Abstract
Toxoplasma gondii is an important opportunistic pathogen in immunocompromised individuals. Successful propagation in an infected host by this obligate intracellular parasite depends on its ability to enter and exit host cells. Egress from the cell can be artificially induced by causing fluxes of calcium within the parasite with the use of calcium ionophores. While this ionophore-induced egress (IIE) has been characterized in detail, it is not known whether it mimics a normal physiological process of the parasite. This is underscored by the fact that mutants in IIE do not exhibit strong defects in any of the normal growth characteristics of the parasite in tissue culture. We have isolated and characterized a T. gondii mutant that along with a delay in IIE exhibits a severe defect in establishing a successful infection in vivo. In tissue culture this mutant displays normal ability to invade, divide within cells and convert into the latent encysted bradyzoite form. Nevertheless, mice infected with this mutant are less likely to die and carry less brain cysts than those infected with wild type parasites. Thus, our results suggest that normal response to calcium fluxes plays an important role during in vivo development of T. gondii.
Keywords: Toxoplasma, Egress, Calcium, Virulence, Bradyzoite
1. Introduction
Toxoplasma gondii is one of the most widespread and successful protozoan pathogens and is a common parasite in humans, where it has become one of the main opportunistic pathogens in AIDS patients [1]. The life cycle of T. gondii is complex and includes both sexual and asexual stages. While the sexual cycle is limited to the gut of felines, the asexual cycle can occur in a wide range of hosts and has two major forms: the rapidly growing tachyzoite and the slow growing bradyzoite. Tachyzoites can invade virtually any nucleated cell, replicate, and exit quickly, thereby disseminating throughout the infected host. In most cases the infection is controlled by the immune system, although some tachyzoites can evade elimination by switching to the dormant bradyzoite form contained within a walled cyst. Bradyzoite cysts can persist within the infected tissue for the life of the host, hidden from the immune system and anti-parasitic drugs. In immunocompromised individuals such as AIDS, leukemia and lymphoma patients, new infections or rupture of pre-existing cysts can lead to toxoplasmic encephalitis [1-3]. Additionally, in congenital infections, the disease can lead to severe neurological problems or even death [4].
A devastating consequence of the uncontrolled growth of T. gondii, and a cause of much of its associated disease, is the lethal lysis of the host cell as tachyzoites exit the parasitophorous vacuole in which they replicate. Egress from the vacuole and host cell is a rapid process, and in vitro (and presumably in vivo) is synchronous for all parasites within a given cell but asynchronous between different cells. What signals the parasite to undergo egress remains unknown, as are the molecular mechanisms by which the parasites exit their host cells. Nevertheless, it has become evident that egress can be influenced by changes in ion homeostasis inside and outside the parasite. For instance, it has been shown that a loss of potassium (K+) from the host cell upon artificial permeabilization can trigger T. gondii egress and that this induction depends on intraparasitic calcium (Ca2+) fluxes [5]. The relation between Ca2+ fluxes and egress is particularly evident in experiments with the calcium ionophore A23187, which induces the parasites to quickly exit their host cell in a process called ionophore-induced egress (IIE)[6]. Similarly, when exposed extracellularly to A23187, parasites activate the secretory and cytoskeletal responses required for invasion [7, 8]. The prolonged exposure to the ionophore while outside the host cell causes T. gondii to irreversibly lose its ability to invade cells, presumably due to the exhaustion of essential invasion factors. The result of this inhibition for the obligate intracellular parasite is death, and thus this phenomenon is known as ionophore-induced death (IID) [8].
Mutants with a pronounced delay in IIE (Iie-) have been isolated and characterized [9, 10]. The analysis of these mutants led us to propose a stepwise model for IIE. First, the ionophore induces the release of intracellular Ca2+ stores within the host cell and the parasites. The parasites respond to these changes in Ca2+ concentrations by first extending their conoid, and then permeabilizing the host plasma membrane and the parasitophorous vacuole by an unknown mechanism. The permeabilization event was shown to be parasite and Ca2+ dependent [9]. Chromium-release assays revealed a delay in the permeabilization of the host cell in the Iie- mutants. Furthermore, the Iie- phenotype of the mutants was rescued by treating the cells with a permeabilizing detergent such as saponin [9]. This important permeabilization step then allows a proposed final egress signal to reach the parasite, inducing them to leave. In addition, Iie- mutants are also defective in early stages of the lytic cycle and some of them exhibit a resistance to IID (Iie-Iid- mutants), suggesting a commonality between IIE, IID and normal physiological processes of the parasite [9]. All but one of the Iie- mutants isolated so far were generated by chemical mutagenesis, and efforts to identify the affected genes in those mutants have failed. Thus the nature of the genetic disruption in all of the chemical Iie- mutants is still unknown. Recently, an insertional mutant with a delay in IIE and a resistance to IID was shown to lack the product of a sodium hydrogen exchanger gene, TgNHE1 [10]. The reason for the Iie- phenotype in this particular mutant was determined to be due to an inability to maintain normal intraparasitic levels of calcium [10].
Interestingly, none of the previously reported mutants with a delay in IIE exhibit a delay in natural (i.e. non-induced) egress [9, 10]. A possible explanation for this is that the difference in timing of IIE between mutant and parental strains is only on the order of minutes. Such a difference would be undetectable during natural egress, which is not synchronized and occurs about 40-55 hours after infection, with a variability of many hours from cell to cell. It is also possible that while sufficient to induce egress, calcium fluxes are not necessary for this process in tissue culture and might play a more critical role in specific tissues or cell types. Indeed, it is not known whether any of the mutants deficient in responding to calcium ionophores have a defect in establishing and propagating an infection in vivo. This is due largely to the fact that all Iie- mutants were established using a particular T. gondii strain, RH, which is not suitable for in vivo experiments given its extreme virulence in mice. Although its rapid growth characteristics make RH a favorite model for in vitro studies, in vivo a single parasite of this strain proves fatal to the mouse host, and the strain is not efficient at differentiating into the encysted form in vivo or in vitro. Consequently, it is not known what role, if any, the response to calcium fluxes plays during infection. Here we describe the isolation and characterization of a novel mutant with a defective response to calcium fluxes. This novel mutant is a derivative of the Pru parasite strain, one of the less virulent T. gondii strains, and therefore better suited for in vivo work. This has allowed us to investigate the effects of Iie- and Iid- phenotypes on virulence. In this study we provide evidence that normal response to calcium fluxes is important in the establishment of a successful infection.
2. Materials and Methods
2.1 Parasite and host cell maintenance and reagents
Parasites were maintained by passage through human foreskin fibroblasts (HFFs) at 37°C and 5% CO2. Normal culture medium was Dubelco's Modified Eagle Medium (DMEM) supplemented with 10% FBS, 2mM L-glutamine and 100 units penicillin/100 μg streptomycin per ml. Ionophore assays were performed using Hanks Balanced Salts Solution (HBSS) supplemented with 1mM MgCl2, 1mM CaCl2, 10mM NaHCO3, 20mM Hepes, pH 7.2 (HBSSc). The calcium ionophore A23187 (Sigma) was dissolved in DMSO at 1 mM to make a stock solution.
2.2 Screen for mutants with delayed IIE
The Pru strain of T. gondii [11] deleted in hypoxanthine-xanthine-guanosine phosphoribosyl transferase (PruΔhpt) and containing a signature-tag was used for creation of the mutant library [12]. For this IIE screen, we used all 4,900 mutants previously generated which are maintained in 96 well plates [12]. In brief, 30 μl from each clone was passed to new HFF monolayers grown in 96 well plates. The parasites were then allowed to grow for 30 hours at which time point all clones were exposed to 1μM A23187 in pre-warmed HBSSc at 37°C for 4 minutes. At this point monolayers were fixed in 100% methanol and stained with Diff-Quik (Dade-Behring). After quick visual inspection of all tested clones we selected a total of 20 clones that showed a higher number of intact vacuoles than expected for this time point. These 20 clones were tested again by performing the detailed IIE assay described below (see Quantitation of IIE). Out of the 20 clones tested only one, 52F11, exhibited a reproducible delay in IIE.
2.3 Quantitation of IIE
The efficiency of egress after calcium ionophore exposure was determined using established protocols [10] with some modifications. In brief, 1 × 105 parasites were added to each well of a 24-well tissue culture plate containing confluent HFFs. After 40 hours of growth, the parasites were incubated at 37°C in HBSSc containing 1μM A23187 calcium ionophore or an equivalent amount of DMSO as a solvent control for time periods ranging from 0 to 4 minutes, after which the cells were fixed in 100% methanol. To visualize intact and lysed vacuoles the cultures were stained using Diff-Quik (Dade-Behring) according to the manufacturer's instructions. Percent egress was determined by dividing the number of lysed vacuoles by the total number of vacuoles for a sample.
Induced egress of mutant 91E4 was assessed using 96-well tissue culture plates of confluent HFFs, to which serial dilutions of Pru or 91E4 parasites were added. Parasites were allowed to grow for 30 hours before egress assays were performed and stained as described. Dilutions of parasites resulting in approximately 200 intact vacuoles per well in HBSSc/DMSO (control)-treated samples were identified. Intact vacuoles were enumerated from wells containing corresponding dilutions of ionophore-treated parasites. The number of lysed vacuoles was derived by subtracting the number of intact vacuoles in an ionophore-treated sample from the number found in the control-treated sample.
2.4 Quantitation of IID
Freshly lysed parasites were incubated in HBSSc containing 1μM A23187 for 0, 10, 30, or 60 minutes at a concentration of 1 × 105 parasites/ml. 1.2 × 103 of the treated parasites for each of the time points were then added directly to a well of a 24-well tissue culture plate containing confluent HFFs in 1ml normal culture medium. Plates were incubated for 5 days at 37°C, after which the cells were methanol fixed and stained with crystal violet to visualize plaques. The number of plaques per well was counted and percentage survival was determined by dividing the number of plaques formed at each time point by the number of plaques formed by non-treated parasites (i.e. 0 minute time point).
2.5 Quantitation of efficiency of plaque formation and invasion
For the plaque assays 1.5 × 103 parasites were added per well of 24-well tissue culture plates containing confluent HFFs. Plates were incubated at 37°C for 6 days at which point the cultures were fixed in 100% methanol. Monolayers were then stained for 5 minutes with crystal violet to visualize and count the total number of plaques per well [13].
The efficiency of invasion was determined by allowing 2 × 106 parasites to invade confluent HFFs grown on coverslips for 15 or 60 minutes. To differentiate those parasites that have entered cells from those that remained outside we utilized an immunofluorescence-based invasion assay. In summary, following fixation with 4% formaldehyde, external parasites were labeled with an antibody against the parasite SAG1 protein generated in rabbits (a gift from J. Boothroyd). Cells were then permeabilized with 0.2% Triton X-100 in PBS, and all parasites were labeled with a second SAG1 antibody that had been generated in mice. The two primary antibodies were visualized with an anti-rabbit IgG secondary antibody with a red fluorescent tag (Alexa Fluor 594 - Molecular Probes) and an anti-mouse IgG secondary antibody with a green fluorescent tag (Alexa Fluor 488 - Molecular Probes) respectively. Thus external parasites were co-labeled red and green, while intracellular parasites were labeled green only. In this manner we determined the number of intracellular parasites in 20 randomly chosen fields of view for each coverslip using a Zeiss Axiovert 40 CFL microscope (400X magnification). The data were expressed as a ratio of the total number of intracellular mutant parasites for 20 fields of view over the number of intracellular Pru⊗hpt strain parasites for 20 fields of view.
2.6 Quantitation of division rate
To compare rate of development of the two strains, 1 × 105 parasites were allowed to invade confluent HFFs for 1 hour in a 24 well plate. Wells were then washed 2 times in PBS, and re-filled with normal culture medium. 24 hours after parasite invasion, the cells were fixed in 4% formaldehyde, and parasites stained using an antibody directed against SAG1 as previously described [14]. The number of parasites per vacuole for a minimum of 100 randomly chosen vacuoles was then counted for each strain using a Nikon Eclipse 2000-5 microscope at 1000X magnification.
2.7 STM screen and infection of mice
The STM library was created by first constructing 60 strains carrying a unique sequence tag insertion (Knoll et al., 2001). Both chemical and insertional mutants were created from each of the tagged strains for a total of 4,900 independent strains. The library was arrayed in 96 well plates in which each clone had a different tag and mutation. To screen for mutants with defects in virulence, the STM strains in each of the 89 established plates were pooled, and either grown as tachyzoites or differentiated to bradyzoites by alkalinization of the media to pH 8.1 for 3 days [15]. Two 9-week old CBA/J mice were gavage fed one half a T75 flask each of in vitro bradyzoites (approximately 2 × 106 bradyzoites per infection). About 5% of the mice became moribund during the acute stage of infection (7-10 days). The remaining 95% of mice were allowed to establish an early chronic infection (22 days), at which time they were sacrificed and their brains removed. Brains were then ground with a hand-held tissue homogenizer with disposable pestles, digested with 0.1 mg/ml pepsin (in 170 mM NaCl, 60 mM HCl) for 1 min at 37°C, neutralized with 94 mM Na2CO3, and allowed to grow on an HFF monolayer for 4-7 days. Genomic DNA was prepared from tachyzoites grown only in cell culture or derived from mouse brains, and used as a template to amplify all tags by radiolabeled PCR as described previously (Knoll et al., 2001). These radiolabeled PCR products were used to probe identical filters of unlabeled PCR products representing all tags present in the plate screened, transferred to Nytran SuPerCharge nylon transfer membrane (Schleicher and Schuell) by a Minifold I dot-blot system (Schleicher and Schuell). Filter hybridizations were done under stringent conditions of 50% formamide at 42°C with washes at 65°C in 0.1 × SSC, 0.1% SDS. In this manner, it can be assessed if a particular mutant clone present in the input was absent in the parasites recovered from the mice, suggesting a defect in establishing an infection in vivo.
To examine 52F11 as a clone during infection, either two or four 9-week old CBA/J mice received 2 × 104 i.p. of 52F11, Pru, PruΔhpt, or the F11 tag strain. Plaque assays were preformed immediately after the inoculation to confirm the number of viable parasites that were injected. Twenty-two days after inoculation (early chronic infection) the mice were sacrificed. Their brains were removed, ground with a mortar and pestle, fixed with 3.0% formaldehyde for 20 minutes, and permeabilized and blocked in 0.2% Triton X-100 with 3.0% bovine serum albumin for 30 minutes. Tissue cysts were stained with Fluorescein labeled Dolichos biflorus agglutinin (Vector Labs), and three 5 μL samples mounted and cyst numbers counted by fluorescence microscopy. The resulting number was multiplied by 26 to represent the total of cysts per brain. Experiments were repeated three times.
2.8 In vitro induction of bradyzoite cysts
The culture medium of confluent HFFs in 24-well tissue culture plates was changed from complete DMEM to switch buffer (RPM-1 tissue culture medium (Gibco), 1% FBS, 50mM Hepes, pH 8.1,) immediately prior to infection with parasites. 5 × 105 parasites were then added to each well. Plates were maintained at 37°C at ambient CO2 in the switch buffer for 7 days. Bradyzoite cysts were identified by staining with an antibody raised against the bradyzoite specific surface protein SRS-9 [16] and a fluorescently labeled secondary antibody (Molecular Probes) using standard immunofluorescence protocols. To determine efficiency of cyst formation, the total number of cysts was counted for 10 randomly selected fields of view at 400x magnification for each well. An anti-SAG 1 antibody was used as a counterstain to identify any parasites that remained as tachyzoites.
2.9 Quantitation of bradyzoite egress
To test bradyzoite cysts for induced egress, bradyzoite differentiation was induced as described above in 24 well plates. After 7 days, wells were washed once with 37°C HBSSc, then incubated in HBSSc (control), 1 μM A23187 in HBSSc, 8% EtOH in HBSSc, 10mM DTT in HBSSc, or 0.005% saponin in HBSSc for 30 min at 37°C. Wells were then fixed and stained with the SRS-9 antibody as previously described. The number of intact cysts was counted for 10 randomly chosen fields of view for each well. The percent intact cysts was defined as the total number of intact cysts for experimental wells divided by the total number of intact cysts for control wells. For comparison to tachyzoites, 1 × 105 parasites were allowed to invade confluent wells of HFFs and develop under conditions of normal serum and CO2. 48 hrs post-invasion wells were washed and treated with EtOH, DTT, or saponin as above. Wells were then fixed and stained using the SAG-1 antibody. Percent intact vacuoles was determined as per percent intact cysts as above.
3. Results
3.1 Isolation of a mutant with a delay defective in responding to calcium fluxes
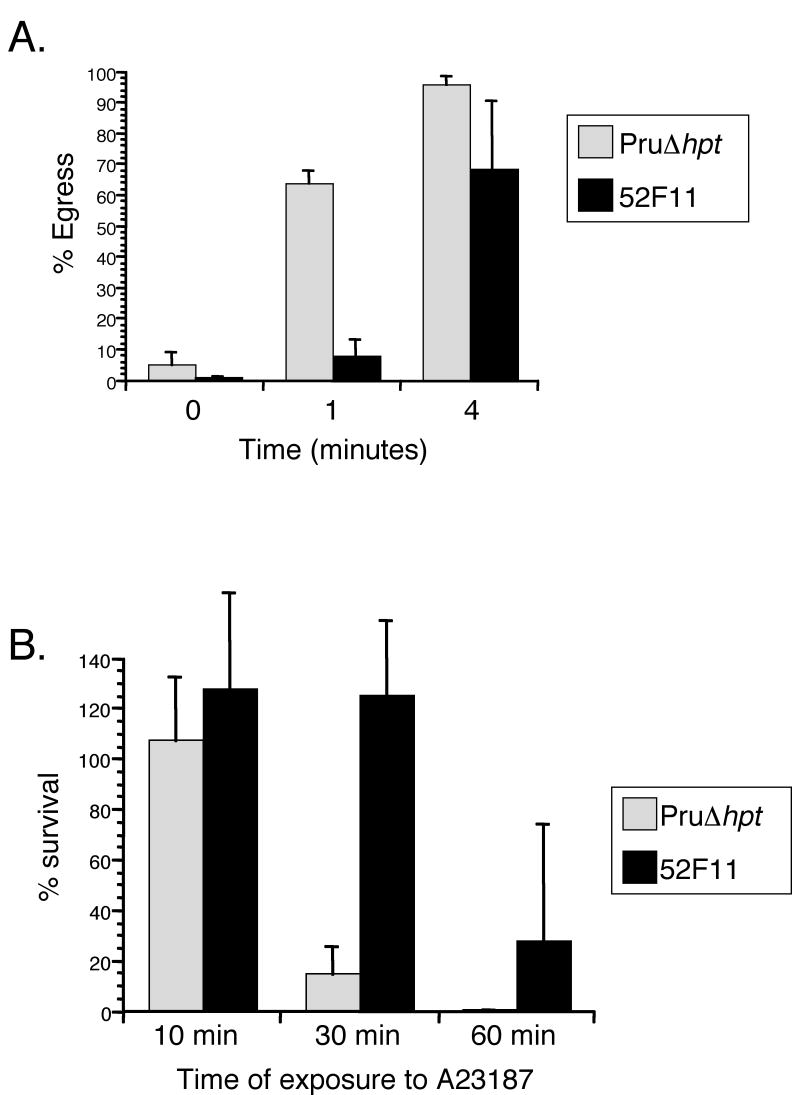
To isolate a type II mutant with a delay in IIE we took advantage of an existing library of independent T. gondii mutants developed using a modified sequence tag mutagenesis (STM) approach [12]. Each of the clones in this library has one of 60 different sequence tags and was mutagenized either chemically or insertionally. For the IIE screen, each of the 4,900 independent clones was exposed to 1μM A23187 for 4 minutes in 96 well plates at 37°C. To determine whether egress had occurred upon induction the cells were fixed and examined by microscopy. After 4 minutes of calcium ionophore exposure 95% of PruΔhpt parasites were outside the cell (Fig. 1A), and thus any clone with over 10% intact vacuoles was identified as a potential Iie- mutant and tested again to confirm the phenotype. In this manner we identified one chemically induced mutant, 52F11, which exhibited a significant delay in IIE as compared to the PruΔhpt strain (Fig. 1A). At 1 min after ionophore exposure, a mean of 7% of 52F11 vacuoles had undergone egress, compared to a mean of 63% for PruΔhpt. By 4 minutes of ionophore exposure, 52F11 vacuoles showed a higher level of egress with a mean of approximately 68% egress, still lower than that seen with PruΔhpt parasites, which exhibited 95% egress by that time point. By 10 minutes both strains exhibited approximately 100% egress (data not shown). Other mutant strains from the library carrying the same sequence tag did not exhibit a delay in egress (data not shown), indicating that it is not the insertion carrying the tag that is responsible for the phenotype but the chemically induced mutation.
Fig. 1. IIE and IID phenotypes of PruΔhpt and the mutant 52F11 strain.
A. Intracellular parasites were exposed to 1μM A23187 Ca2+ ionophore for the indicated time period. Percentage egress represents the number of lysed vacuoles divided by the total number of vacuoles (lysed + intact). Each data point represents the average of three independent experiments and the error bars represent the standard deviation. At least 100 vacuoles were counted per experiment. B. Extracellular parasites were exposed to 1μM A23187 Ca2+ ionophore for the indicated time period before being added to cells. Percentage survival was calculated by dividing the number of plaques formed by parasites treated with A23187 divided by the number of plaques formed by untreated parasites. For each treatment all plaques in a well of a 24-well plate were counted. Data bars represent the mean of 3 independent experiments and the error bars represent the standard deviation.
As mentioned above, most RH strain mutants resistant to IIE also exhibit a resistance to exposure to A23187 while outside their host cell [9, 10]. To determine whether this connection between IIE and IID was also true for the Pru strain mutants such as 52F11 we tested its sensitivity to extracellular exposure to the calcium ionophore (Fig. 1B). After 10 minutes of exposure to 1μM A23187 there was no reduction in the efficiency of invasion or plaque formation for either strain. However, after 30 minutes of incubation in the presence of A23187 PruΔhpt strain parasites exhibited only 14% survival (Fig. 1B). At the same time point, 52F11 parasites exhibited a level of survival equivalent to unexposed controls. Similarly, after 60 minutes of A23187 exposure, the mutant strain 52F11 showed 27% survival, at which time point no survivors were ever detected with the PruΔhpt strain (Fig. 1B).
3.2 Mutant strain 52F11 shows reduced formation of brain cysts in vivo
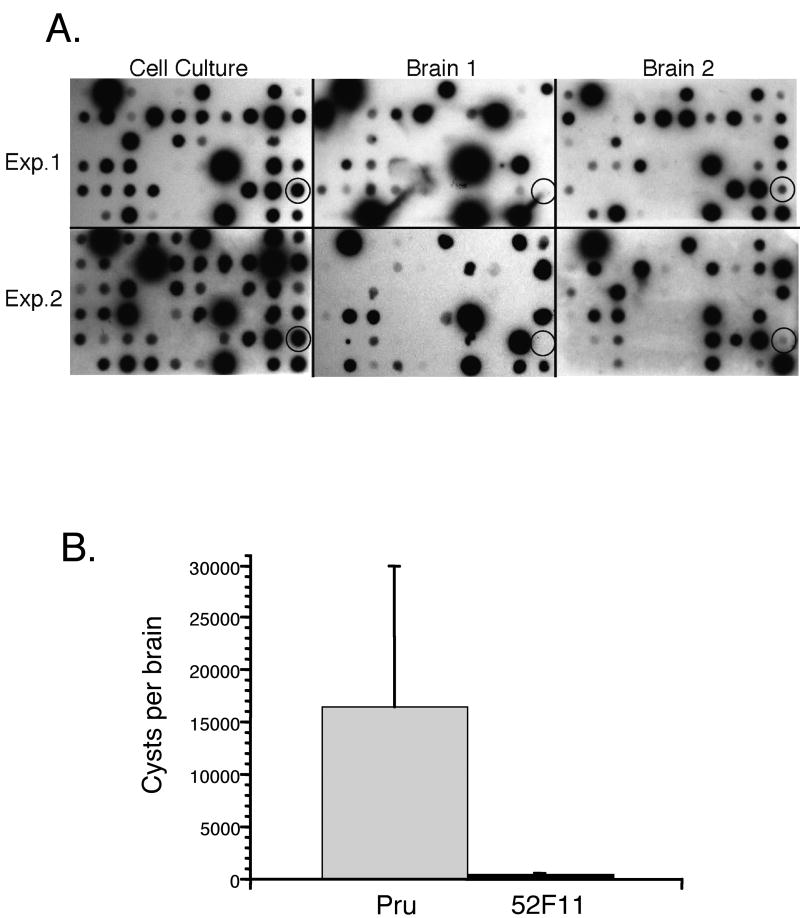
Previous to the IIE screen, the library of 4900 mutant parasites was screened in mice to identify mutants that were defective in their ability to establish a chronic infection (Knoll and Boothroyd unpublished results). Accordingly, mutants carrying different tags were pooled and inoculated into mice. Comparison of the input parasites to those recovered from the mice after the establishment of a chronic infection allows for the identification of those mutants unable to establish a successful infection when in competition with other strains. Interestingly, in such an assay, the 52F11 mutant was less competitive in a pool of T. gondii within mice by this STM screen. This was seen by a reduced hybridization signal for the tag in 52F11 in parasites recovered from mouse brains after 22 days of infection compared to cell culture tachzyoites in two independent experiments of two mice each (Fig. 2A). This reduced hybridization signal suggested that the 52F11 mutant was likely defective in virulence as well as ionophore-induced egress.
Fig. 2. In vivo phenotypes of 52F1.
A. STM screen of plate containing clone 52F11. Cell culture blots represent the pooled parasites from the plate grown one passage as tachyzoites. The cell culture blot was directly compared to blots from the brains of two mice, 22 days after gavage inoculation with in vitro developed bradyzoites. Shown are two independent experiments of plate 52 (Exp. 1 and Exp. 2). The circled spot shows hybridization from the 52F11 mutant that is reduced in the mouse brains. B. In vivo bradyzoite cyst formation by Pru and 52F11 strains. The number of cyst in brains from mice infected intraperitonially with either Pru or 52F11 parasites were counted in three 5μl samples as to determine the total number of cysts per brain. The data bars represent the average number of cysts found in the brains of the surviving mice. Ten total mice were infected for each strain from 3 independent experiments. The error bars represent the standard deviation.
To further characterize the in vivo phenotype of 52F11, we infected mice with parasites of either the Pru or the 52F11 mutant strain. Three independent experiments were performed in which either two or four mice were infected by intraperitoneal (i.p.) inoculation with 2 × 104 parasites of either strain. Two of the total of 10 mice infected with Pru died during acute infection, while all 10 of the mice infected with 52F11 survived. To analyze the level of infection in the surviving mice we sacrificed them and determined the mean number of cysts per brain of all surviving mice. While mice infected with Pru parasites had an average of 16,000 cysts per brain, those infected with 52F11 parasites had an average of 93 cysts per brain (Fig. 2B). Moreover, three of the mice infected with 52F11 had no cysts at all. Serum for all of these three mice tested positive for T. gondii antibodies, confirming that these animals were successfully infected, even though no cysts could be detected (data not shown). In addition, no significant differences were seen in cyst numbers between mice infected with Pru, PruΔhpt, or F11 tag strain, indicating that the phenotype in 52F11 is not caused by either the lack of HPT or the tag (data not shown). The results from the in vivo experiments strongly indicate that 52F11 has a reduced ability to complete a successful infection in mice.
3.3 Strain 52F11 does not have a reduced rate of bradyzoite cyst formation in cultured HFFs
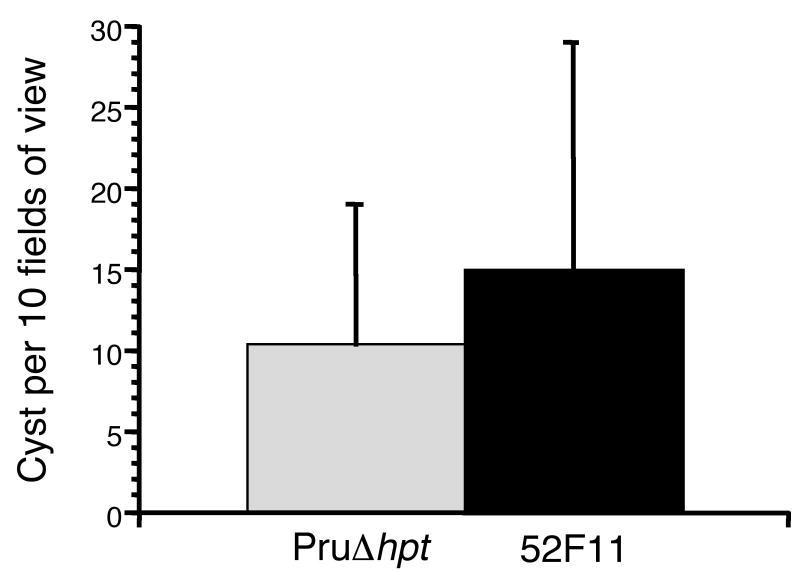
Given the low number of cysts in the brains of animals infected with 52F11, we tested whether 52F11 could be induced to form bradyzoite cysts in tissue culture using a high pH, low serum culture medium. The efficiency of bradyzoite cyst formation was assessed by staining with an antibody for the bradyzoite-specific SRS-9 protein. In addition, we simultaneously utilized an antibody against the tachyzoite specific surface protein SAG1 to detect any tachyzoites that had not successfully switched to bradyzoites. Under the switch conditions used, all surviving parasites for both strains were bradyzoites by 7 days of induction, as indicated by a positive SRS-9 stain and a negative SAG1 stain. In addition, we determined the number of cysts formed by either strain in 10 randomly chosen fields of view. Despite its decreased number of bradyzoite cysts in mice in vivo, 52F11 tachyzoites were able to switch to bradyzoites with the same efficiency as PruΔhpt parasites in vitro as indicated by the number of cysts formed (Fig. 3). These results suggest that 52F11 tachyzoites retain the wild type ability to differentiate into bradyzoites in vitro.
Fig. 3. In vitro bradyzoite cyst formation by PruΔhpt and 52F11 strains.
Bradyzoite cysts were defined as those vacuoles with parasites expressing the bradyzoite specific antigen SRS9 and not the tachyzoite specific antigen SAG1 as detected by IFA. Data bars represent mean total number of cysts in ten randomly chosen fields of view for 3 independent experiments. The error bars represent the standard deviation.
3.4 52F11 does not have a deficiency in invasion or propagation in host cells
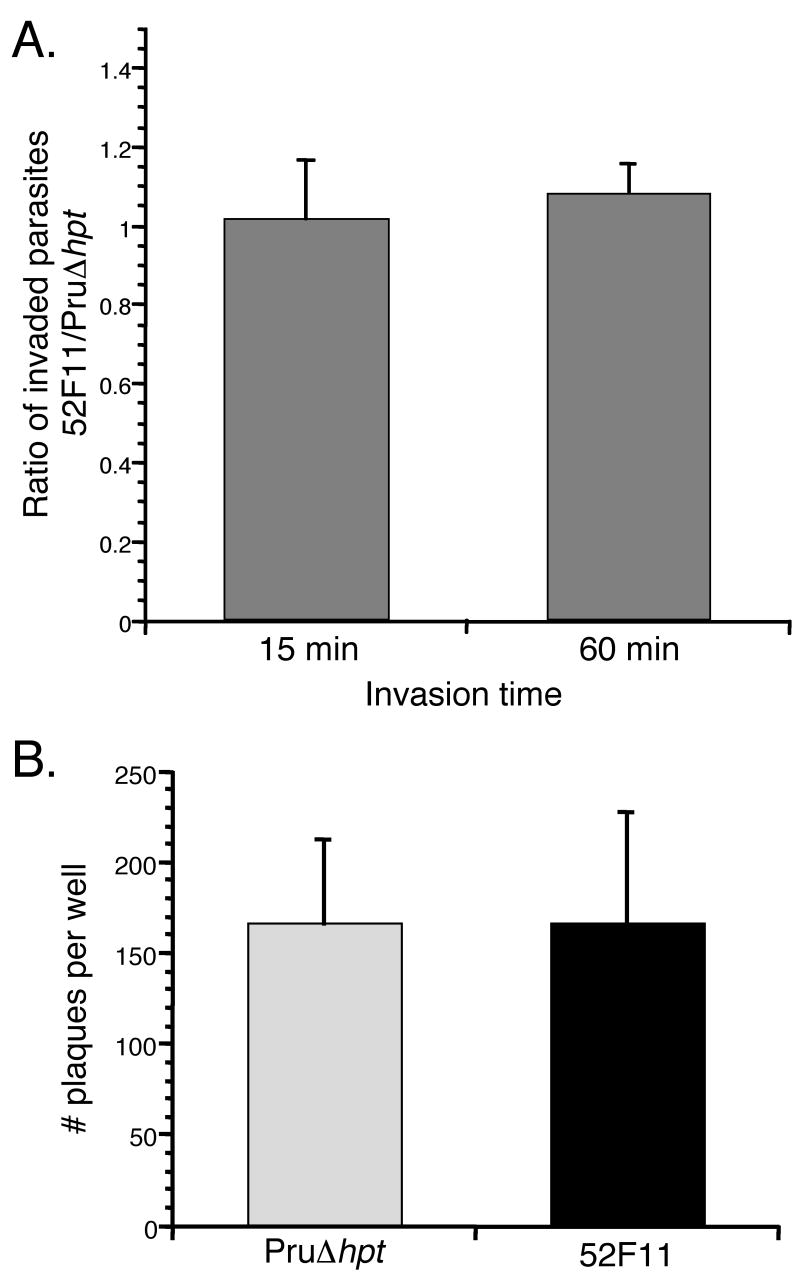
To investigate whether the defect in virulence of 52F11 could be ascribed to a deficiency in the lytic cycle of the parasite we tested the in vitro invasion efficiency of the mutant. For this purpose we exposed equal numbers of 52F11 and PruΔhpt parasites to HFFs for either 15 or 60 minutes and compared their success in invading host cells during those limited time periods. Since at these time points invaded and extracellular parasites are indistinguishable, we identified those parasites inside host cells by using a double staining method in which parasites were differentially labeled with antibodies before permeabilization of HFFs (which would only stain extracellular parasites) and after permeabilization (which would stain both intra- and extracellular parasites) [17]. Utilizing this method we determined the number of intracellular parasites in an equal number of fields of view for both parasite strains and expressed our results as the ratio of the number of intracellular 52F11 parasites over the number of intracellular PruΔhpt parasites. As Fig. 4A shows, this ratio was very close to 1 for both the 15 and 60 minute invasion times in three independent experiments, indicating that the PruΔhpt and 52F11 parasite strains had equal rates of invasion of host cells.
Fig. 4. Efficiency of invasion and plaque formation by PruΔhpt and 52F11 strains.
A. Equal numbers of PruΔhpt and 52F11 strains were allowed to invade HFFs for 15 or 60 minutes. The data was expressed as the ratio of the total number of invaded 52F11 parasites divided by the total number of invaded PruΔhpt parasites, with a ratio of 1 thus indicating no difference in invasion efficiency between the two strains. Data bars represent the mean ratio values for 3 independent experiments and the error bars represent the standard deviation. B. Equal numbers of PruΔhpt and 52F11 strains were allowed to invade HFFs and propagate for 6 days, at which point the cells were fixed, stained, and the total number of plaques per well of a 24-well plate was counted. Data bars represent the mean total number of plaques for 3 independent experiments and the error bars indicate the standard deviation.
As a separate measure of invasion in tissue culture, we tested the ability of the PruΔhpt and 52F11 strains to form plaques. Because there is no limit on invasion time, this test depends not solely on the rate of initial invasion, but on the ability of the parasite to divide and reinvade after exiting the cell naturally. Equal numbers of 52F11 and PruΔhpt parasites were added to wells of 24-well plates containing confluent HFFs. After 6 days the total number of plaques per well was counted. As shown in figure 4B, there was no difference in the number of plaques formed by the 52F11 and PruΔhpt strains, indicating that invasion did not differ between the two strains as seen with the IFA based assay (Fig. 4A). Moreover, visual examination of the plaques formed by either strain did not reveal any significant size difference in the plaques formed by either strain (data not shown). This strongly suggests that the mutant strain divides and propagates at the same rate as PruΔhpt.
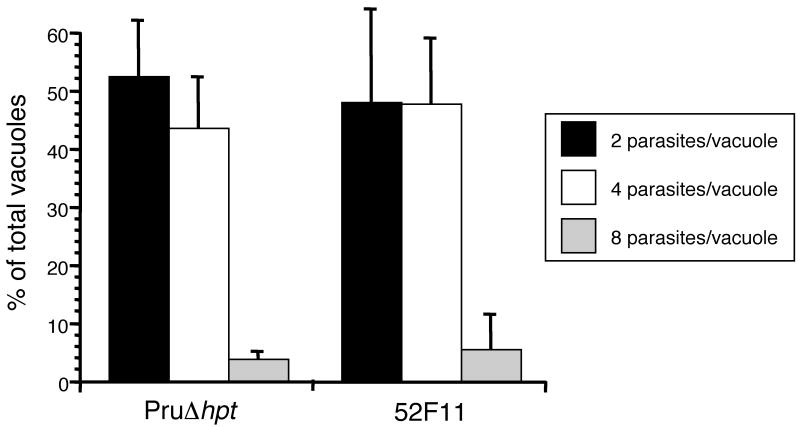
To confirm that 52F11 parasites divide at the same rate as the PruΔhpt strain we infected host cells with parasites from either strain and allowed them to divide for 24 hours. At that time point we fixed the cells, stained them with SAG1 primary antibody and a fluorescent secondary antibody, and counted the number of parasites per vacuole for at least 100 vacuoles per sample. After 24 hours of growth, vacuoles from PruΔhpt and 52F11 parasites show similar distribution of vacuole sizes (Fig. 5). For PruΔhpt we observe 42% two parasite vacuoles, 53% four parasite vacuoles, and 5% eight parasite vacuoles, while for 52F11 we find 42% two parasite vacuoles, 54% four parasite vacuoles, and 4% eight parasite vacuoles. These results confirm that 52F11 has a normal division rate in cultured cells.
Fig. 5. Number of parasites per vacuole for PruΔhpt and 52F11 strains.
Parasites of the PruΔhpt and mutant strains were allowed to invade and divide for 24 hours. The number of vacuoles with 2, 4 or 8 parasites were determined after visualizing the parasites by IFA. At least 100 vacuoles were examined per strain. The data is expressed as the percentage of vacuoles containing the indicated number of parasites. Data bars represent the mean percentages for 3 independent experiments and the error bars represent the standard deviation.
3.5 Mature in vitro bradyzoite cysts do not respond to inducers of tachyzoite egress
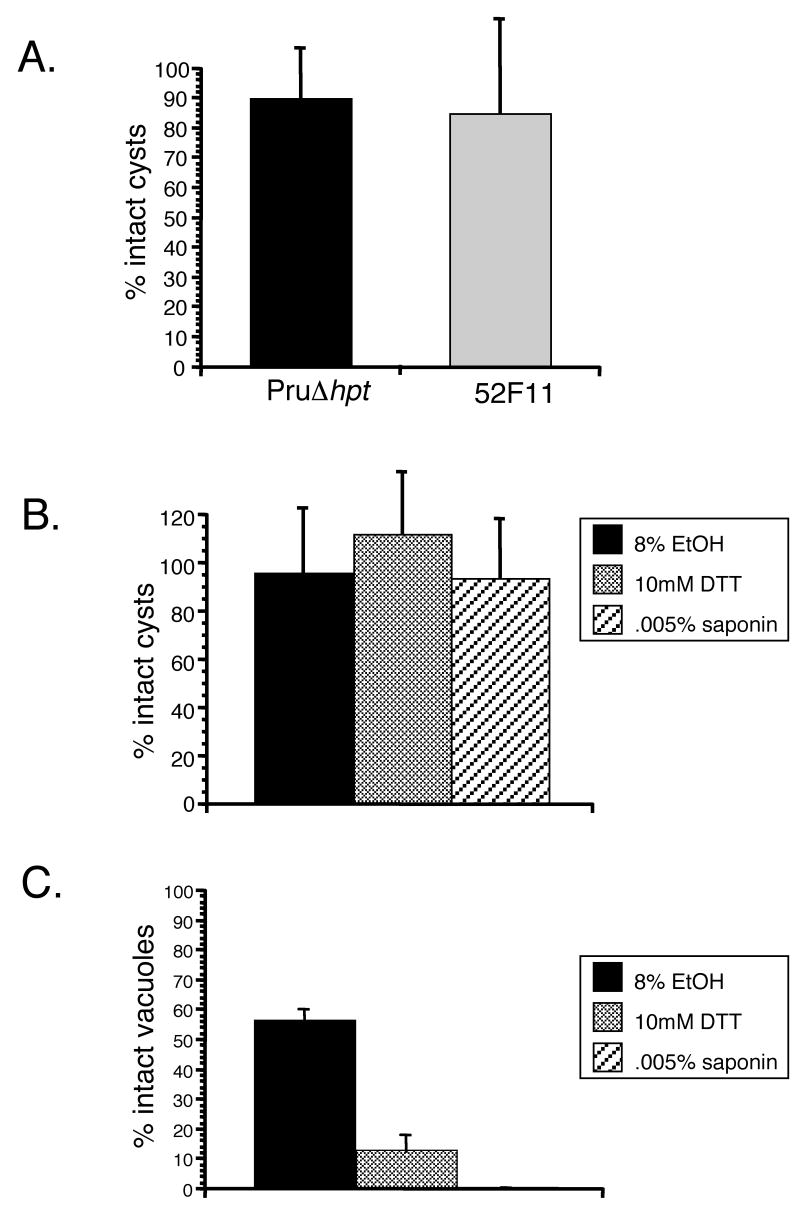
To test whether bradyzoites cysts from 52F11 parasites may respond to egress cues in a different manner than the PruΔhpt strain, and thus account for the lack of tissue cysts in vivo, we examined the response of 52F11 and PruΔhpt strain in vitro cysts to the A23187 calcium ionophore. For this assay 7 days old cysts were exposed to 1μM A23187 for 30 min, exactly as we did with tachyzoites in IIE assays. Cells were then fixed and stained with the SRS-9 antibody to identify bradyzoites. Neither the PruΔhpt strain nor the 52F11 mutant exhibited any significant number of lysed cysts upon ionophore treatment (Fig. 6A). This result suggests that bradyzoite cysts do not respond to A23187 in the same manner as tachyzoites, which lyse their vacuole effectively after less than 5 minutes of exposure.
Fig. 6. Induced egress of in vitro bradyzoite cysts.
A. Seven day old bradyzoite cysts of PruΔhpt and 52F11 strains were exposed to 1μM A23187 for 30 min or to regular media as a control. The percent of intact cysts after ionophore exposure was determined by dividing the number of intact cysts in 10 fields of view of ionophore treated samples by the total number of cysts in 10 fields of view of control samples (no ionophore exposure). B. Seven day old PruΔhpt cysts were exposed to 8% EtOH, 10mM DTT, or 0.005% saponin for 30 min. To determine percent intact cysts, the total number of cysts in the treated samples was divided by the total number of cysts in the untreated one. C. 48 hrs post-invasion PruΔhpt tachyzoite vacuoles were exposed to 8% EtOH, 10mM DTT, or 0.005% saponin for 30 min. To determine percent intact vacuoles, the total number of vacuoles in the treated samples was divided by the total number of vacuoles in the untreated one. In all graphs (A-C) data bars represent the mean for 3 independent experiments and the error bars indicate the standard deviation.
To explore whether the insensitivity to A23187 is specific to the ionophore or represents a general non-responsiveness to calcium fluxes, we examined whether other inducers of tachyzoite egress would induce egress of in vitro bradyzoite cysts. Accordingly, 7 day old differentiated bradyzoite cysts of the PruΔhpt strain were exposed to either 8% EtOH, 10mM DTT, or 0.005% saponin, all known inducers of tachyzoite egress [5, 9, 18]. Regardless of the inducer used, the percentage of intact vacuoles after treatment is nearly 100 (Fig. 6B). By contrast, all of these chemicals were able to induce egress of PruΔhpt tachyzoites (Fig. 6C). In conjunction these results suggest that, at least for 7 day old in vitro cysts, bradyzoites do not respond to host-cell permeablilization or Ca2+-activated egress in the same manner as tachyzoites.
3.6 A second independent mutant is defective in vivo and in response to calcium fluxes
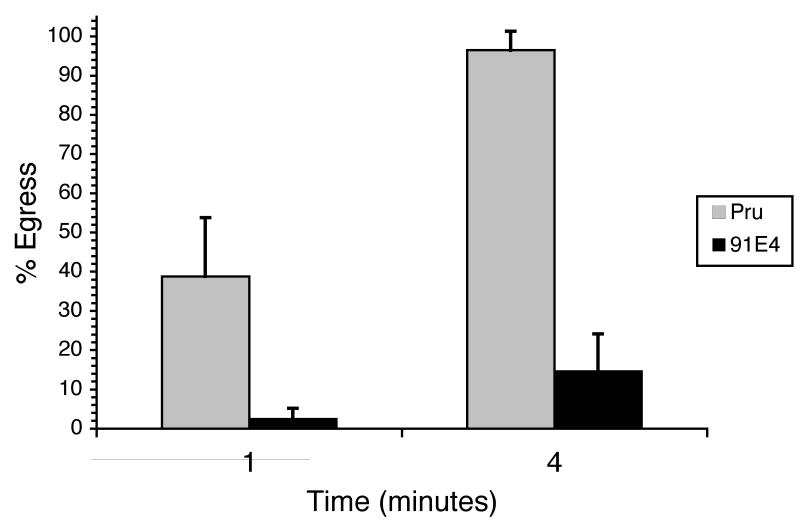
The two main phenotypes manifested by 52F11, the delay in IIE and the inability to establish a chronic infection, could be the consequence of one mutation or of two independent mutations. Several efforts at complementing the mutant phenotype in 52F11 have been unsuccessful and thus the identity of the disruption is not known. Nevertheless, to address whether these two phenotypes are genetically linked we analyzed the IIE response of a panel of mutants that are defective in their ability to establish a chronic infection as identified from a screen of a new STM library [19]. One avirulent mutant in particular, 91E4, which only forms 1% of the number of cyst as compared to the parental strain in mice [19], shows a delay in IIE (Fig. 7). At 1 min after ionophore exposure, a mean of 2% of 91E4 vacuoles had undergone egress, compared to a mean of 39% for Pru parasites. By 4 minutes of ionophore exposure, 91E4 vacuoles showed approximately 15% egress, at which time point the parental strain exhibited nearly 100% egress.
Figure 7. IIE phenotype of Pru and mutant strain 91E4.
Intracellular parasites were exposed to 1μM A23187 Ca2+ ionophore for the indicated time period. Percent egress was determined by dividing the number of lysed vacuoles in treated samples by the total number of intact vacuoles in control-treated samples. Each data point represents the average of four independent experiments. The error bars correspond to the standard deviation.
Discussion
Toxoplasma gondii's lytic cycle is influenced by intraparasitic calcium fluxes [18]. This is most evident by the fact that calcium ionophores such as A23187 will induce intracellular parasites to undergo egress [6] and extracellular ones to initiate invasion related events such as cytoskeletal rearrangements, secretion and motility [7, 8, 20, 21]. If the extracellular ionophore-induced effects occur in the absence of host cells, the parasite eventually loses it ability to invade, and hence dies. In the studies described above, we isolated a mutant strain, 52F11, which exhibits a resistance to both effects of the calcium ionophore A23187: ionophore induced egress (IIE) and ionophore-induced extracellular death (IID).
While several mutants with both Iie- and Iid- phenotypes have been isolated and described before [9], 52F11 is derived from the Pru strain, which is genotypically distinct from the RH strain used to generate previous mutants. Genetic analysis has shown that the vast majority of European and North American T. gondii isolates fall within three canonical genotypes, known as types I, II and III [22, 23]. Our current study shows that Pru, which is a type II strain, is sensitive to the induction of calcium fluxes both intracellulary and extracellularly, just as has been previously reported for the type I strain RH. When comparing the results described here for Pru parasites to those previously reported for RH [9], we observe similar IIE kinetics for both strains: over 50% of vacuoles lysed after 1 minute of exposure to 1 μM A23187 and over 95% by 4 minutes of exposure. This similarity in sensitivity to the ionophore between the type I and the type II strains is also true for IID. With both strains, 100% of parasites are dead after 60 minutes of extracellular exposure to A23187 (our results and [9]). Thus, although Pru parasites exhibit different growth and invasion kinetics as compared to RH parasites, both strains are identically sensitive to the effects of ionophore.
A genetic connection between IIE and IID was previously established for the RH strain through a series of genetic selections [9]. Regardless of what phenotype (Iie- or Ied-) RH mutants were selected for, most of them were both Iie-/Iid-. The fact that 52F11, which was isolated for its delay in IIE, also exhibits IID resistance indicates that a relation between these two phenomena also exists in type II strains. Additionally, 52F11 was screened for from a limited collection of mutants rather than selected for from a population, as were all the RH mutants, indicating that a mutant with both phenotypes can be generated without the selective pressure imparted by the ionophore. Thus, the identification of 52F11 further strengthens the hypothesis that the disruption of one gene is responsible for both phenotypes. Nevertheless, despite various attempts at complementing 52F11 and the other Iie-/Iid- mutants with cDNA, genomic and cosmid libraries, we do not as yet know the identity of the genes disrupted.
The strength of the IIE and IID phenotypes of 52F11 (i.e. how much survival and how little egress upon ionophore exposure) is comparable to that seen with the RH based mutants mbe1.1 and mbd 2.1 [9]. Nevertheless, while all RH mutants that harbor both Iie-/Ied- phenotypes also exhibit a low efficiency of forming plaques as compared to the PruΔhpt strain, we show here that 52F11 has normal invasion and plaque forming efficiency. Whether this is due to 52F11 carrying a mutation in a gene different than the one in the RH mutants is not yet known. It is also possible that the same gene is affected in all mutants, and that the exact nature of the mutation is different or that there is a different role for the gene depending on the strain.
Because only 1 parasite of a type I strain will kill a mouse, partial reduction in virulence in these strains cannot be detected and type I mutant strains are not ideal for virulence studies. Consequently, the previously described Iie- and Iid- mutants have not been analyzed in terms of virulence. Given that the 52F11 mutant is derived from a type II strain, we could now study the consequence of defects in responses to calcium fluxes in an in vivo system. Interestingly, 52F11 shows a strong and consistent in vivo phenotype. Mice infected with 20,000 Pru parasites were more likely to die than those infected with an equal number of 52F11 parasites. Moreover, the number of cysts formed by the 52F11 mutant in vivo was nearly 200-fold reduced compared to Pru.
The delay in IIE and defect in establishing chronic infection in mice are seen not only in mutant 52F11, but also in the independently generated and isolated mutant 91E4. The fact that two independent mutants exhibit defects during in vivo growth and in IIE strongly suggests that these two phenotypes are genetically linked. Given that 52F11 was the only mutant out of over 4,000 from the original library that had a consistent egress defect and that a virulence defect of this magnitude was present in less than 1% of STMs examined, it is unlikely that two separate mutations are causing these phenotypes. Thus, the independent isolation of two mutants with both phenotypes strongly indicates that response to calcium fluxes and virulence are linked.
The lack of cysts in the brain, which is considered the end result of a successful infection, could be due to a defect in converting from the tachyzoite to the bradyzoite stage in the mice. While we cannot completely exclude this possibility, 52F11 tachyzoites can be induced to form bradyzoite cysts in vitro at the same rate as the PruΔhpt strain, suggesting that the in vivo phenotype is unlikely to be due to a complete inability of 52F11 tachyzoites to convert to bradyzoites. The only difference found between the 52F11 and the PruΔhpt strain in vitro was in their ability to respond to calcium fluxes, as indicated by the mutant Iie- and Iid- phenotypes of 52F11. Given the important role of calcium signaling in various events in the life cycle of the parasite (i.e. attachment, invasion, division, egress, motility, etc) it is possible that this mutant has defects in some of the steps required for propagation but that the phenotype is not manifested in tissue culture. It is also possible that this mutant is unable to invade and replicate in a particular tissue such as the brain. Since we see a reduction in lethality of the 52F11 mutant, it is most likely that the 52F11 is defective during early stages of infection as well. The inability of the 52F11 strain to propagate well as a tachyzoite would reduce the number of parasites that are in the brain to become cysts. Regardless, it is evident that efficient response to calcium fluxes by T. gondii is important for the completion of a successful infection in mice. Future work will address the particular stages at which these mutants fail during an in vivo infection.
Acknowledgments
We thank Seon Kim and John Boothroyd for providing us with the SRS-9 antibody, and Gregg Furie and Casey Scott-Weathers for technical assistance. This work was supported by NIH grants from the NCRR Center of Biomedical Research Centers P20 RR15587 (G.A.), the NIAID K22 program AI061293-01 (G.A.), an NIH COBRE award (G.A.), and the NIAID R01 A1054603 (L.J.K.). M.D.L. is supported by the Idaho IDEA Network of Biomedical Research Excellence grant from the NIH/NCRR (P20 RR016454). Some of the initial experiments for the work presented here were performed at Dr. John C. Boothroyd's laboratory at Stanford University School of Medicine, which is funded by NIH grants AI41014 and AI45057.
Abbreviations
- IIE
ionophore-induced egress
- IID
ionophore-induced death
- HFF
human foreskin fibroblast
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark D. Lavine, Department of Microbiology, Molecular Biology and Biochemistry and Center for Reproductive Biology, University of Idaho, Life Sciences South Room 142, Moscow, ID 83843, USA.
Laura J. Knoll, University of Wisconsin-Madison, Dept Medical Microbiology and Immunology, 1300 University Avenue MSC 495, Madison, WI 53706.
Peggy J. Rooney, University of Wisconsin-Madison, Dept Medical Microbiology and Immunology, 1300 University Avenue MSC 495, Madison, WI 53706
Gustavo Arrizabalaga, Department of Microbiology, Molecular Biology and Biochemistry and Center for Reproductive Biology, University of Idaho, Life Sciences South Room 142, Moscow, ID 83843, USA.
References
- 1.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–22. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 2.Israelski DM, Remington JS. Toxoplasmosis in patients with cancer. Clin Infect Dis. 1993;17 2:S423–35. doi: 10.1093/clinids/17.supplement_2.s423. [DOI] [PubMed] [Google Scholar]
- 3.Slavin MA, Meyers JD, Remington JS, Hackman RC. Toxoplasma gondii infection in marrow transplant recipients: a 20 year experience. Bone Marrow Transplant. 1994;13:549–57. [PubMed] [Google Scholar]
- 4.Wong SY, Remington JS. Toxoplasmosis in pregnancy. Clin Infect Dis. 1994;18:853–61. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]
- 5.Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem. 2001;276:41492–501. doi: 10.1074/jbc.M106154200. [DOI] [PubMed] [Google Scholar]
- 6.Endo T, Sethi KK, Piekarski G. Toxoplasma gondii: calcium ionophore A23187-mediated exit of trophozoites from infected murine macrophages. Exp Parasitol. 1982;53:179–88. doi: 10.1016/0014-4894(82)90059-5. [DOI] [PubMed] [Google Scholar]
- 7.Carruthers VB, Giddings OK, Sibley LD. Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell Microbiol. 1999;1:225–35. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 8.Mondragon R, Frixione E. Ca2+-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J Eukaryot Microbiol. 1996;43:120–7. doi: 10.1111/j.1550-7408.1996.tb04491.x. [DOI] [PubMed] [Google Scholar]
- 9.Black MW, Arrizabalaga G, Boothroyd JC. Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol Cell Biol. 2000;20:9399–408. doi: 10.1128/mcb.20.24.9399-9408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrizabalaga G, Ruiz F, Moreno S, Boothroyd JC. Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. J Cell Biol. 2004;165:653–62. doi: 10.1083/jcb.200309097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zenner L, Darcy F, Cesbron-Delauw MF, Capron A. Rat model of congenital toxoplasmosis: rate of transmission of three Toxoplasma gondii strains to fetuses and protective effect of a chronic infection. Infect Immun. 1993;61:360–3. doi: 10.1128/iai.61.1.360-363.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knoll LJ, Furie GL, Boothroyd JC. Adaptation of signature-tagged mutagenesis for Toxoplasma gondii: a negative screening strategy to isolate genes that are essential in restrictive growth conditions. Mol Biochem Parasitol. 2001;116:11–6. doi: 10.1016/s0166-6851(01)00295-x. [DOI] [PubMed] [Google Scholar]
- 13.Roos DS, Donald RG, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Boothroyd JC. Toxoplasma gondii: stable complementation of sag1 (p30) mutants using SAG1 transfection and fluorescence-activated cell sorting. Exp Parasitol. 1995;80:46–53. doi: 10.1006/expr.1995.1006. [DOI] [PubMed] [Google Scholar]
- 15.Soete M, Camus D, Dubremetz JF. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–70. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 16.Kim SK, Boothroyd JC. Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J Immunol. 2005;174:8038–48. doi: 10.4049/jimmunol.174.12.8038. [DOI] [PubMed] [Google Scholar]
- 17.Huynh MH, Rabenau KE, Harper JM, Beatty WL, Sibley LD, Carruthers VB. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. Embo J. 2003;22:2082–90. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrizabalaga G, Boothroyd JC. Role of calcium during Toxoplasma gondii invasion and egress. Int J Parasitol. 2004;34:361–8. doi: 10.1016/j.ijpara.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Frankel MB, Mordue DG, Knoll LJ. Discovery of parasite virulence genes reveals a unique regulator of chromosome condensation 1 ortholog critical for efficient nuclear trafficking. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0701893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchot A, Zierold K, Bonhomme A, Kilian L, Belloni A, Balossier G, Pinon JM, Bonhomme P. Tachyzoite calcium changes during cell invasion by Toxoplasma gondii. Parasitol Res. 1999;85:809–18. doi: 10.1007/s004360050637. [DOI] [PubMed] [Google Scholar]
- 21.Kieschnick H, Wakefield T, Narducci CA, Beckers C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem. 2001;276:12369–77. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- 22.Grigg ME, Bonnefoy S, Hehl AB, Suzuki Y, Boothroyd JC. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science. 2001;294:161–5. doi: 10.1126/science.1061888. [DOI] [PubMed] [Google Scholar]
- 23.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–6. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]