5HT-induced inward current that is mediated by a reduction in K+ conductance. In contrast, Fig. 4B shows that currents evoked by 5HT in the presence of Ba2+ followed a relatively linear I-V relation and was associated with a chord conductance increase of 0.63 ± 0.16 nS (n = 3). Ba2+-insensitive 5HT current had an estimated reversal potential of -19 ± 23 mV, suggesting a mixed cation conductance. Taken together, these results suggest that 5HT can evoke inward currents by opening a cation conductance and by closing a K+ conductance. Moreover, our data show that both mechanisms may operate concurrently in the same STN neuron. Similar dual mechanisms have been described for inward currents evoked by muscarine and substance P in locus coeruleus neurons (Shen and North, 1992a; Shen and North, 1992b).
Dual modulation of burst firing by 5HT
Because firing patterns of STN neurons are known to greatly influence muscle tone and motor function (Benedetti et al., 2004; Magariños-Ascone et al., 2000), we were interested in examining the effect of 5HT on burst firing. While recording under current-clamp conditions, we evoked burst firing in STN neurons by perfusing slices with NMDA (10 - 20 μM), as we have reported previously (Zhu et al., 2004). As shown in Fig. 5, each burst consisted of 20 - 40 spikes and was followed by an interburst hyperpolarization. We divided STN neurons into two groups based upon whether 5HT evoked an outward or an inward current when recording under voltage-clamp at -70 mV. In neurons in which 5HT evoked inward currents (Fig. 5A), 5HT (30 μM) significantly increased the frequency of bursts, from a control value of 0.20 ± 0.03 Hz to 0.40 ± 0.05 Hz in 5HT (P < 0.01, paired t-test; n = 5). 5HT also caused membrane depolarization, which was associated with prolongation of burst duration. But in neurons in which 5HT evoked outward currents (Fig. 5B), 5HT reduced the frequency of bursts, from a control value of 0.35 ± 0.18 Hz to 0.23 ± 0.13 Hz in 5HT (P = 0.059, paired t-test; n = 3). These results demonstrate that 5HT can significantly modify the firing pattern of STN neurons.
Fig. 5.
Dual modulation of NMDA-induced burst firing by 5HT. Current-clamp recordings show effects of 5HT on burst firing that was evoked by NMDA (20 μM). (A) 5HT (30 μM) potentiates burst firing in this neuron in which 5HT also evoked an inward current under voltage clamp (shown in right trace). (B) 5HT (30 μM) slows the frequency of burst discharges in this STN neuron in which 5HT also evoked an outward current under voltage clamp (shown in right trace).
DISCUSSION
Our studies have shown that stimulation of 5HT1A receptors in STN neurons evokes an inhibitory current that is mediated by an increase in K+ conductance. However, in other STN neurons, 5HT had a predominantly excitatory effect that was mediated by both 5HT2C and 5HT4 receptors. Activation of these receptor subtypes evoked inward currents that were mediated by the combined influences of a reduction in a K+ conductance and an increase in a mixed cation conductance. In addition, we found that 5HT can either increase or decrease NMDA-induced burst firing, and these effects could be predicted based upon whether 5HT produced an inward or outward current at -70 mV. We conclude that 5HT acts via multiple receptor subtypes and ionic mechanisms that can significantly influence firing patterns of STN neurons.
Distinct actions of 5HT are mediated via different receptor subtypes
Although we found that 5HT produced an excitatory effect (inward current at -70 mV) in the majority of STN neurons, a sizeable proportion of cells (36%) showed either an inhibitory effect (outward current at -70 mV) or a mixed response to 5HT. Our results with selective 5HT antagonists indicate that inhibitory responses of 5HT are mediated by 5HT1A receptors, whereas 5HT2C and 5HT4 receptors mediate excitatory responses. Moreover, our data showing that inward currents can be evoked by 5HT when outward current is blocked by a 5HT1A antagonist suggest that multiple 5HT receptor subtypes can be co-expressed in an STN neuron. These findings agree with the work of Stanford et al. (2005) who studied effects of selective 5HT agonists and antagonists on spontaneous firing rates of STN neurons in slices of mouse brain. However, our results differ from those of Xiang et al. (2005) who only found excitatory actions of 5HT in STN neurons that were mediated by 5HT2C and 5HT4 receptors. Because expression of 5HT receptor subtypes are known to be age-dependent (Béïque et al., 2004), it is possible that Xiang et al. found no evidence of 5HT1A receptor-dependent outward current because they used brain tissue from young (P16-23) as opposed to adult rats. Co-expression of different 5HT receptors that have opposing effects on membrane potential has been reported in many central neurons including those in hippocampus (Andrade and Nicoll, 1987; Roychowdhury et al., 1994) and cerebral cortex (Araneda and Andrade, 1991; Davies et al., 1987).
5HT modulates multiple conductances via different receptor subtypes
Our data show that activation of 5HT1A receptors evokes an outward current that is associated with an increase in chord conductance. This outward current is mediated by K+ because it was blocked by Ba2+, and changes in external K+ concentration shifted its reversal potential. Activation of 5HT1A receptors has also been reported to increase K+ conductances in neurons in the hippocampus (Sodickson and Bean, 1998), cerebral cortex (Davies et al., 1987), and dorsal raphe nucleus (Katayama et al., 1997). However, when a 5HT1A antagonist blocked outward current, we found that 5HT evoked inward currents at all test potentials. Inward currents, which were activated by stimulation of 5HT2C and 5HT4 receptors, showed two types of I-V relation. One type of I-V relation (type 1) showed a negative slope conductance at potentials more hyperpolarized than -110 mV. Moreover, Ba2+ transformed the type 1 I-V relation into one that was nearly linear. These data suggest that Ba2+ occludes a 5HT-induced current that is mediated by a reduction in K+ conductance. This result agrees with the study by Xiang et al. (2005) who showed that 5HT excites STN neurons by closing a Ba2+-sensitive K+ conductance. Because the Ba2+-sensitive 5HT current was larger at more hyperpolarized potentials, this is consistent with the hypothesis that 5HT reduces an inwardly rectifying K+ current. 5HT has also been shown to block inwardly rectifying K+ currents in motoneurons (Larkman and Kelly, 1998; Takahashi and Berger, 1990) and in nucleus accumbens neurons (North and Uchimura, 1989). Concerning the Ba2+-insensitive inward current evoked by 5HT, it had a nearly linear I-V relation (type 2) with positive slope conductance that we estimated would reverse at -19 mV. These findings suggest that the Ba2+-insensitive inward current is produced by a mixed cation conductance. Thus, our data suggest that inward currents induced by 5HT are the result of the combined influences of a cation conductance increase and a K+ conductance decrease. This conclusion agrees with the work of others who showed that 5HT utilizes both of these mechanisms to evoke inward currents in trigeminal motoneurons (Hsiao et al., 1997) and ventrolateral medulla neurons (Hwang and Dun, 1999). Because we found that 5HT can evoke inward and outward currents in the same cell, the net effect of 5HT will be the result of these combined influences. For example, in STN neurons in which 5HT evoked substantial outward currents, co-activation of a mixed cation conductance most likely explains why the outward current evoked by 5HT reversed at a potential that was more positive than that predicted by the Nernst equation for K+. Whether or not 5HT produces an excitatory or inhibitory effect on neuronal activity will depend upon the degree to which these different ionic mechanisms can be activated in a given cell. Although STN neurons may therefore exhibit variable responses to 5HT, we suggest that the STN contains a continuous spectrum of neurons that vary in their 5HT response, rather than discrete subpopulations of neuron that can be identified by expression of 5HT receptor subtype.
Functional significance
Excessive burst firing of STN neurons has been implicated in the expression of symptoms of Parkinson’s disease (Hashimoto et al., 2003; Bevan et al., 2002). The physiology of burst firing can be complex, and both intrinsic and extrinsic influences may be involved. Because many of these mechanisms are voltage-dependent, burst firing in STN neurons can be influenced by relatively small shifts in membrane potential (Beurrier et al., 1999; Zhu et al., 2002). By showing that 5HT has multiple effects on membrane currents, our data would predict that 5HT can influence firing pattern. Indeed, this hypothesis is supported by our data showing that 5HT has differential effects on NMDA-induced burst firing. Because we found that burst firing was inhibited by 5HT1A-dependent currents and facilitated by 5HT2C- and 5HT4-dependent currents, our data support the hypotheses that agonists at 5HT1A receptors and antagonists at 5HT2C or 5HT4 receptors might be useful in the treatment of Parkinson’s disease (Di Giovanni et al., 2006; Nicholson and Brotchie, 2002). In contrast, we would predict that non-selective ligands such as 5HT releasing agents and 5HT reuptake inhibitors would lack clinical efficacy due to the fact that the present study shows that STN neurons can express multiple 5HT receptors that exert opposing influences on neuronal excitability.
Acknowledgements
This study was supported by USPHS grant NS38175 and by the Portland VA Parkinson’s Disease Research, Education, and Clinical Center.
Abbreviations
- 5HT
5-hydroxytryptamine
- I-V
current-voltage
- NMDA
N-methyl-D-aspartate
- STN
subthalamic nucleus
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section Editor: Dr. Yoland Smith (neuropharmacology)
REFERENCES
- Andrade R, Nicoll RA. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol (Lond) 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neurosci. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Barwick VS, Jones DH, Richter JT, Hicks PB, Young KA. Subthalamic nucleus microinjections of 5-HT2 receptor antagonists suppress stereotypy in rats. NeuroReport. 2000;11:267–270. doi: 10.1097/00001756-200002070-00009. [DOI] [PubMed] [Google Scholar]
- Béïque J-C, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Pazo JH. Turning behaviour induced by stimulation of the 5-HT receptors in the subthalamic nucleus. Eur J Neurosci. 2004;19:346–355. doi: 10.1111/j.0953-816x.2003.03125.x. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nature Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci. 1999;19:599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trend Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. The serotonergic inhibitory postsynaptic potential in prepositus hypoglossi is mediated by two potassium currents. J Neurosci. 1995;15:223–229. doi: 10.1523/JNEUROSCI.15-01-00223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinvels AT, Palacios JM, Hoyer D. Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn-Schmiedeberg’s Arch Pharmacol. 1993;347:569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA. Afferent connections of the subthalamic nucleus: a combined regrograde and anterograde horseradish peroxidase study in the rat. Brain Res. 1990;513:43–59. doi: 10.1016/0006-8993(90)91087-w. [DOI] [PubMed] [Google Scholar]
- Compan V, Daszuta A, Salin P, Sebben M, Bockaert J, Dumuis A. Lesion study of the distribution of serotonin 5-HT4 receptors in rat basal ganglia and hippocampus. Eur J Neurosci. 1996;8:2591–2598. doi: 10.1111/j.1460-9568.1996.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Davies MF, Deisz RA, Prince DA, Peroutka SJ. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res. 1987;423:347–352. doi: 10.1016/0006-8993(87)90861-4. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trend Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Benigno A, Esposito E. Serotonin involvement in the basal ganglia pathophysiology: could the 5-HT2C receptor be a new target for therapeutic strategies? Current Medicinal Chemistry. 2006;13:3069–3081. doi: 10.2174/092986706778521805. [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K, Lucki I, Chesselet M-F. A role for the subthalamic nucleus in 5-HT2C-induced oral dyskinesia. Neurosci. 1996;72:117–128. doi: 10.1016/0306-4522(95)00548-x. [DOI] [PubMed] [Google Scholar]
- Fagni L, Dumuis A, Sebben M, Bockaert J. The 5-HT4 receptor subtype inhibits K+ current in colliculi neurones via activation of a cyclic AMP-dependent protein kinase. Br J Pharmacol. 1992;105:973–979. doi: 10.1111/j.1476-5381.1992.tb09087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Rosales MG, Hernández S, Sierra A, Aceves J. 5-Hydroxytryptamine increases spontaneous activity of subthalamic neurons in the rat. Neurosci Lett. 1995;192:17–20. doi: 10.1016/0304-3940(95)11597-p. [DOI] [PubMed] [Google Scholar]
- Guridi J, Herrero MT, Luquin MR, Guillen J, Ruberg M, Laguna J, Vila M, Javoy-Agid F, Agid Y, Hirsch E, Obeso JA. Subthalamotomy in parkinsonian monkeys: Behavioural and biochemical analysis. Brain. 1996;119:1717–1727. doi: 10.1093/brain/119.5.1717. [DOI] [PubMed] [Google Scholar]
- Hallworth NE, Bevan MD. Globus pallidus neurons dynamically regulate the activity pattern of subthalamic nucleus neurons through the frequency-dependent activation of postsynaptic GABAA and GABAB receptors. J Neurosci. 2005;25:6304–6315. doi: 10.1523/JNEUROSCI.0450-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol. 1998;44:622–628. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- Hwang LL, Dun NJ. 5-HT modulates multiple conductances in immature rat rostral ventrolateral medulla neurones in vitro. J Physiol (Lond) 1999;517:217–228. doi: 10.1111/j.1469-7793.1999.0217z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama J, Yakushiji T, Akaike N. Characterization of the K+ current mediated by 5-HT1A receptor in the acutely dissociated rat dorsal raphe neurons. Brain Res. 1997;745:283–292. doi: 10.1016/s0006-8993(96)01141-9. [DOI] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Characterization of 5-HT-sensitive potassium conductances in neonatal rat facial motoneurones in vitro. J Physiol (Lond) 1998;508:67–81. doi: 10.1111/j.1469-7793.1998.067br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid A-L. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Eng J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Magariños-Ascone CM, Figueiras-Mendez R, Rivera-Meana C, Córdoba-Fernández A. Subthalamic neuron activity related to tremor and movement in Parkinson’s disease. Eur J Neurosci. 2000;12:2597–2607. doi: 10.1046/j.1460-9568.2000.00127.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Price DL, Geyer MA. Subthalamic 5-HT1A and 5-HT1B receptor modulation of RU 24969-induced behavioral profile in rats. Pharmacol Biochem Behav. 2002;71:569–580. doi: 10.1016/s0091-3057(01)00704-3. [DOI] [PubMed] [Google Scholar]
- Mori S, Takino T, Yamada H, Sano Y. Immunohistochemical demonstration of serotonin nerve fibers in the subthalamic nucleus of the rat, cat and monkey. Neurosci Lett. 1985;62:305–309. doi: 10.1016/0304-3940(85)90566-x. [DOI] [PubMed] [Google Scholar]
- Ni Z-G, Bouali-Benazzouz R, Gao D-M, Benabid A-L, Benazzouz A. Time-course of changes in firing rates and firing patterns of subthalamic nucleus neuronal activity after 6-OHDA-induced dopamine depletion in rats. Brain Res. 2001;899:142–147. doi: 10.1016/s0006-8993(01)02219-3. [DOI] [PubMed] [Google Scholar]
- Nicholson SL, Brotchie JM. 5-Hydroxytryptamine (5-HT, serotonin) and Parkinson’s disease - opportunities for novel therapeutics to reduce the problems of levodopa therapy. Eur J Neurol. 2002;3(Suppl):1–6. doi: 10.1046/j.1468-1331.9.s3.1.x. [DOI] [PubMed] [Google Scholar]
- North RA, Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. J Physiol (Lond) 1989;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati L-N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J Physiol (Lond) 1993;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, Hass H, Anderson EG. 5-HT1A and 5-HT4 receptor colocalization on hippocampal pyramidal cells. Neuropharmacol. 1994;33:551–557. doi: 10.1016/0028-3908(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW. Presynaptic dopamine D2 and muscarine M3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. J Physiol (Lond) 2000;525:331–341. doi: 10.1111/j.1469-7793.2000.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K-Z, North RA. Muscarine increases cation conductance and decreases potassium conductance in rat locus coeruleus neurones. J Physiol (Lond) 1992a;455:471–485. doi: 10.1113/jphysiol.1992.sp019312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K-Z, North RA. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neurosci. 1992b;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. Neurotransmitter activation of inwardly rectifying potassium current in dissociated hippocampal CA3 neurons: interactions among multiple receptors. J Neurosci. 1998;18:8153–8162. doi: 10.1523/JNEUROSCI.18-20-08153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Kantaria MA, Chahal HS, Loucif KC, Wilson CL. 5-Hydroxytryptamine induced excitation and inhibition in the subthalamic nucleus: action at 5-HT2C, 5-HT4 and 5-HT1A receptors. Neuropharmacol. 2005;49:1228–1234. doi: 10.1016/j.neuropharm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol (Lond) 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin-1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Wang L, Kitai ST. Modulation of spontaneous firing in rat subthalamic neurons by 5-HT receptor subtypes. J Neurophysiol. 2005;93:1145–1157. doi: 10.1152/jn.00561.2004. [DOI] [PubMed] [Google Scholar]
- Zhu Z-T, Munhall A, Shen K-Z, Johnson SW. Calcium dependent subthreshold oscillations determine bursting activity induced by N-methyl-D-aspartate in rat subthalamic neurons in vitro. Eur J Neurosci. 2004;19:1296–1304. doi: 10.1111/j.1460-9568.2004.03240.x. [DOI] [PubMed] [Google Scholar]
- Zhu Z-T, Shen K-Z, Johnson SW. Pharmacological identification of inward current evoked by dopamine in rat subthalamic neurons in vitro. Neuropharmacol. 2002;42:772–781. doi: 10.1016/s0028-3908(02)00035-7. [DOI] [PubMed] [Google Scholar]




