Abstract
The protein tyrosine phosphatase SHP2 is a positive effector of EGFR signaling. To improve our understanding of SHP2's function, we searched for additional binding proteins of SHP2. We found that Annexin II is an SHP2 binding protein. Physical interactions of SHP2 with Annexin II were confirmed in vivo. Furthermore, binding of SHP2 with Annexin II was regulated somewhat by EGF treatment and the extracellular Ca2+ chelator, EGTA. Previously, we reported that HSP70 levels can influence the binding of SHP2 with EGFR. Interestingly, increased HSP70 levels also inhibited the binding of SHP2 with Annexin II after EGF treatment in vivo. In addition, immunostaining experiments indicated that a fraction of SHP2 and Annexin II co-localized in the cell membrane region after EGF treatment. Our findings indicate that Annexin II is binding partner of SHP2 and the binding of SHP2 with Annexin II is affected by EGF stimulation, extracellular calcium levels and the levels of HSP70.
Keywords: SHP2, Annexin II, HSP70, calcium chelators
Introduction
SHP2 is a non-receptor phosphatase containing two SH2 domains, a PTP domain, tyrosyl phosphorylation sites and a proline-rich motif. SHP2 is required for activation of the ERK pathway and for receptor-evoked functions, including cell proliferation, differentiation, and migration in the biological responses to growth factors, hormones, cytokines, and cell adhesion molecules [1].
SHP2 binds to several RTK [2, 3], adaptor proteins [4–12], and SHPS-1 [13]. These interactions are essential for growth factor- or cytokine-induced activation of the multiple receptor-evoked functions. However, the complete mechanism(s) of action and other binding proteins of SHP2 underlying this regulation have remained elusive. Recently using mass spectrometry we identified several binding partners of SHP2 amongst which HSP70 was found to be a new binding protein of SHP2 [14]. HSP70 was not a substrate of SHP2 [14]. In this manuscript we characterize another candidate binding protein of SHP2, namely Annexin II.
Annexins are a family of calcium binding proteins and play a pivotal role in membrane/cytoskeleton linking, the maintenance of cell junctions and the exo- and endo-cytosis [15]. Annexin II is a member of the Annexin protein family, which is defined by the unique structural and biochemical features of its members. Annexin II exists either as a 36 kD monomer or a heterotetramer with the protein S100A10 (p11), which is a member of the Ca2+-binding EF-hand protein S100-protein family [16]. Annexin II is mainly localized in the cytoplasm of the cells and in the nucleus may effect DNA synthesis and cell proliferation [17].
It was reported that Annexin II co-immunoprecipitates with SHP-2 from membrane extracts from confluent endothelial cells in a cholesterol-dependent mechanism [18]. Furthermore, recent studies have demonstrated that Annexin II is downregulated in prostate cancer [19] and inhibits prostate cancer cell migration [20]. Therefore, Annexin II has become a candidate to act as a scaffolding protein to organize signalling proteins and control cellular processes involved in proliferation, differentiation and apoptosis.
The current study reports that Annexin II binds to SHP2 in vivo and that the binding of these two proteins was influenced by extra cellular calcium not by intra cellular calcium. Interestingly, HSP70 expression levels affect the SHP2 binding with Annexin II. These results indicate that the interaction between Annexin II and SHP2 is potentially regulated by HSP70 and extra cellular calcium levels.
Materials and Methods
Cells, Cell culture, Antibodies, and Plasmid construction
The cell type used in this study was COS-1 cells. COS-1 was grown in Dulbecco's modified Eagle's medium supplemented with 10% FBS, and was maintained at 37°C with 7% CO2. EGF and anti- FLAG monoclonal antibody were purchased from Sigma; anti-Annexin II monoclonal antibody was from BD Laboratories; anti-GAB1 polyclonal and anti-Src monoclonal antibodies were from Cell Signaling; anti-HSP70 antibody was from Stressgen; anti-PTP1D monoclonal antibody was from Transduction Laboratories; anti-EGFR polyclonal antibody and anti-pY (4G10) monoclonal antibody were from Upstate Biotechnology; The polyclonal antibody to SHP2 was raised by injection of rabbits with a glutathione S-transferase (GST) fusion of the N-terminal region [21]. Horseradish peroxidase-conjugated secondary antibodies were purchased from Amersham. Construction of plasmids that express HSP70, wild-type SHP2 (WT-SHP2), D425A, C459S -SHP2 (DM-SHP2), or N-terminal FLAG-tagged WT/DM SHP2 proteins was described previously (FSHP2WT, FSHP2DM) [3, 14]. C-terminal 3x FLAG tagging Annexin II was a gift from Dr. S. Tsirka (SUNY at Stony Brook, New York, USA).
Isolation of FLAG· SHP2 complexes
COS-1/FLAG-WT or -DM SHP2 cells (5 × 100mm culture dishes) were cultured 36 hours, and then treated with 100ng/ml EGF for 10 minutes. An equal number of cells transfected with vector alone were cultured as a negative control. Cells were disrupted in RIPA buffer (150 mM NaCl, 10 mM Tris-HCl, pH 7.5, 1% triton X-100, 0.5% deoxycholate, 0.1% SDS, and 1 mM EDTA) containing a mixture of protease inhibitors. Clarified cellular lysates were incubated with 400 μl of anti-FLAG M2 agarose gel (Sigma) for 3 hours at 4 °C. The resin was collected by centrifugation and was washed with TBS three times. FLAG-SHP2 complexes were eluted from the resin by incubation for 60 minutes at 4 °C with 300 ng/μl FLAG peptide (Sigma) in TBS and acetone precipitated. The precipitated proteins were redissolved in loading buffer and resolved by gel electrophoresis on 10% SDS-PAGE gels.
Mass Spectrometry
The Mass Spectrometry analysis was carried out as described previously [14]. In brief, visible protein bands were excised from the colloidal blue-stained gel and chopped into 1-mm3 pieces. LC/MS/MS experiments were performed by the Proteomics Center at the Stony Brook University on an API Qstar Pulsar i quadrupole-TOF LC/MS/MS mass spectrometer. Protein identification was done by using the ProID software to search the Swissprot protein sequence database.
Cell transfection and preparation of lysates
Cells were transfected with various constructs by using the FuGene transfection reagent as recommended by the manufacturer (GIBCO-BRL), incubated for <36 hours, serum starved for <12 hours, and then stimulated with 100 ng/ml of EGF for the indicated time. After being washed twice with ice-cold PBS, cells were lysed in RIPA buffer with protease inhibitors for 30 minutes on ice and centrifuged at 5,000g for 30 minutes at 4°C. The supernatant was analyzed as described.
Immunoprecipitation and Immunoblotting
The immunoprecipitation (IP) analysis was carried out as described previously [14]. In brief, cells were lysed in the RIPA buffer with protease inhibitors for 30 minutes on ice and centrifuged at 5,000g for 30 minutes at 4°C. The supernatants were incubated with the indicated antibodies for 3 hours, at 4°C or overnight with an additional incubation for 2 hours after addition of protein A- or G-Sepharose beads (Amersham Pharmacia). Immunocomplexes captured on Sepharose beads were washed three times with RIPA buffer, eluted by being boiled with SDS gel-loading buffer. The immunoblot analysis was carried out as described previously [14].
Immunocytochemistry Analysis
COS-1 cells were used in immunocytochemistry analysis. Cells were rinsed with PBS, fixed in 3.5% paraformaldehyde in PBS for 10 minutes at room temperature, permeabilized with 0.1% triton X-100 for 20 minutes, and incubated with the primary antibodies in 3% BSA for 1 hour. After a brief washing, the cells were incubated with the secondary antibodies conjugated with FITC or TRITC, mounted in a mounting solution with DAPI, and observed with a fluorescence microscope. The FITC- or TRITC-conjugated secondary antibody was diluted to 1:200.
Results
Purification of SHP2 Complex
SHP2's functions in various signal pathways are still not clear. Therefore, we decide to try and identify additional SHP2 binding proteins. As reported previously we used FLAG-SHP2 expression vectors involving a trapping mutant of SHP2 for affinity purification of SHP2 complexes [3; 14]. FLAG-SHP2 protein complexes were eluted by the addition of a peptide consisting of the FLAG epitope (FLAG peptide) and resolved by SDS-PAGE (Fig. 1A). Increased levels of tyrosine phosphorylated proteins are present in the FSHP2DM transfected sample compared with the two other samples, suggesting that these bands represent SHP2 interacting proteins that are specifically trapped by this mutant construct (Fig 1A).
FIG. 1. Binding capacity of SHP2 with Annexin II after EGF treatment.
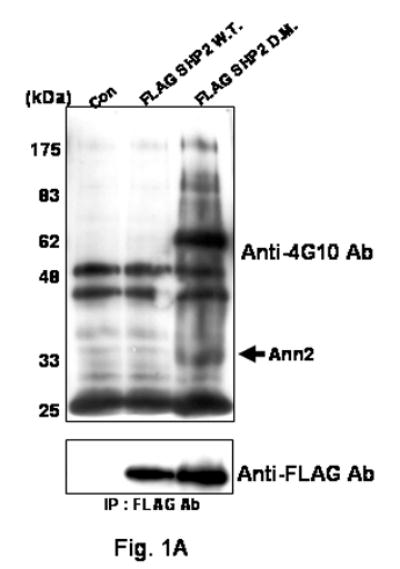
(A) COS-1 cells engineered to express FSHP2WT or FSHP2DM were incubated with EGF (100 ng/ml) for 10 minutes. Cleared cellular lysates were immunoprecipitated with anti-FLAG M2 agarose. Immunoprecipitates were washed, and FLAGSHP2 complexes were eluted with FLAG peptide. After acetone precipitation, FSHP2DM and associated proteins were resolved by SDS-PAGE on a 10% acrylamide gradient gel. The presence of bands was assessed by Western blotting with antibodies directed against 4G10 Ab. Bottom panel, expression of FSHP2WT or FSHP2DM was confirmed by western blotting using an antibody directed against the FLAG epitope.
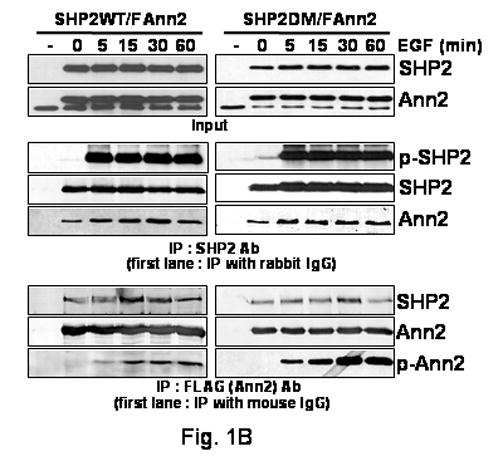
(B)COS-1 cells were treated for the indicated times with EGF (100 ng/ml). Western blot analysis was carried out with lysates of the COS-1 cells transfected with SHP2WT or SHP2DM with Annexin II constructs (Input). The binding level of SHP2 with Annexin II was assessed by co-IP. Cellular lysates were immune precipitated with SHP2 or FLAG antibodies and then SHP2, Annexin II, FLAG and 4G10 antibodies were used for western blot. As a control, cell lysates were immunoprecipitated with normal rabbit serum or an irrelevant anti- mouse monoclonal antibody, first lanes.
Identification of Annexin II in SHP2 Complexes
The band of ∼36kDa (p36) (Fig. 1A) in the FSHP2DM transfected sample was subjected to analysis. This band was excised from the gel and digested with trypsin. The resulting tryptic peptides were subjected to mass spectrometry analysis. SEQUEST was used to match MS with proteins in the Swissprot protein sequence database. Based upon these analyses, the p36 band contained Annexin II (Data not shown).
Annexin II Is Associated with SHP2
To confirm that the Annexin II protein could associate with SHP2, co-IP experiments were carried out. COS-1 cells that had been transfected transiently with the FSHP2WT or FSHP2DM were untreated or treated for 10 minutes with EGF. These cells were lysed with RIPA buffer and subjected to IP using anti-FLAG monoclonal antibody. Monoclonal antibodies against either SHP2 or Annexin II proteins were then used for detection of proteins present in the immunoprecipitates. Supplementary Figure shows that the monoclonal antibody against Annexin II detected co-immunoprecipitation of this protein with SHP2 protein. In addition to these transfection experiments, as shown below (Fig. 3A lane 1–3), endogenous SHP2 could also be demonstrated to bind with endogenous Annexin II in non-transfected COS-1 cells. These results indicate that SHP2 interacts with Annexin II in vivo.
FIG. 3. HSP70 expression and EGF treatment regulates binding of SHP2 with Annexin II in vivo.
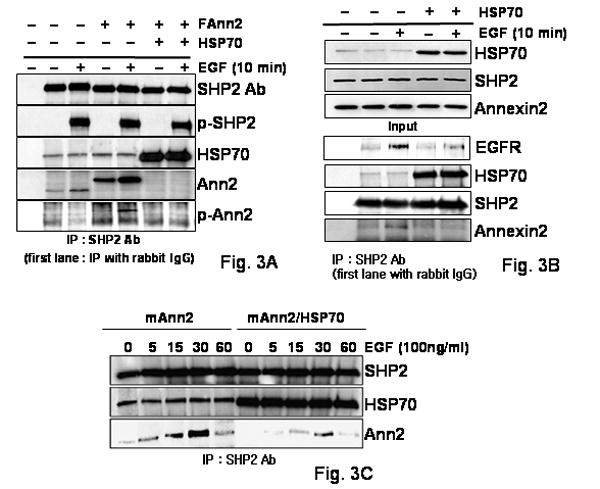
(A) The cell lysates were obtained from COS-1 cells that had been transfected transiently with the Annexin II and HSP70 constructs and then treated with EGF (100 ng/ml) or not for 10 min. The binding level of SHP2 with HSP70 or Annexin2 was assessed by IP with anti-SHP2 antisera. After IP, SHP2, HSP70, Annexin II and 4G10 antibodies were used for western blot. As a control, cell lysates were immunoprecipitated with normal rabbit serum, first lanes.
(B) The cell lysates were obtained from COS-1 cells that had been transfected transiently with the HSP70 construct or not in EGF (100ng/ml) treated or not for 10 min. The cell lysates were immunoblotted with either anti-HSP70, -Annexin II or -SHP2 antibodies. The binding level of SHP2 with EGFR, HSP70 or Annexin2 was assessed by IP with anti-SHP2 antibodies. After IP, anti-EGFR, -SHP2, -HSP70, and Annexin II antibodies were used for western blot. As a control, cell lysates only were immunoprecipitated with anti- rabbit polyclonal antibodies used at first lane.
(C) SHP2 was immunoprecipitated from COS-1 cells that were transfected with HSP70 or vector control, followed by EGF (100ng/ml) treatment for the indicated times. SHP2, HSP70, and Annexin II antibodies were used for western blot after IP with anti-SHP2 antisera.
We decided to determine if SHP2 binds with Annexin II in a time dependent manner following addition of EGF. We were concerned that the tagging of the SHP2 constructs may affect the results, so non-tagged SHP2 constructs were used for all future experiments. SHP2WT or SHP2DM with FLAG tagged Annexin II (FAnn2) were expressed in COS-1 cells and lysates from these cells were immunoprecipitated with SHP2 or FLAG antibodies after EGF treatment for the indicated times. SHP2 was rapidly tyrosine phosphorylated as soon as EGF treatment commences, (Fig. 1B, 5 min middle panel), Annexin II was only slowly phosphorylated on tyrosine and phosphorylation peaks around 30 minutes of EGF treatment and the binding of SHP2 with Annexin II had peaked by that time (Fig. 1B bottom panel). This may mean that the kinases using these proteins as substrates are different. Annexin II binding with SHP2WT only slightly increased after 5 min of EGF treatment and there was a similar small increase in SHP2DM binding with Annexin II after EGF treatment (Fig. 1B middle panel). These results indicate that Annexin II binds SHP2 weakly before EGF stimulation and this interaction is increased slightly on EGF treatment. The increase in tyrosine phosphorylation of Annexin II in cells expressing SHP2DM compared to WTSHP2 (Fig. 1B bottom panels) may indicate that Annexin II is a substrate of SHP2 in EGF signaling pathway.
Binding of Annexin II with SHP2 Is Changed after Extracellular Ca2+ Chelator Treatment
We wondered if the Annexin II and SHP2 interaction was regulated by Ca2+ because of reports that Annexin II function is regulated by Ca2+ concentration. So we employed calcium chelators to investigate the potential role of Ca2+ on binding of Annexin II with SHP2. COS-1 cells were treated with EGTA to reduce the availability of extracellular calcium or with BAPTA/AM to chelate intracellular calcium. SHP2WT/Annexin II expressed in COS-1 cells were treated with BAPTA/AM or EGTA for the indicated times after ∼12h serum starvation. Expression levels of SHP2, Annexin II, and Src proteins are essentially unchanged after treatment with these calcium chelators (Fig. 2). Cell lysates were immunoprecipitated with the SHP2 antiserum after EGF treatment for the indicated times (Fig. 2). These chelators did not effect the tyrosine phosphorylation of SHP2 induced by EGF treatment (Fig. 2). Chelating intracellular calcium had essentially no effect on the binding of SHP2 with Annexin II before or after EGF treatment (Fig. 2). In contrast, chelating extracellular calcium appeared to almost eliminate the increase in binding of SHP2 with Annexin II following EGF treatment (Fig. 2). This result indicates that the EGF dependent association of Annexin II with SHP2 is affected by extracellular calcium concentration.
FIG. 2. Binding of Annexin II with SHP2 was affected by extra cellular calcium chelator.
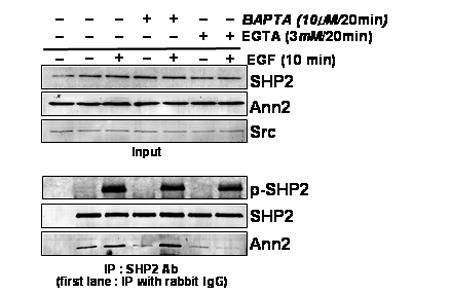
Western blot analysis was carried out with lysates of the COS-1 cells pre-treated with the indicated calcium chelators for 20 min, and then either untreated or treated with EGF (100 ng/ml) for 10 min. SHP2, Annexin II or Src antibodies were used in western blots to detect the levels of the specific proteins (Input). The cell lysates were immunoprecipitated with anti-SHP2 polyclonal antibodies, followed by western blot analysis using anti -SHP2, -Annexin II or -4G10 antibodies. As a control, cell lysates were immunoprecipitated with normal rabbit serum, first lanes in lower panel.
HSP70 Regulates SHP2 Binding with Annexin II
Previously we demonstrated that HSP70 expression levels influences SHP2 binding to EGFR and GAB1 [14] and in transfection experiments we found that increased HSP70 expression also affected SHP2 binding with Annexin II (data not shown). Therefore we decided to determine if HSP70 can also affect the binding of endogenous SHP2 with Annexin II, A comparison of non-transfected, FAnn2 only, or FAnn2 plus HSP70 transfected cell lysates were IP with anti-SHP2 antiserum either with or without EGF treatment (Fig. 3A). SHP2 levels are similar in all cases and endogenous SHP2 could be shown to bind to endogenous Annexin II (Fig. 3A lane 1–3) and more clearly to exogenous Flag-tagged Annexin II which appeared to compete out the endogenous binding (fig 3A, lanes 4–5). Interestingly, Annexin II binding to SHP2 was hardly detected in the cell lysates in which HSP70 was transfected (Fig. 3A).
To confirm HSP70 may affect binding of endogenous SHP2 with Annexin II, a comparison of non-transfected or HSP70 transfected cells with or without EGF treatment was performed using immune precipitation with anti-SHP antiserum (Fig 3B). Endogenous HSP70, Annexin II and SHP2 levels are similar in all samples (Fig 3B, upper panel). HSP70 protein binding with SHP2 depends on HSP70 level of expression. EGFR binding with endogenous SHP2 is decreased in HSP70 over-expressing cells (Fig 3B). Moreover, Annexin II binding with SHP2, which increased on EGF addition, almost disappeared in HSP70 transfected cell lysates (Fig 3B). These data indicate that the increased expression of HSP70 regulates SHP2 binding with Annexin II.
To determine if the effect of HSP70 varied over time following EGF treatment cells were immunoprecipitated with the anti-SHP2 antiserum after EGF treatment for the indicated times, (Fig 3C). There was almost no change in the binding of SHP2 with HSP70 after EGF treatment for the indicated times (Fig. 3C). In addition, as shown above, SHP2 binds with HSP70 even in cells not treated with EGF (Fig. 3C). In Annexin II only transfected cells, Annexin II binding with SHP2 increases slightly and then the binding decreased slightly after 30 minutes of EGF treatment. In Annexin II/HSP70 co-transfected cells, Annexin II binding with SHP2 is very low before or also after EGF treatment. These data indicate that HSP70 levels can regulate the interaction of SHP2 with Annexin II.
Annexin II Binds with SHP2 in the Cell Membrane after EGF Treatment
To confirm that the SHP2 and Annexin II proteins are binding in cells, and also to try and localize the region within the cell where they interact, COS-1 cells were co-immunostained with antibodies specific to the SHP2 protein and Annexin II with or without EGF treatment. Endogenous Annexin II and SHP2 are localized in the cytosol and the nucleus before EGF treatment (Fig 4, upper). Therefore because of the diffuse cytoplasmic nature of these signals it was not possible to determine if there was co-localization. However, when cells are treated with EGF, a sub-population of Annexin II clearly co-localized with SHP2 in the cell plasma membrane region (Fig 4, lower). This colocalization of SHP2 and Annexin II after EGF treatment remained when HSP70 was expressed (data not shown). It appears that at least a sub-population of these molecules are affected by EGF treatment in their intracellular colocalization
FIG. 4. Co-localization of the SHP2 with Annexin II protein.
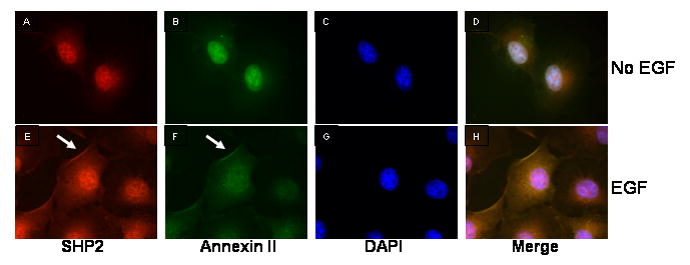
COS-1 cells were co-immunostained with antibodies against SHP2 (A and E) and Annexin II (B and F). The nucleus was stained with DAPI (C and G). Panel D and H are merged images. Cells in panels E-H were treated with EGF (100ng/ml) for 10 min.
Discussion
In the present study we have investigated the binding of SHP2 with Annexin II. Our findings demonstrate that SHP2 binds with Annexin II in vivo. Furthermore, we have shown that this binding was regulated to some extent by EGF treatment and extra cellular Ca2+ concentration. In addition, our results demonstrate a role for HSP70 levels in regulating the binding of SHP2 with Annexin II. These data are consistent with a previous report which indicated that Annexin II co-immunoprecipitates with SHP2 from membrane extracts in a cholesterol- and cell confluence- dependent manner [18].
Based on the immunofluorescence experiments it also appears that a subpopulation of Annexin II may go to the cell membrane after EGF treatment. This indicates that EGF can affect the localization of Annexin II and in doing so increase its ability to bind with SHP2 located in the membrane. This localized binding may regulate an Annexin II-dependent signaling pathway. Generally, Annexin II has been shown to be involved in the regulation of membrane organization (i.e. stabilization of lipid rafts), membrane-cytoskeleton linkage, membrane trafficking, and endocytosis [22]. These functions of Annexin II are related to its Ca2+-dependent binding of the protein to phospholipids [23]. Surprisingly the binding of SHP2 with Annexin II was influenced by the extra cellular Ca2+ concentration. This may indicate that SHP2 can regulate some of Annexin II's functions in trafficking, end-, exo-cytosis, and membrane-cytoskeleton linkage.
Previously, we reported that increased HSP70 levels effect the interaction of SHP2 with EGFR and Gab1 [14]. We report here that the binding of Annexin II with SHP2 was also affected by HSP70 levels. Whether the involvement of HSP70 in these interactions is related to a stress-response is unclear. Similarly the physiological meaning of SHP2's binding with Annexin II also needs to be elucidated. Nevertheless, this study demonstrates that Annexin II is a binding partner of SHP2, and that this binding can be regulated by extra cellular Ca2+ and HSP70.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (MJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SQ, Tsiaras WG, Araki T, Wen G, Minichiello L, Klein R, Neel BG. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol Cell Biol. 2002;22:4062–4072. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol. 2003;23:7875–7886. doi: 10.1128/MCB.23.21.7875-7886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gale NW, Kaplan S, Lowenstein EJ, Schlessinger J, Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- 5.Kuhne MR, Pawson T, Lienhard GE, Feng GS. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993;268:11479–11481. [PubMed] [Google Scholar]
- 6.Myers MG, Jr, Wang LM, Sun XJ, Zhang Y, Yenush L, Schlessinger J, Pierce JH, White MF. Role of IRS-1-GRB-2 complexes in insulin signaling. Mol Cell Biol. 1994;14:3577–3587. doi: 10.1128/mcb.14.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardelli A, Longati P, Gramaglia D, Stella MC, Comoglio PM. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15:3103–3111. doi: 10.1038/sj.onc.1201561. [DOI] [PubMed] [Google Scholar]
- 8.Carlberg K, Rohrschneider LR. Characterization of a novel tyrosine phosphorylated 100-kDa protein that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid cells. J Biol Chem. 1997;272:15943–15950. doi: 10.1074/jbc.272.25.15943. [DOI] [PubMed] [Google Scholar]
- 9.Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, Hirano T. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 11.Ong SH, Guy GR, Hadari YR, Laks S, Gotoh N, Schlessinger J, Lax I. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol Cell Biol. 2000;20:979–989. doi: 10.1128/mcb.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machida K, Matsuda S, Yamaki K, Senga T, Thant AA, Kurata H, Miyazaki K, Hayashi K, Okuda T, Kitamura T, Hayakawa T, Hamaguchi M. v-Src suppresses SHPS-1 expression via the Ras-MAP kinase pathway to promote the oncogenic growth of cells. Oncogene. 2000;19:1710–1718. doi: 10.1038/sj.onc.1203497. [DOI] [PubMed] [Google Scholar]
- 14.Yoo JC, Hayman MJ. HSP70 binds to SHP2 and has effects on the SHP2-related EGFR/GAB1 signaling pathway. Biochem Biophys Res Commun. 2006;351:979–985. doi: 10.1016/j.bbrc.2006.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Rothermund CA, Ayala-Sanmartin J, Vishwanatha JK. Nuclear annexin II negatively regulates growth of LNCaP cells and substitution of ser 11 and 25 to glu prevents nucleo-cytoplasmic shuttling of annexin II. BMC Biochem. 2003;4:10. doi: 10.1186/1471-2091-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberhard DA, Karns LR, VandenBerg SR, Creutz CE. Control of the nuclear-cytoplasmic partitioning of annexin II by a nuclear export signal and by p11 binding. J Cell Sci. 2001;114:3155–3166. doi: 10.1242/jcs.114.17.3155. [DOI] [PubMed] [Google Scholar]
- 18.Burkart A, Samii B, Corvera S, Shpetner HS. Regulation of the SHP-2 tyrosine phosphatase by a novel cholesterol- and cell confluence-dependent mechanism. J Biol Chem. 2003;278:18360–18367. doi: 10.1074/jbc.M210701200. [DOI] [PubMed] [Google Scholar]
- 19.Chetcuti A, Margan SH, Russell P, Mann S, Millar DS, Clark SJ, Rogers J, Handelsman DJ, Dong Q. Loss of annexin II heavy and light chains in prostate cancer and its precursors. Cancer Res. 2001;61:6331–6334. [PubMed] [Google Scholar]
- 20.Liu JW, Shen JJ, Tanzillo-Swarts A, Bhatia B, Maldonado CM, Person MD. Annexin II expression is reduced or lost in prostate cancer cells and its re-expression inhibits prostate cancer cell migration. Oncogene. 2003;22:1475–1485. doi: 10.1038/sj.onc.1206196. [DOI] [PubMed] [Google Scholar]
- 21.Park CY, Hayman MJ. The tyrosines in the bidentate motif of the env-sea oncoprotein are essential for cell transformation and are binding sites for Grb2 and the tyrosine phosphatase SHP-2. J Biol Chem. 1999;274:7583–7590. doi: 10.1074/jbc.274.11.7583. [DOI] [PubMed] [Google Scholar]
- 22.Hayes MJ, Rescher U, Gerke V, Moss SE. Annexin-actin interactions. Traffic. 2004;5:571–576. doi: 10.1111/j.1600-0854.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 23.Gerke V, Moss SE. Annexins and membrane dynamics. Biochim Biophys Acta. 1997;1357:129–154. doi: 10.1016/s0167-4889(97)00038-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


