Abstract
We determined whether a two part space-conforming polyethyleneglycol/dopa polymer-based gel promoted healing of contaminated wounds in mice. This silver-catalysed gel was previously developed to be broadly microbiocidal in vitro while being biocompatible with human wound cell functioning. Full-thickness wounds were created on the backs of mice. The wounds were inoculated with 104 CFU of each of four common skin wound contaminants, Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumanii and Clostridium perfringens. The wounds were then treated with our multifunctional polymer-based gel, the commercially-available NewSkin product, or left to heal untreated. The untreated wounds were overtly infected, and presented detectable bacterial loads over the entire 21 day healing period, while the gel and NewSkin groups presented significantly smaller rises in bacterial levels and were cleared of detectable colonies by the third week, with the gel group clearing the bacteria earlier. While all three groups healed their wounds, the polymer-based gel treated group demonstrated signficantly earlier re-epithelialization and dermal maturation (P < 0.05). This was reflected in a quick regain of tensile strength. This accelerated dermal maturation and regain in strength was noted in mice treated with the polymer-based gel when compared to wound treated with the commercially-available Aquacel-Ag dressing (P < 0.05). What distinguishes the polymer-based gel from these other products is that is incorporated within the healing wound. These preclinical studies show that the anti-microbial polymer gel not only supports but also accelerates healing of bacterially contaminated wounds.
Introduction
Wound healing is intricate, orchestrated process involving the interactions of various cells and matrix components to first establish a provisional tissue and then remodel this while forming the mature replacement [1]. Initially, the hemostatic platelet plug re-establishes the infection- and dessication-limiting barrier, and elicits the first wave of cellular infiltrates. This consists mainly of leukocytes that provide both innate and acquired immunity. These cells produce enzymes and biocidal molecules to eliminate microbial contamination; however, these same defense mechanisms are detrimental to the keratinocytes, fibroblasts and endothelial cells required to regenerate the lost tissue. Thus, as healing proceeds, the events and processes of the inflammatory phase need to regress. A particular challenge is offered in the case of skin wound repair, which occurs at a contaminated surface. It is well documented that if a wound becomes infected, the normal healing is disrupted as the inflammatory phase becomes chronic suppressing the regenerative phase. Further, the enzymes elaborated by both the microbes and leukocytes break down the wound tissue as well as surrounding skin. Thus, it is critical to proper healing to prevent infections being established by normal skin wound contaminants.
In view of this concern, we conceived of a multifunctional polymer based gel that would present intrinsic antimicrobial properties [2]. As this was designed to be space conforming, two aqueous-based fluid parts contribute to the gelation in situ. The catalyst for gelation included silver, which is also widely used as an antimicrobial agent [3]. Silver has a very broad spectrum of activity against bacteria, fungi and yeast, all common skin wound contaminants [4, 5]. It also exerts its effect on biofilms [6]. This activity is brought about by the positive silver ions binding with the negatively charged proteins on the microbial surface and interfering with their replication and mitochondrial electron transport [7-9]. In a proof of concept study, we demonstrated that the silver used to catalyze the gelation was sufficient to act also as a poly-antimicrobiocide that killed Gram positive and negative bacteria as well as yeast [2]. However, in vivo effects may be limited by the wound healing process.
Antimicrobial activity is just one part of promoting wound healing. Any wound gel must support the subsequent tissue regeneration. In the case of superficial wounds, most agents simply act to protect the wound bed; however, in deeper wounds, the gel should shape a space to be replaced by tissue. We used a biodegradable, DOPA-crosslinked gel built from polyethylene glycol (PEG) and lysine diisocyanate (LDI). Incorporated into this gel was collagen type I (ColI) to provide hemostasis. In addition, ColI, a major physiologic constituent of the dermis, promotes the migration of keratinocytes, fibroblasts and endothelial cells [10], that is crucial to the regenerative phase of healing. This gel was found to be biocompatible with human primary keratinocytes and dermal fibroblasts in vitro, and to allow fibroblast penetration within the gel [2]. However, we needed to determine whether in a wound situation, this gel would be replaced by tissue or would remain as a potential foreign body, detrimental to the repair and regeneration.
Full thickness skin wounds were made on the dorsum of young, healthy mice. These wounds were contaminated with a light to moderate level of four common skin pathogens, the Gram-positive Staphlococcus aureus, Gram-negative Pseudomonas aeruginosa, the soil commensural Acinetobacter baumanii, and the anaerobe Clostridium perfringens that causes gangrene. While such contaminations of wounds in healthy mice are usually cleared after a short time, they do cause a delay in healing along with deficient repair [11, 12]. This enables us to determine whether our proposed wound healing gel promotes repair and combats infection.
Materials and Methods
Wound dressings
NewSkin (J&J, New Brunswick, NJ) was obtained as an over-the-counter product and Aquacel and Aquacel-Ag (ConvaTec, Skillman, NJ) by pharmacy purchase. The polymer-based gel was generated from individual constituents as described previously [2]. In brief, a polyethyleneglycol-DOPA/collagen I prepolymer was dissolved in 0.1M Na2B4O7 aqueous solution (0.3gm prepolymer per ml solution). This underwent polymerization initiated by the redox initiation system AgNO3/K4P2O8 (7 μl per ml gel), yielding 20 μg of Ag/gm polymer gel wet weight. Silver leached from the gel in detectable amounts for at least 10 days, with approximately half the silver leaving the gel in the first 24 hours with a further loss of about 10% per day for the next 3 days. Specifics of polymer stability and silver diffusion have been published previously [2].
Mouse models for wound healing
Female FVB mice, between 7−9 weeks of age, were used for the experiment. All the mice were housed singly, to prevent fighting and attacks on the wounds, and given food and water ad libitum. They were maintained on a 12- hour light and dark cycle at room temperature. They were all acclimatized to the setting at least a week before using them for our experiments. Whenever the mice were handled, caps, sterile gloves, gowns and shoe covers were worn. The mice were maintained according to the regulations of the Laboratory Animal Welfare Act and amendments and the regulations of the Guide for the Care and Use of Laboratory animals, prepared by the Institute of Laboratory Animal Resources. These studies were deemed approved by the Institutional Animal Care and Use Committee of the Pittsburgh VAMC (Protocol 02481), where the mice were housed.
After shaving the hair on the back of the mice a full thickness wound of size 1.5 cm in diameter was created. Each wound was inoculated with 40 μl of broth mix containing 104 CFU (colony forming units) each of the four microorganisms (Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumanii, and Clostridium perfringens) [13]. After inoculation, we waited 5 minutes before applying the commercially available 0.5 ml NewSkin (J&J, New Brunswick, NJ), 0.5 ml final of our gel (our gel was added as a two part mixture through a Y-adapter or pipette tip and allowed to polymerize in the wound [2]), cut-to-size Aquacel or Aquacel-Ag (ConvaTec, Skillman, NJ), or left the wound untouched. 30 Minutes after the treatments, we covered the wounds with Tegaderm (3M, St.Paul, MN) in all the three groups to maintain uniformity and prevent the mice from removing the treatments. Based on initial experiments [14], we examined the wounds at 7, 14 and 21 days post-wounding so as not to disturb the wound infection (the non-contaminated wounds were measured every other day as they did not have this limitation). This was done to capture the transitions from inflammatory to regenerative and regenerative to resolving phases of wound healing [15]. Animals were euthanized by CO2 inhalation and the wounds assessed. For each experiment, we had four individuals in each group at each time point. Each wound was measured and then removed from the animal, with unwounded skin taken from the contralateral dorsum as a control. Each biopsy was bisected with three of the halves going to tensiometry and histology, and two of the halves being used for quantitative microbial load determinations. The wound healing study was repeated in triplicate for confirmation.
Wound closure measurement
Immediately after creating the wounds, tracings were taken. For uncontaminated wounds, wound size was determined every second day. For the contaminated wounds, at days 7, 14 and 21 the mice were euthanized and tracings of the wound edges were made. Wound areas were determined using the Macintosh Adobe Photoshop software Histogram Analysis. The % of wound contraction was calculated using:
% Wound contraction= (A0-At)/A0 x 100 [16]
Where A0 is the original wound area and At is the area of wound at the time of biopsy on day 7, 14 and 21 accordingly.
Microbial inoculation
Four microorganisms commonly associated with human wound infections were selected to comprise the polymicrobial solution. The microorganisms were obtained from the Clinical Microbiology Laboratory of the UPMC Presbyterian/Shadyside Hospital (these are unlinked to any patient identification and provided as excess pathologic specimens exempted (4e) from human studies as determined by the University of Pittsburgh IRB). The isolates of Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumanii, and Clostridium perfringens were all recovered from human wounds. The initial inoculum was prepared by growing the aerobic bacteria in Trypticase Soy Broth (TSB) overnight at 37°C; the anaerobic bacteria (C. perfringens) was grown in the Chopped meat extract anaerobic Broth at 37°C. After 24 hours the broths were centrifuged at 1000 rpm for 15 minutes and resuspended in TSB with 15% glycerol in case of aerobic bacteria and Chopped meat extract with 15% glycerol in case of anaerobic bacteria. The concentration was adjusted to yield 104 CFU/10 ul, and stored at −70°C. Prior to applying to the wound, the four bacterial stocks were mixed so that 40 ul would deliver 104 CFU of each bacteria. The microbial load was confirmed by direct plating and CFU enumeration after the freeze-thaw in parallel with inoculations.
The inoculum was delivered by sterile pipette to the center of the open wounds. In parallel, an aliquot was taken and quantitatively cultured for the four different bacteria to ensure level of inoculum. At the times of euthanization (days 7, 14, and 21) two of the bisected tissue segments were analyzed for microbial load using the protocol for human wound biopsy culture as stated in the UPMC Clinical Microbiology Laboratory Procedure Manual. The tissue biopsies were weighed and placed in 1.5ml of TSB and homogenized in a tissue grinder. A single drop of the homogenate was placed on the slide and Gram stained for rough assessment (if one or more bacteria are present per oil-immersion field then the expected count in the tissue should be at least 105 CFU/gram). Serial dilutions were made with the tissue homogenate 1:10 (0.1+0.9) in the dilution blanks using distilled water. The CFU/gram of tissue was calculated by:
CFU/Gram= Plate Count × (1/dilution) × 10/ Wt. of Homogenized Tissue
Tensometry
Bisected biopsies taken on days 7, 14 and 21 were wrapped in foil and snap frozen in liquid nitrogen and then stored at −80 deg C; tensiometry was obtained on 3 of each group at each time point. To measure the tensile strength according to established procedures [14], the frozen specimens were trimmed of subcutaneous fat and any muscle that was taken along with the biopsy. The cross-sectional area of each specimen was measured with calipers. Then the specimen was clamped in the tensiometer and force was exerted until the skin tore. The measurements were recorded by a computer and tensile strength was calculated as:
Tensile strength (TS) = Maximum Tensiometer Reading (converted to g)/ Cross-sectional Area (sq-mm)
The TS for the wound was compared to unwounded dorsal skin from the same animal to obtain a relative TS (TS-wound / TS-skin = relative TS-wound).
Histology
Tissue biopsies taken for histology were fixed in formalin, processed and then embedded in paraffin. Sections were stained by routine procedures hematoxylin&eosin for general analyses, and Masson's trichrome and picrosirius red for collagen evaluation. For microscopic assessment each of the slides was coded by a technician, and then read blinded to the sample identification. The samples were scored on a scale of 0 to 4 for epidermal healing (0= no migration, 1= partial migration, 2= complete migration with partial keratinization, 3= complete keratinization, 4= hypertrophic epidermis) and dermal healing (0= no healing, 1= inflammatory infiltrate, 2= granulation tissue present- fibroplasias and angiogenesis, 3=collagen deposition replacing granulation tissue >50%, 4= hypertrophic fibrotic response).
Gram-stained slides were analyzed under oil immersion 1000X magnification for histological estimation of bacterial load. This was required in addition to the quantitative counting as anaerobic bacteria are not enumerated as above. For the Gram reaction (gram-positive or gram-negative), morphology (eg, coccus, rod,) or (eg, formation of chains or clusters), and number of organisms seen per field were determined. Gram stains were considered positive if organisms were seen; the morphology of the organisms (ie, gram-positive cocci in clusters resembling Staphylococcus species, gram-negative rods resembling Pseudomonas, gram-negative cocci resembling Acinetobacter species, gram-positive cocci in chains resembling Streptococcus resembling species), resulted in presumptive classification, though we recognize that any organism could also have derived from the mouse skin or local environment.
Statistical Analysis
All the statistics presented here are shown as mean ± s.e.m. The differences in the groups were considered significant at P < 0.05 using the paired Student's t-test at each time point.
Results
Wound closure in mice treated with polymer-based gel
Initially, we examined whether the polymer gel promoted wound healing in wounds made in an aseptic manner (Fig 1a). We did not note any statistical difference between untreated wounds and the NewSkin or gel-treated wound, with all closing by day 15 or so. This was not unexpected, as healthy young mice are known to heal skin wounds efficiently, and there is likely little room for significant improvement. However, when the wounds were contaminated, a different situation was noted. Untreated contaminated wounds basically closed in all animals by 21 days, which is about six days longer than we note in uncontaminated wounds in mice (Fig 1b) [14]. This suggested that a gel that controlled the bacterial load may provide benefit in wound healing. Interestingly, the area of the untreated wounds increased over the first week (∼35%); such an increase in wound size is not seen in uncontaminated wounds, suggesting that an active infection is occurring in these wounds and disrupting the normal early wound contraction. Still, wound closure was accelerated in both treated groups; while neither showed the early wound retraction of the untreated group, the wound size was stagnant at the first week's measurement, unlike in the uncontaminated wounds. At the intermediary time point of 14 days, the wound size in the polymer group was the smallest of the three groups (P < 0.05). However, all groups attained full closure by the end of the third week.
Figure 1.
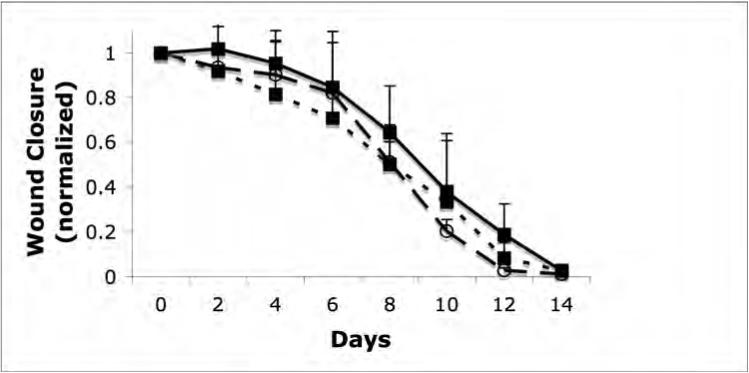
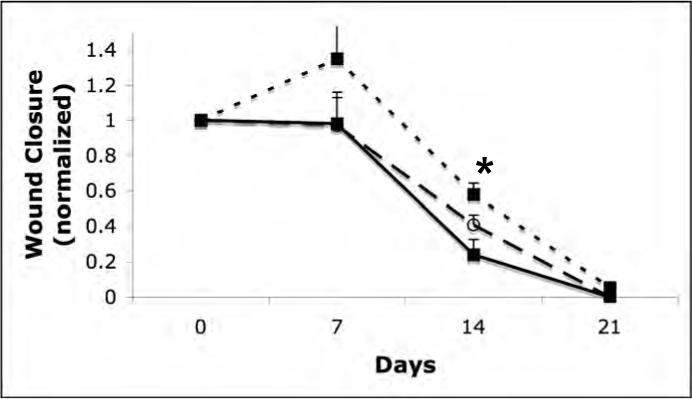
Closure of clean and contaminated wounds. 1.5cm full thickness wounds were measured from time of wounding until closures. The healed area was compared to the initial wound size to determined wound closure by tracing the wound. (A) Closure of full thickness aseptic wounds showed no significant difference in the rate of healing between the untreated (square, dotted line), NewSkin (open circle, dashed line), or polymer gel-treated (solid square, line) wounds. All wounds were closed by day 15. (B) Full thickness wounds that were contaminated with a mixture of microorganisms increased in size initially, while the NewSkin and polymer gel-treated wounds lacked the initial expansion and the polymer gel-treated wounds closed somewhat faster with full closure by day 21. Shown are the mean ± s.e.m. for two separate experiments, each in quadruplicate. * P < 0.05 polymer gel compared to NewSkin at day 14; at a ll days, polymer gel was significantly different from untreated wounds.
Microbial loads in treated wounds
The increase in wound size in the untreated contaminated wounds and the lack of closure during the first week in the NewSkin and polymer gel treatment groups suggested active wound infection. This was supported by quantitative assessment of wound flora (Table 1; Fig 2). The initial inoculum of approximately 104 CFU/10ul of each organism peaked at about 108 CFU/gram in the control and treated wounds by day 7. Some of the wounds in the untreated group overtly appeared infected. In the treatment groups, while the bacterial loads were similarly high, the wounds did not appear macroscopically to be infected. By days 14 and 21, the bacteria levels dropped in all three groups, though the polymer gel group showed the most rapid decrease with a statistically significant difference at the day 14 timepoint.
Table 1.
Bacterial load. Average of four mice at each time point, shown per organism; control refers to initial inoculum for each mouse.
| Organism | Condition | Day | Bacterial count |
|---|---|---|---|
| S.aureus | Control | 0 | 5.52 × 104 CFU/10ul |
| 7 | 6.94 × 108 CFU/g | ||
| 0 | 5.5 × 104 CFU/10ul | ||
| 14 | 8.92 × 108 CFU/g | ||
| 0 | 5.41 × 106 CFU/10ul | ||
| 21 | 79 CFU/g | ||
| New skin | 0 | 5.4 × 104 CFU/10ul | |
| 7 | 2.1 × 106 CFU/g | ||
| 0 | 5.52 × 104 CFU/10ul | ||
| 14 | 3.46 × 105 CFU/g | ||
| 0 | 5.42 × 104 CFU/10ul | ||
| 21 | 0 CFU/g | ||
| Gel | 0 | 5.58 × 104 CFU/10ul | |
| 7 | 3.28 × 108 CFU/g | ||
| 0 | 5.36 × 104 CFU/10ul | ||
| 14 | 3.11 × 104 CFU/g | ||
| 0 | 5.28 × 104 CFU/10ul | ||
| 21 | 0 CFU/g | ||
| P.aeruginosa | Control | 0 | 5.35 × 104 CFU/10ul |
| 7 | 4.07 × 108 CFU/g | ||
| 0 | 5.51 × 104 CFU/10ul | ||
| 14 | 5.78 × 108 CFU/g | ||
| 0 | 5.58 × 104 CFU/10ul | ||
| 21 | 60 CFU/g | ||
| New skin | 0 | 5.41 × 104 CFU/10ul | |
| 7 | 1.91 × 108 CFU/g | ||
| 0 | 5.47 × 104 CFU/10ul | ||
| 14 | 3.21 × 105 CFU/g | ||
| 0 | 5.44 × 104 CFU/10ul | ||
| 21 | 0 CFU/g | ||
| Gel | 0 | 5.46 × 104 CFU/10ul | |
| 7 | 2.34 × 108 CFU/g | ||
| 0 | 5.50 × 104 CFU/10ul | ||
| 14 | 2.3 × 104 CFU/g | ||
| 0 | 5.45 × 104 CFU/10ul | ||
| 21 | 0 CFU/g | ||
| A.baumanii | Control | 0 | 5.45 × 104 CFU/10ul |
| 7 | 4.76 × 108 CFU/g | ||
| 0 | 5.33 × 104 CFU/10ul | ||
| 14 | 6.40 × 108 CFU/g | ||
| 0 | 5.18 × 104 CFU/10ul | ||
| 21 | 68 CFU/g | ||
| New skin | 0 | 5.5 × 104 CFU/10ul | |
| 7 | 1.12 × 108 CFU/g | ||
| 0 | 5.55 × 104 CFU/10ul | ||
| 14 | 2.77 × 105 CFU/g | ||
| 0 | 5.24 × 104 CFU/10ul | ||
| 21 | 0 CFU/g | ||
| Gel | 0 | 5.45 × 104 CFU/10ul | |
| 7 | 1.06 × 108 CFU/g | ||
| 0 | 5.48 × 104 CFU/10ul | ||
| 14 | 2.08 × 104 CFU/g | ||
| 0 | 5.61 × 104 CFU/10ul | ||
| 21 | 0 CFU/g |
Figure 2.
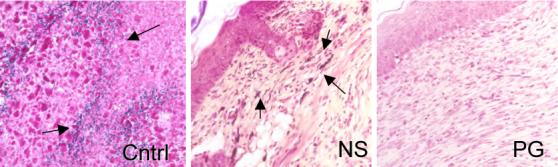
Tissue gram stain evaluation of wounds. Wound biopsy of the infected mice untreated or treated with NewSkin or polymer gel were gram stained at days 7, 14, and 21. Mice treated with the polymer gel displayed a redu ced identification of gram positive or negative microorganisms at day 14 compared to the untreated group which presented visible microorganism that is indicated by the violet color of the rods and red cocci (arrows). Shown are representative gram stains on day 14, w ith arrows pointing to bacterial clusters in the tissue (representative of two experiments, each in triplicate). Original magnifications taken at ×400; the photomicrographs are 300 microns on each edge.
Quantitative counts of C. perfringens were not attempted as culturing anaerobes from skin wounds is not performed in clinical practice and is fraught with under-enumeration due to the aerobic nature of the site. As such we evaluated the wounds using tissues gram stains to determine if anaerobes were contaminating the skin in excess to that counted by quantitation of aerobes (Fig 2). Quantitation of the number of gram positive and negative organism, per high-power field, in the upper dermis reflected the quantitative cell counts. It is not surprising that the treated groups reduced bacterial loads more quickly as they contain antimicrobial agents. NewSkin contains the bio-incompatible compounds 8-hydroxyquinolone, acetone, and alcohols, while the polymer gel contains the clinically used antimicrobial silver.
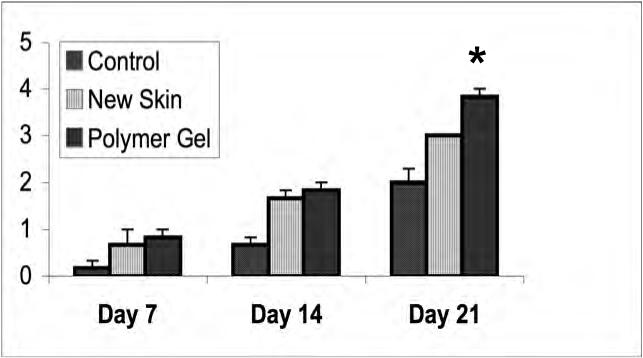
Tensile strength in healing wounds
A key parameter to healing involves regaining strength of the regenerated dermal matrix. While this takes months to reach a final level [17], the trajectory of strength gain over the early weeks is a good indicator of eventual repair. Tensiometry was performed in all the three groups at days 7, 14 and 21 (Fig 3). The treated wounds demonstrated superior strength properties compared to the control wounds at each time point; this was expected as the untreated wounds retracted during the first week. Additionally, the polymer gel-treated wounds were significantly, though only marginally stronger than the NewSkin-treated wounds. Interestingly, the polymer gel-treated wounds were able to be stretched before tearing approximately 3.5 times compared to the control group. This indicates that the gel group exhibited greater flexibility and elasticity compared to the control group.
Figure 3.
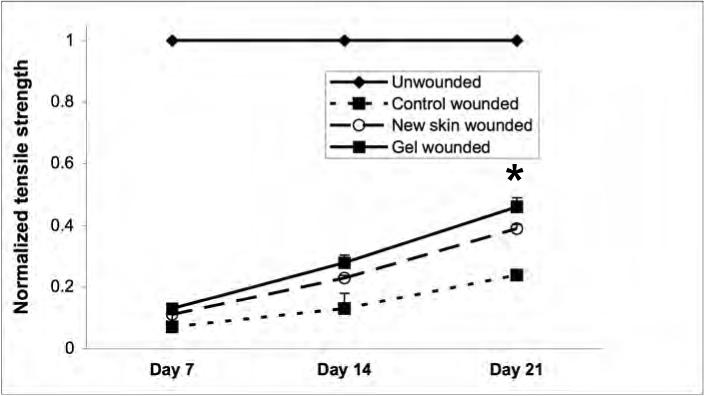
Dermal strength determinations in variously treated wounds. Full thickness wounds, untreated, NewSkin, and polymer gel-treated, were biopsied at days 7, 14, and 21 post-wounding and tensile strength measured. The polymer gel-treated wounds were significantly increased in tensile strength over the NewSkin and untreated group. Shown are the mean ± s.e.m. for two separate experiments, each in triplicate. * P < 0.05 polymer gel compared to NewSkin at day 21; at all days, polymer gel was significantly different from untreated wounds.
Evaluation of dermal and epidermal maturation.
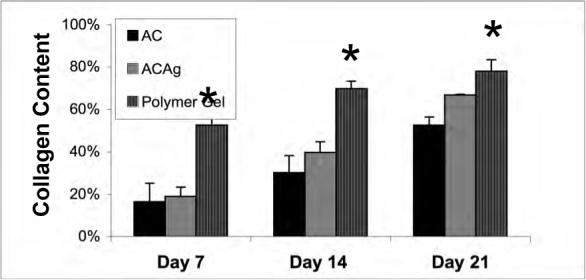
The above data demonstrating enhanced wound contraction and tensile strength suggests that the treated wounds present a more mature dermal matrix. We examined this via routine histological analyses (Fig 4). Dermal maturation is normally assessed in the three stages of proliferation, remodeling, and maturation. The polymer gel-treated wounds exhibited accelerated advancement in all three of these stages. At the cellular level, the polymer gel had advanced to the fibroblast-rich stage when the control and NewSkin-treated wounds still presented a continuous infiltration of neutrophils and polymorphonuclear cells between days 14 and 21. These wounds revealed a unstructured type of dermis wherein the tissues were unorganized in contrast to the polymer gel-treated wounds in which the dermis was organized and well layered.
Figure 4.
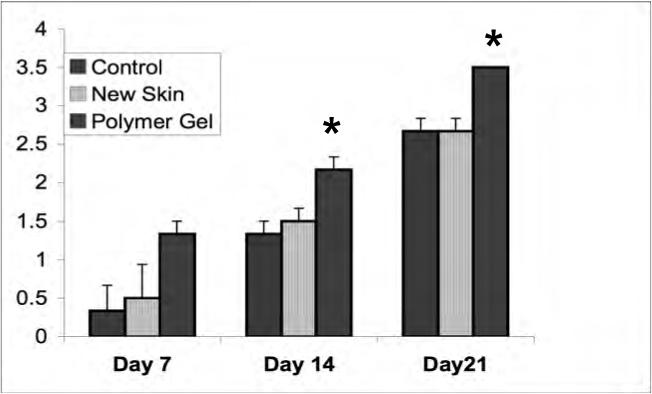
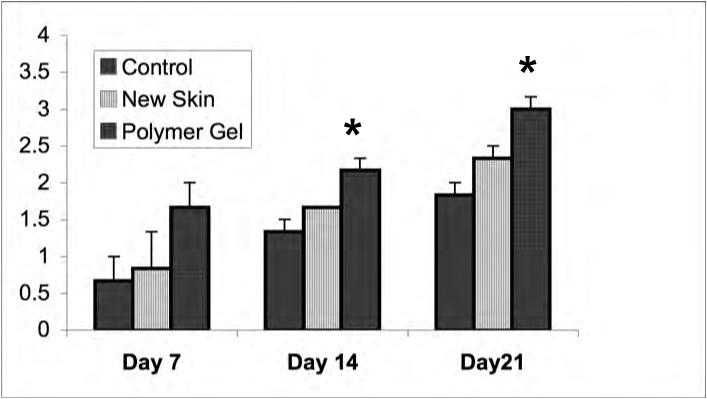
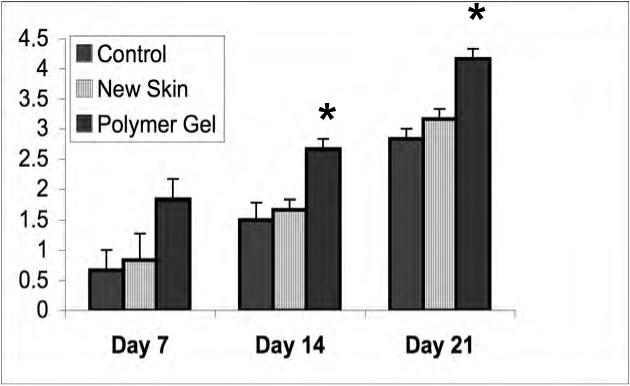
Dermal maturation in mouse wounds. Histological evaluation of the wounds from day 7 to 21 revealed enhanced healing patterns in the polymer gel-treated wounds. (A) Dermal maturation in all wounds at the various time periods was assessed within the parameters of proliferating, remodeling, and maturation. Polymer gel-treated wounds exhibited late remodeling and early maturation by 14 days post wounding. (B) This advancement correlates with the fibroblast infiltration into the wounded area which was scored base on their maturity from reactive to normal. The polymer gel-treated wounds again show great progression over the untreated group in fibroblast maturity. (C) Collagen synthesis was also advanced in the polymer gel-treated wounds. All histological parameters were scored blinded to sample identity (for representative low power H&E stained sections see Fig 6b). Shown are the mean ± s.e.m. for two separate experiments, each in triplicate. * P < 0.05 polymer gel compared to NewSkin; at all days, polymer gel was significantly different from untreated wounds.
Masson's trichrome and picrosirus red staining highlighted the collagen remodeling and maturation (Fig 5). At each time point, the polymer gel-treated wounds had more mature collagen development compared to both the control and NewSkin-treated wounds, though the difference was only in the range of 15% over NewSkin (77 ± 1% of unwounded skin for PG versus 66 ± 1% for NS at day 21; P < 0.05). In toto, the histology of the polymer gel-treated wounds was reminiscent of dermis undergoing aseptic remodeling.
Figure 5.
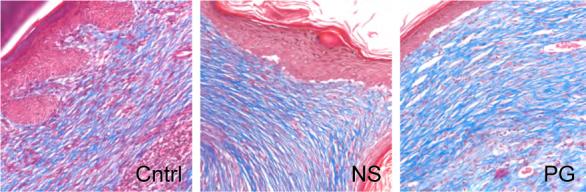
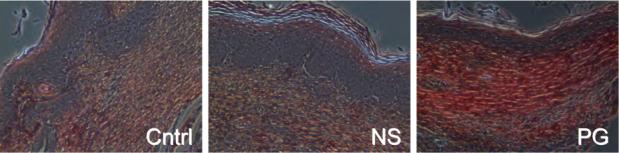
Collagen maturation in wounds. (A) Collagen content was visualized using Masson's Trichrome staining to show distinguishable patterns of collagen between the untreated, NewSkin, and gel-treated. MetaMorph analysis of the wounds confirmed that the polymer gel-treated wounds contained significantly more than that of the other groups (Fig 4c). (B) Quanlitative analysis of the collagen alignment and fibril maturity showed accelerated maturation in the polymer gel-treated wounds. Picrosirius red staining showed longer, mature collagen fibers in the scar area of the polymer gel-treated wounds as shown by the change shift from red to green color. MetaMorph analysis demonstrated that the polymer gel-treated wounds were advanced compared to NewSkin on days 14 and 21 (mean ± s.e.m., n = 2 each in triplicate, p <0.05). Representative microphotographs (of six) are shown of day 21. Original magnifications, ×400 the photomicrographs are 300 microns on each edge.
The accelerated healing also was noted in the epidermal compartment (Fig 6). Epithelialization was initiated by day 7 in the polymer gel-treated wounds but not the other wounds. Polymer gel-treated wounds were multilayered as in normal skin and fully mature by day 21. Keratinization and regeneration of the epithelium showed no signs of irregularity whereas the control and new skin displayed impairment in overall epidermal maturation in comparison to the gel group.
Figure 6.
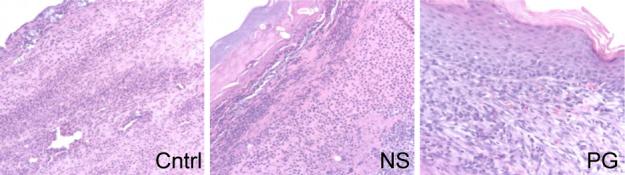
Epidermal maturation. Epidermal maturation was score histologically from ‘no migration’ ( 0 ) to ‘completed migration with keratinization’ ( 4 ). (A) Representative photograph of day 14 wounds showed the polymer gel-treated wounds to h ave completed the reepithelialization process with well attached multilayered cells and normal keratinization. The untreated and NewSkin groups appeared less structured, lacking a strong attachment of the epidermal layer to the dermis at day 14. (B ) Quantitative analysis revealed the polymer gel-treated wounds to present more reepithelialization throughout in comparion to the untreated and NewSkin groups (mean ± s.e. m., n = 2 each in triplicate, p < 0.05) . Original magnifications, ×400 the photomicrographs are 300 microns on each edge.
Comparasion of wound maturation between silver-containing dressings
The NewSkin dressing contains antimicrobial chemicals as evidenced by accelerated clearing of bacteria (Table 1), but is not directly analogous to the space-filling and silver-containing polymer gel dressing. There are a number of commercially available wound dressings that present silver and are meant to conform to a space deficit in the skin [18]. While these dressings have been developed for a different usage, as are to be changed in distinction to the biodegradable polymer gel, they do provide for an efficacy equivalency comparison. Aquacel and Aquacel-Ag (silver containing) were selected as representative of this class of dressings [18]. Contaminated full thickness wounds were generated as previously with subsequent treatment with a singular application after wounding of Aquacel, Aquacel-Ag, or polymer gel; the Aquacel and Aquacel-Ag were cut to fit the wound margins. It appeared that closure was similar in all three treatment groups and similar to the prior experiments with polymer gel, though this could not be reliably quantitated as removal of the dressings disturbed some of the wound margins. However, tensometric and histopathologic measurements could be evaluated by a blinded pathologist.
In all three treatment groups the bacterial count was reduced from 7 to 21 days, with a statistically significant reduction at days 7 and 14 in wounds treated with the silver-containing groups when compared to Aquacel; there was no difference between Aquacel-Ag and polymer gel (data not shown). However, the polymer gel-treated wounds appeared to mature more rapidly as there was significantly increased number and maturity of collagen fibers after the first week as determined histologically (Fig 7). This was also reflected in increased tensile strength at these time points. Interestingly, epidermal maturation appeared unaffected by treatment regimen (Fig 7), though it should be noted that in this series of wounds, the dermal maturation was not as rapid as the prior series comparing the polymer gel to NewSkin (Fig 6). These data demonstrate that polymer gel treatment of wounds is not inferior to that of commercially-available space-filling and silver-containing dressings.
Figure 7.
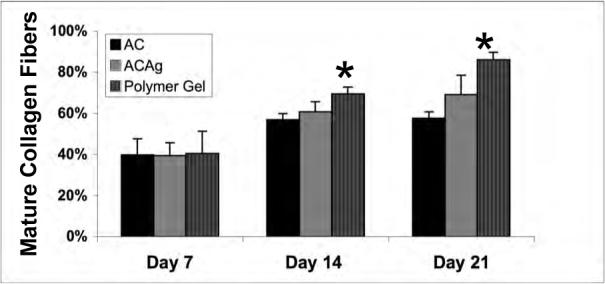
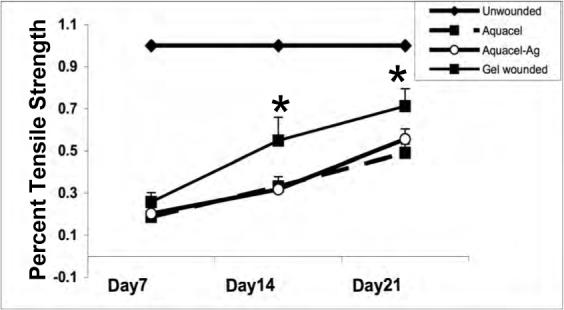
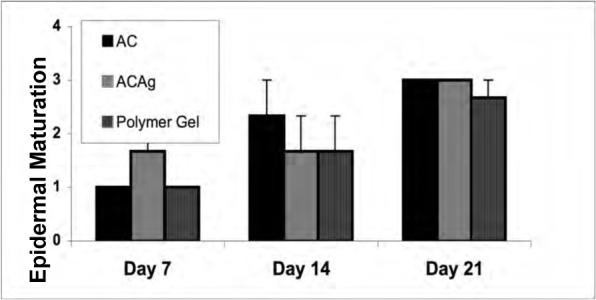
Effects of polymer gel and Aquacel dressings on wound healing. Histological evaluation of wounds treated once after wounding with sized Aquacel, Aquacel-Ag or polymer gel. Scoring is as described in the other figure legends. Shown are evaluation by a blinded pathologist for (A) collagen content, (B) bundling into mature fibers, (C) tensile strength, and (D) epidermal maturation. Shown are mean ± s.e.m., n = 3 each in triplicate evaluation. *P < 0.05 comparing the polymer gel to Aquacel-Ag or Aquacel; the differences in (D) are not significant.
Relationship between regenerative tissue and wound coatings
The polymer gel is proposed to be used in penetrating dermal wounds from which the gel would be difficult to extricate; as such all components and constituents are individually biocompatible and approved for internal clinical use. This is in distinction to NewSkin and Aquacel tested herein, and other similar products that are designed only as wound coverings or dressings that are to be removed from the wound. However, if the polymer gel is to be used in non-removable situations, then it needs to be replaced with the healing tissue. While the depth of wounds in mouse skin often leads to sloughing of applied materials, in about half of the cases the gel remained in the wound. In these situations, we note leukocytes and mesenchymal cells infiltrating the polymer gel (Fig 8). This polymer appears to be replaced by day 21, consistent with the in vitro half-life studies [2]. This is in distinction to the NewSkin which forms a barrier to the provisional matrix and its cellular constitutents, usually attached to, and sloughing with the eschar.
Figure 8.
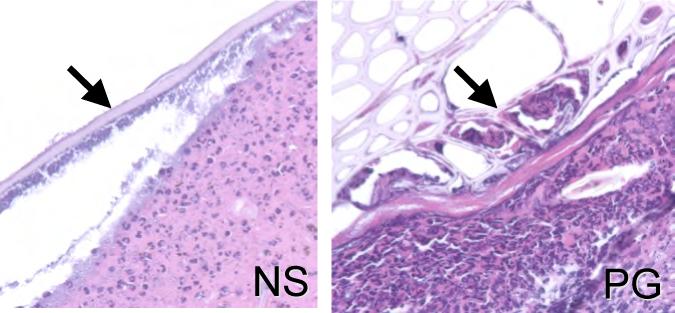
Relationship of dressing to regenerative wound tissue. Histological H&E stained sections of wounds at day 7 displayed the differences between the fate of NewSkin and the polymer gel in the healing wounds. The polymer gel appears to be integrated into the wound with fibroblastoid-appearing cells infiltrating (arrows), whereas NewSkin only provide a superficial layer over the provisional matrix (arrow). Shown are ones of three similar sections. Original magnifications, ×400 the photomicrographs are 300 microns on each edge.
Discussion
A wound dressing should address all aspects of healing. This should not only promote tissue regeneration but also induce hemostasis and limit microbial infection, as these latter two are critical as failure to accomplish these immediate and early steps prevents the subsequent repair. Herein, we report on the in vivo capabilities of such a biocompatible polymer-based gel [2] that incorporates hemostatic (polymer gel and collagen), antimicrobial (silver), and skin cell supportive (collagen) elements. This mixture, shape-forming in situ, appears to accelerate healing of contaminated wounds in a mouse model of skin injury.
The likely key element in our polymer-based gel is the antimicrobial activity. Both acute and chronic infection disrupt the healing cascade. Silver was chosen as it also catalyzes the gellation of the polymer subunits [2]. Silver is a broad-spectrum antimicrobial that limits growth of bacteria and yeast [19, 20]. We have shown that the silver in the polymer-based gel is cytotoxic and cytostatic to many wound contaminants in vitro [2]. Herein, we find that this gel also accelerates the clearing of contaminants in vivo. Two caveats should be noted in this present study. First, even the untreated wounds were cleared of the contaminating microbes, which was expected as healthy animals have robust antimicrobial defenses; still, the polymer-based gel and NewSkin improved the microbial clearing. Second, we have not isolated the antimicrobial activity to the silver per se, as removal of the silver from the gel would not allow for gellation with the result that the agent would not be retained in the wound. However, extrapolating form the in vitro studies, and others investigating silver [3], we feel confident that a major part of the antimicrobial activity is due to the silver.
Silver is widely used as a broad-spectrum antimicrobial in many topical and biomaterials applications [20, 21]. This is generally well tolerated, however, recently two emerging issues have led to questions about its use. First, silver-resistant microbes have emerged. The fact that these have arisen mainly in hospital settings, while cautionary, is less concerning to the proposed ‘field’ use as the soil and skin microbes are less likely to be resistant as there is an absence of selective pressures in these settings. One caution to using silver in an absorbable polymer is the development of argyria, an uncommon side effect that involves both the cosmetically important but nonmorbid condition of skin discoloration and the morbid condition of peripheral neurotoxicity [21]. This condition has been been reported upon use of silver-impregnated dressings [22, 23]. However, we do not consider this side effect limiting, as it is still uncertain that silver causes neurotoxicity [24] and that the cases of argyria involved repeated uses of silver-impregnated dressings over relatively large body areas. Still, these two issues of emergence of microbial resistance and toxicity must be followed.
Many space-filling wound adjuvants have been evaluated. These include synthetic polymers, including polyvinyls and polyurethanes, and natural gels, such as collagens, hydrogels and chitins [25-28]. However, most of these other complex adjuvants focus on providing specific capabilities. Our polymer-based gel aims to fulfill the multifaceted properties of promoting wound healing while being a biocompatible matrix that is resorbed into the healing wound. A special aspect involves the use of this gel in a contaminated wound field. This is in distinction to many such space-filling gels developed for use in sterile fields [29, 30]. A second category includes space-filling dressings that may incoporate silver as a broad spectrum antimicrobial [18]. These are constructed as replaceable dressings for chronic wounds and other longer-term wounds whose treatment involves repeated care. In contradistinction to these two different classes of wound adjuvants, we propose that our polymer-based gel can be used in ‘field’ settings in which wound debridement, cleansing and ongoing care is not possible or desireable. The biodegradability of the polymer over a period of weeks [2], contributes to this use paradigm as it prevents the gel from being established as a foreign body that might serve as a nidus for infection or biofilm.
Pain control is another important factor, particularly in ‘field’ settings. We have incorporated the widely used topical anesthetic lidocaine into the polymer-based gel. Lidocaine acts via nociceptive pathways to stabilize the neuronal membrane by inhibiting the fluxes that regulates ionic flow that initiate and conduct the pain impulses from the wound site. As pain limits use and function of the wounded body part beyond the inherent wound deficity, it is essential to produce anaesthetic effects to maximize the healing process. While we did not assess analgesic effect in this initial wound healing study, the presence of diffusible lidocaine at pharmacologic concentrations is presumed to provide such activity, though this will need be tested in further studies. However, it is important to note that inclusion of this agent did not prevent in situ gellation or the promotion of wound healing.
In conclusion, we present herein a multifunctional polymer-based wound healing gel. This gel incorporates both antimicrobial and hemostatic agents as integral parts that perform double duty. Collagen promotes both hemostasis and the survival and ingrowth of skin fibroblasts, endothelial cells and keratinocytes. Silver is the gellation catalyst and antimicrobial agent. The use of multifunctional agents minimizes the number of molecules for which biocompatibility needs to established and the possibilities of adverse reactions. While a full toxicity study remains to be performed, the use of a small number of clinically-used individual agents bodes well.
Acknowledgements
This work was supported by grants from the DoD to the National Tissue Engineering Center in Pittsburgh, and the National Institute of General Medical Sciences, and in-kind materials and aid from the Veteran's Administration Medical Center in Pittsburgh. We sincerely express our gratitude to Dr. Bruce McClane for providing C. perfringens. We thank Diane George, Pam Matey, Noreen Keane, Mckingley Blair, Shawn Ward, Roger Sembrat VMD, and Nicholas Squeglia for their help with the animal experiments in VA hospital, Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CCY and DW contributed equally to this work.
References
- 1.Singer AJ, Clark RAF. Cutaneous wound healing. New England Journal of Medicine. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Babu R, Zhang J, Beckman EJ, Virji M, Pasculle AW, Wells A. Antimicrobial activities of silver used as a polymerization catalyst for a wound healing matrix. Biomaterials. 2006;27:4304–14. doi: 10.1016/j.biomaterials.2006.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ip M, Lui SL, Poon VK, Lung I, Burd A. Antimicrobial activities of silver dressings: an in vitro comparison. Journal of Medical Microbiology. 2006;55:59–63. doi: 10.1099/jmm.0.46124-0. [DOI] [PubMed] [Google Scholar]
- 4.Coutts P, Sibbald RG. The effect of a siver-containing Hydrofiber dressing on superficial wound bed and bacterial balance of chronic wounds. International Wound Journal. 2005;2:348–56. doi: 10.1111/j.1742-4801.2005.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver S, lePhung T, Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. Journal of Industrial Microbiology and Biotechnology. 2006;33:627–34. doi: 10.1007/s10295-006-0139-7. [DOI] [PubMed] [Google Scholar]
- 6.Chaw KC, Manimaran M, Tay FE. Role of silver ions in destabilization of intermolecular adhesion forces measured by atomic force microscopy in Staphylococcus epidermidis biofilms. Antimicrobial Agents and Chemotherapy. 2005;49(12):4853–9. doi: 10.1128/AAC.49.12.4853-4859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt KB, Bard AJ. Interaction of silver(I) ions with the respiratory chain of Eschericia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+ Biochemistry. 2005;44(39):13214–23. doi: 10.1021/bi0508542. [DOI] [PubMed] [Google Scholar]
- 8.Wang HL, Yuan K, Burgett F, Shyr Y, Syed S. Adherence of oral microorganisms to guided tissue membranes:an in vitro study. Journal of Periodontology. 1994;65(3):211–8. doi: 10.1902/jop.1994.65.3.211. [DOI] [PubMed] [Google Scholar]
- 9.Darouiche RO. Anti-infective efficacy of silver-coated medical prostheses. Clinics in Infectious Disease. 1999;29(6):1371–7. doi: 10.1086/313561. [DOI] [PubMed] [Google Scholar]
- 10.Torres DS, Freyman TM, Yannas IV, Spector M. Tendon cell contraction of collagen- GAG matrices in vitro: effect of cross-linking. Biomaterials. 2000;21:1607–19. doi: 10.1016/s0142-9612(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 11.Ovington L. Bacterial toxins and wound healing. Ostomy Wound Management. 2003;49(7A):S8–12. [PubMed] [Google Scholar]
- 12.Singer AJ, McClain SA. Persistent wound infection delays epidermal maturation and increases scarring in thermal burns. Wound Repair and Regeneration. 2002;10(6):372–7. doi: 10.1046/j.1524-475x.2002.10606.x. [DOI] [PubMed] [Google Scholar]
- 13.Holder IA, Brown RL, Greenhalgh DG. Mouse models to study wound closure and topical treatment of infected wounds in healing-impaired and normal healing hosts. Wound Repair and Regeneration. 1997;5:198–204. doi: 10.1046/j.1524-475X.1997.50213.x. [DOI] [PubMed] [Google Scholar]
- 14.Hebda PA, Whaley DL, Kim H-G, Wells A. Absence of inhibition of cutaneous wound healing in mice by oral doxycycline. Wound Repair and Regeneration. 2003;11:373–9. doi: 10.1046/j.1524-475x.2003.11510.x. [DOI] [PubMed] [Google Scholar]
- 15.Babu M, Wells A. Dermal-epidermal communication in wound healing. Wounds. 2001;13:183–9. [Google Scholar]
- 16.Kirker KR, Luo Y, Nielson JH, Shelby J, Prestwich GD. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials. 2002;23(17):3661–71. doi: 10.1016/s0142-9612(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 17.Muehlberger T, Moresi JM, Schwarze H, Hristopoulos G, Laenger F, Wong L. The effect of topical tretinoin on tissue strength and skin components in a murine incisional wound model. Journal of the American Academy of Dermatology. 2005;52(4):583–8. doi: 10.1016/j.jaad.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 18.Burd A, Kwok CH, Hung SC, Chan HS, Gu H, Lam WK, Huang L. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound Repair and Regenration. 2007;15:95–104. doi: 10.1111/j.1524-475X.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 19.Wright JB, Lam K, Hansen D, Burrell RE. Efficacy of topical silver against fungal burn wound pathogens. American Journal of Infection Control. 1999;27(4):344–50. doi: 10.1016/s0196-6553(99)70055-6. [DOI] [PubMed] [Google Scholar]
- 20.Brett DW. A discussion of silver as an antimicrobial agent: alleviating the confusion. Ostomy Wound Management. 2006;52(1):34–41. [PubMed] [Google Scholar]
- 21.Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Current Problems in Dermatology. 2006;33:17–34. doi: 10.1159/000093928. [DOI] [PubMed] [Google Scholar]
- 22.Walker M, Cochrane CA, Bowler PG, Parsons D, Bradshaw P. Silver depostion and tissue staining associated with wound dressings containing silver. Ostomy Wound Management. 2006;52(1):42–50. [PubMed] [Google Scholar]
- 23.Trop M, Novak M, Rodl S, Hellbom B, Kroell W, Goessler W. Silver-coated dressing Acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. Journal of Trauma. 2006;60(3):648–52. doi: 10.1097/01.ta.0000208126.22089.b6. [DOI] [PubMed] [Google Scholar]
- 24.Lansdown AB. Critical observations on the neurotoxicity of silver. Critical Reviews in Toxicology. 2007;37(3):237–50. doi: 10.1080/10408440601177665. [DOI] [PubMed] [Google Scholar]
- 25.Gomathi K, Gopinath M, Ahmed R, Jayakumar R. Quercetin incorporated collagen matrices for dermal wound healing processes in rat. Biomaterials. 2003;24:2767–72. doi: 10.1016/s0142-9612(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 26.Muzzarelli RAA, Guerrieri M, Goteri G, Muzzarelli C, Armeni T, Ghiselli R, Cornelissen M. The biocompatibility of dibutyryl chitin in the context of wound dressings. Biomaterials. 2005;26:5844–54. doi: 10.1016/j.biomaterials.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Balakrishnan B, Mohanty M, Umashanker PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;24:6335–42. doi: 10.1016/j.biomaterials.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Park SN, Kim JK, Suh H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials. 2004;25:3689–98. doi: 10.1016/j.biomaterials.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 29.Patino MG, Neider ME, Andreana S, Noble B, Cohen RE. Collagen as an implantable material in medicine and dentristry. Journal of Oral Implantology. 2002;28:220–5. doi: 10.1563/1548-1336(2002)028<0220:CAAIMI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Seminars in Cell and Developmental Biology. 2002;13(5):377–83. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]


