Abstract
Infiltration by dendritic cells (DCs) is a common feature of most human tumors. Prior studies evaluating the interaction of DCs with tumors have focused largely on their immunologic properties (for review see Banchereau, J., and R.M. Steinman. 1998. Nature. 392:245–252). In this study, we show that the clonogenicity of several human tumor cell lines and primary tumor cells from myeloma patients is enhanced by their interactions with DCs. Myeloma cells cultured in the presence of DCs have an altered phenotype with an increased proportion of cells lacking terminal plasma cell differentiation marker CD138. DC–tumor interaction also leads to the up-regulation of B cell lymphoma 6 expression in myeloma cells. Effects of DCs on myeloma cells are inhibited by blockade of the receptor activator of NF-kB (RANK)–RANK ligand and B cell–activating factor–APRIL (a proliferation-inducing ligand)-mediated interactions. Together, these data suggest that tumor–DC interactions may directly impact the biology of human tumors, particularly multiple myeloma, and may be a target for therapeutic intervention.
DCs have been studied extensively in the context of immunity to tumors (for reviews see references 1, 2). Several studies have documented the increased infiltration of human tumors by DCs and often correlated this with adverse prognosis (3–5). This has generally been interpreted in terms of the potential ability of tumor-infiltrating DCs to induce immune tolerance. However, whether DCs interact more directly with human tumors/tumor progenitor cells or alter their growth or differentiation has not been described.
Multiple myeloma (MM) is a B cell tumor characterized by the clonal expansion of malignant plasma cells in the bone marrow (6). A typical feature of MM, which is responsible for the term “multiple” myeloma, is the multifocal growth of tumors in the bone marrow, illustrating the potential importance of specific niches in the marrow. The tumor microenvironment consists of several distinct elements, including immune effector cells, myeloid cells, bone cells, and fibroblasts. However, most studies of the tumor microenvironment in myeloma have used ill-defined stromal elements (6, 7). Therefore, the relative contribution of distinct elements of the microenvironment on the biology of tumor cells remains to be clarified.
Some studies have suggested a role for tumor-associated macrophages in regulating the growth of tumors (5, 8). However DCs are biologically distinct from monocytes or macrophages and have unique functional properties (for review see reference 1). Prior studies have shown that DCs play an important role in normal B and plasma cell differentiation and survival (9–11). Therefore, we hypothesized that DCs may also directly impact the growth and differentiation of myeloma tumor cells.
RESULTS AND DISCUSSION
DCs enhance the clonogenicity of tumor cell lines in vitro
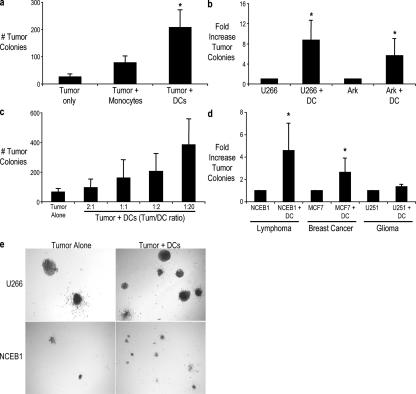
To test whether human DCs could alter the clonogenic growth of human myeloma cell lines, we plated tumor cells alone or with purified monocytes or monocyte-derived DCs in methylcellulose cultures. Plating tumor cells alone in this assay results in the growth of discrete tumor colonies with an efficiency of ∼1% of cells plated. The addition of DCs to these cultures led to a greater number of tumor colonies in a dose-dependent manner compared with tumor cells alone or cocultures with monocytes (Fig. 1, a–c). This growth-promoting effect of DCs on human tumors is not restricted to myeloma, as the clonogenic growth of two other tumors tested (lymphoma and breast cancer) was also enhanced (Fig. 1 d). In contrast, DCs had only a minor impact on the growth of glial tumors. The enhanced number of tumor colonies was mostly evident as an increased number rather than size of individual colonies, suggesting an effect on cloning efficiency or survival (Fig. 1 e). Therefore, interactions of tumor cells and DCs can directly promote the clonogenicity of several but not all human tumors.
Figure 1.
Enhancement of the clonogenicity of human tumor cells by Mo-DCs. (a) Comparison of DCs versus monocytes. U266 myeloma tumor cells were plated with and without purified CD14+ monocytes or Mo-DCs in a clonogenic assay at a ratio of 1:2. (b) Myeloma cell lines (U266 and ARK) were plated with or without DCs at a ratio of 1:2 in a clonogenic assay. (a and b) The numbers of colonies were enumerated microscopically after 3 wk of culture. (c) U266 cells were plated with Mo-DCs at an increasing ratio of DC/tumor in a clonogenic assay. The numbers of tumor colonies were enumerated microscopically after an incubation of 3 wk. (a–c) Results are representative of three separate experiments. (d) Mantle cell lymphoma (NCEB1), breast cancer (MCF-7), or glioma (U251) cells were cultured alone or with DCs in a clonogenic assay. The numbers of tumor colonies were enumerated microscopically after 3 wk of culture. Results are the mean ± SD (error bars) of the aggregate of three separate experiments. *, P < 0.05. (e) Appearance of colonies from a U266 myeloma cell line (top) or NCEB1 mantle cell lymphoma cell line (bottom) in the presence or absence of DCs. Micrographs show the appearance of colonies in methylcellulose gels at low power.
Coculture of myeloma cell lines with DCs leads to an increase in the CD138− subpopulation of tumor cells
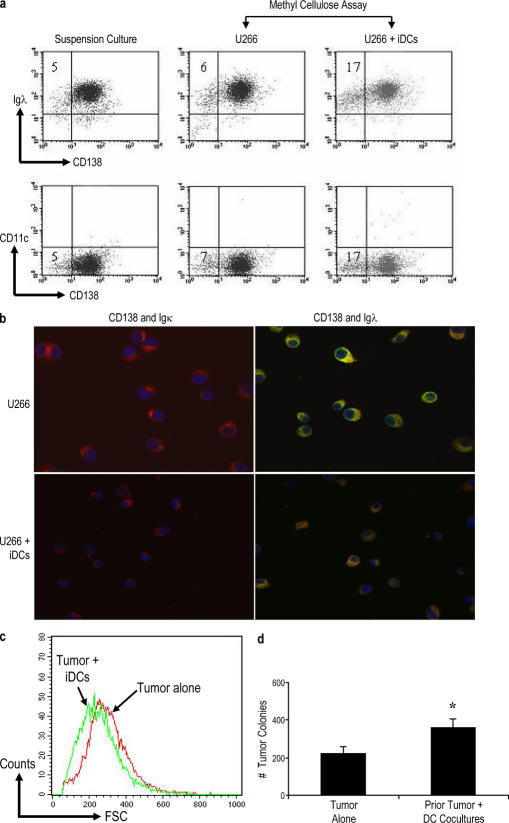
The phenotype of tumor colonies in the clonogenic assay was monitored by flow cytometric detection of CD138 and CD11c, and the presence of myeloma cells was identified by the presence of cells expressing the appropriate cytoplasmic Ig light chain (λ light chain in the case of U266 cells; Fig. 2 a). Flow cytometry data were also confirmed by immunofluorescence microscopy (Fig. 2 b). The majority of the tumor cells in these cultures had a typical plasma cell phenotype with the expression of CD138 and light chain restriction. However, the culture of tumor cells in the presence of DCs led to a mild but consistent increase in the proportion of cells lacking CD138, a marker of terminally differentiated plasma cells (Fig. 2 a). Upon immunofluorescence microscopy, tumor cells grown in the presence of DCs were somewhat smaller in size with less cytoplasm compared with clonogenic cultures of tumor cells grown alone (Fig. 2 b). This is also evident as lower forward scatter of these tumors on flow cytometry (Fig. 2 c). Therefore, DC-mediated enhancement of myeloma clonogenicity is associated with an altered phenotype of tumors. A prior study has suggested that the CD138− subpopulation of MM cell lines is enriched for the clonogenic growth in serial replating assays (12). Thus, we tested whether this altered phenotype was associated with enhanced clonogenicity in serial replating assays. Cells initially cultured with DCs had higher cloning efficiency in replating assays, suggesting that the observed alteration in phenotype is associated with the enhancement of clonogenicity (Fig. 2 d).
Figure 2.
Phenotypic evaluation of tumor colonies of clonogenic assays. (a) Tumor colonies from clonogenic assays were harvested and stained with various antibodies for flow cytometric evaluation. U266 cells grown under different conditions (suspension culture, U266 alone, and with Mo-DCs in clonogenic assays) were analyzed after a 3-wk culture for the expression of cell surface CD138, CD11c, and intracellular Ig light chain. Data shown are gated for the live population. Numbers represent percentages of CD138−Igλ+ cells. Note that CD11c+ DCs are no longer evident at this time point. (b) Phenotype of CD138+ tumor cells in clonogenic assays. Cytospins of tumor cells from cultures of tumor cells alone, or tumor–DC cocultures were stained with anti–CD138-PE and Igλ/Igκ-FITC-AlexaFluor488 and analyzed by immunofluorescence microscopy. Red, CD138; green, Ig light chain (κ or λ); blue, DAPI nuclear stain. U266 cells are λ light chain restricted. (c) Histogram of forward scatter (FSC) on flow cytometric evaluation to analyze the size of tumor cells cultured as tumor cells alone or with DCs. (a–c) Results are representative of three separate experiments. (d) Enhanced clonogenicity of cells from tumor–DC cocultures. Tumor cells were harvested from the clonogenic assays of U266 cells originally plated with and without DCs (as in Fig. 1 b) and were serially replated without additional fresh DCs. The numbers of colonies were enumerated microscopically after a further incubation of 3 wk. Results are representative of two separate experiments. Error bars represent SD. iDC, immature DC. *, P < 0.05.
Mechanism of DC-mediated enhancement of clonogenicity
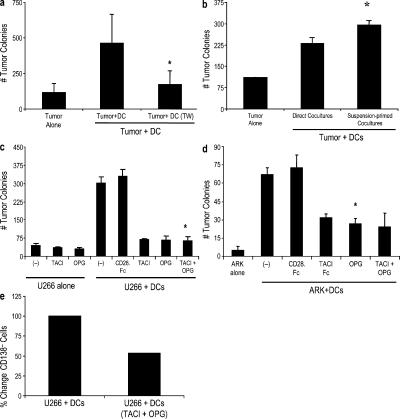
DC-mediated enhancement of tumor clonogenicity in this system required short-range interactions between tumor cells and DCs, as it was not evident when the two cell populations were separated in a Transwell (Fig. 3 a). To optimize cell–cell contact, we also evaluated the initial coculture of DCs and tumor cells in suspension culture for 24 h before plating them in methylcellulose. Adding this step led to a further increase in tumor colonies compared with direct cocultures, supporting the need for cellular proximity (Fig. 3 b). DCs express several molecules that are implicated in B cell differentiation as well as costimulatory molecules and integrins that may be important for the observed effects (11). We focused on two of these pathways involving TNF superfamily members. Both B cell–activating factor (BAFF)–APRIL (a proliferation-inducing ligand; references 13–15) and receptor activator of NF-kB (RANK)–RANK ligand (RANK-L) pathways (16) have been previously implicated in the survival of myeloma cells. Blockade of BAFF–APRIL-mediated interactions with TACI-Fc (transmembrane activator calcium modulator and cyclophilin ligand interactor-Fc chimera) or blockade of RANK–RANK-L–mediated interactions with osteoprotegerin (OPG; reference 17) led to the inhibition of DC-mediated enhancement of tumor clonogenicity in both myeloma cell lines tested (Fig. 3, c and d). This was also associated with less enrichment of the CD138− subpopulation in these cocultures (Fig. 3 e). TACI-Fc or OPG did not alter the clonogenicity of tumor cells alone. We were unable to demonstrate a synergy between these ligands under the conditions tested. Therefore, DC-mediated enhancement of myeloma clonogenicity is mediated, in part, by RANK-L and BAFF–APRIL-mediated interactions.
Figure 3.
Mechanism of DC-mediated enhancement of the clonogenic growth of tumor cells. (a) Requirement for cell–cell contact. U266 cells were plated in the clonogenic assay with or without DCs as in Fig. 1. DCs were either plated along with U266 cells (DC/tumor ratio of 2:1) or separated from U266 cells by a Transwell insert. Data are representative of three similar experiments. (b) The myeloma cell line (U266) was cocultured with Mo-DCs at a ratio of 1:2 overnight in suspension culture in a 96-well plate (suspension primed) before plating in a clonogenic assay and was compared with cell lines plated directly with and without Mo-DCs as in Fig. 1 b. Results are representative of two separate experiments. (a and b) The numbers of colonies were enumerated after 3 wk. (c and d) Tumor cells (U266 and ARK) and Mo-DCs were cultured in the presence of 1 μg/ml TACI-Fc,0.5 μg/ml osteoprotegerin (OPG), or 1 μg/ml CD28-Fc chimera as a control. The numbers of colonies for U266 (c) and ARK (d) were enumerated microscopically after an incubation of 3 wk. Data are representative of three similar experiments. (e) Tumor cells were harvested from clonogenic assays of U266 cells cocultured with DCs in the presence or absence of 1 μg/ml TACI-Fc and 0.5 μg/ml OPG at the end of 3 wk of incubation and were characterized using flow cytometry. Cells were stained for cell surface CD138 (PE) and intracellular Ig light chain (FITC). Data shown are gated for the live population and represents the percent decrease in CD138− cells in cocultured tumor cells in the presence of TACI and OPG compared with the control cultures without TACI/OPG. Error bars represent SD. *, P < 0.05.
DC-mediated changes in tumor cells
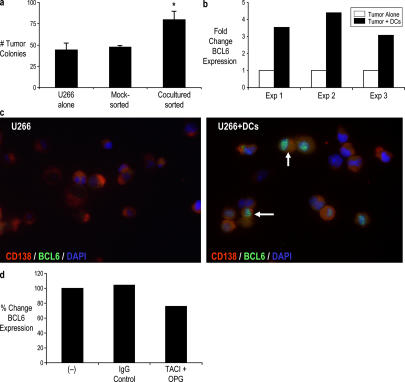
To gain insights into the early events during tumor–DC interaction, we cocultured DCs and tumor cells for 24 h before separating tumor cells by FACS sorting to >99% purity and plated them in clonogenic assays without DCs. Tumor cells from these short cocultures demonstrated enhanced clonogenic growth compared with mock-sorted tumor cells cultured alone under similar conditions (Fig. 4 a). This suggested that even short-term interactions between tumor cells and DCs can alter the behavior of tumor cells. Pilot microarray experiments suggested B cell lymphoma 6 (BCL6) as one of the major genes consistently up-regulated in tumor cells purified from these cocultures (unpublished data). A prior study has suggested an important role for BCL6 in survival and self-renewal of germinal center B cells (18). Thus, we analyzed the expression of BCL6 in these tumor cells by real-time RT-PCR (TaqMan) to confirm these results. Coculture of tumor cells with DCs was associated with an induction of BCL6 messenger RNA (mRNA) in sorted tumor cells compared with tumors cultured alone (Fig. 4 b). This was also confirmed at the protein level by immunofluorescence microscopy. U266 tumor cells cultured alone do not express BCL6; however, clear intranuclear staining for BCL6 was observed in tumor cells cocultured with DCs (Fig. 4 c). As TACI-Fc and OPG inhibited the DC-mediated enhancement of tumor clonogenicity, we also tested their effect on BCL6 up-regulation. The addition of TACI and OPG led to the modest but detectable inhibition of DC-mediated BCL6 mRNA up-regulation in tumor cells (Fig. 4 d). Therefore, the short-term coculture of DCs and tumor cells is associated with an up-regulation of BCL6 on tumor cells.
Figure 4.
Effect of DCs on tumor cells during tumor–DC cocultures. (a) Tumor cells (U266) were cocultured with Mo-DCs overnight at a ratio of 1:2 in suspension culture in a 96-well plate before sorting CD138+ tumor cells by flow cytometry. U266 cells cultured alone were mock sorted as a control. Sorted tumor cells were plated in clonogenic assays and were compared with untreated U266 cells. The numbers of colonies were enumerated after 3 wk. Error bars represent SD. *, P < 0.05. (b) U266 tumor cells were cultured alone or with pure populations of DCs at a tumor/DC ratio of 1:2. After 24 h, CD138+ tumor cells were sorted by flow cytometry to >99% purity. The expression of BCL6 mRNA in purified tumor cells was analyzed by real-time RT-PCR (TaqMan) and normalized to the expression of the housekeeping gene GAPDH. (c) Immunofluorescence analysis of BCL6 protein in U266 tumor cells cultured alone or with DCs. Acetone-fixed cells on poly-lysine–coated slides were stained with anti-BCL6 mAb followed by AlexaFluor488 and CD138 (PE). DAPI was used as a nuclear stain. Note the intranuclear staining for BCL6 in tumor cells from tumor–DC cocultures. Arrows indicate cells expressing intranuclear BCL6. (d) U266 tumor cells were cultured alone or with pure populations of DCs at a tumor/DC ratio of 1:2 in the presence or absence of 1 μg/ml TACI and 0.5 μg/ml OPG. Cells cultured in the presence of IgG1 were used as controls. After 24 h, CD138+ tumor cells were sorted by flow cytometry to >99% purity. The expression of BCL6 mRNA in purified tumor cells was analyzed by real-time RT-PCR (TaqMan) and normalized to the expression of the housekeeping gene GAPDH. The effect of TACI + OPG on DC-induced BCL6 expression in tumor cells was analyzed as the percent change compared with control cocultures.
DCs enhance the clonogenic growth of primary myeloma cells
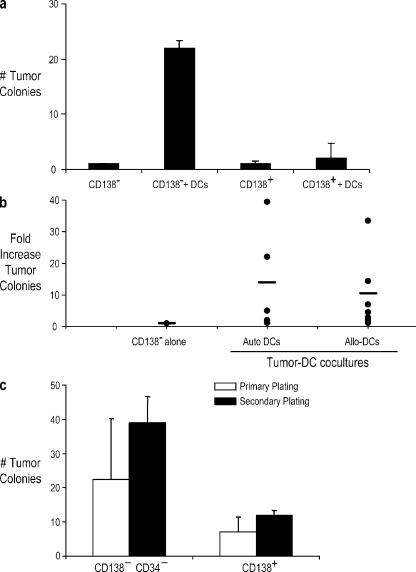
To extend these observations on cell lines to primary cells from patients, we obtained bone marrow samples from patients with myeloma and preneoplastic gammopathy (MGUS). A prior study has shown that clonogenic growth of tumor cells is enriched in CD34−CD138− subpopulations (12). Bone marrow mononuclear cells (MNCs) from myeloma (n = 9) or MGUS (n = 3) patients were separated into CD138+ and CD34−CD138− subpopulations and cultured in the presence or absence of autologous or allogeneic DCs in clonogenic assays. The addition of DCs to these subpopulations led to a more than twofold enhancement of tumor colonies from the CD34−CD138− fraction in 4/5 patients (two MGUS and three MM) using autologous DCs and 7/11 patients (two MGUS and nine MM) using allogeneic DCs (Fig. 5, a and b). There were no major differences between MGUS and myeloma samples. To further assess their clonogenicity, tumor cells harvested from some of these assays were replated in fresh assays. Tumor cells could be successfully passaged in serial assays from both subpopulations (Fig. 5 c). Importantly, coculture with DCs allowed clonogenic growth even from the purified CD138+ subpopulation from myeloma patients, which normally does not grow well in vitro by itself (12). Therefore, DCs lead to the enhanced clonogenic growth of primary tumor cells from myeloma patients, and this coculture system may be a useful model system for the growth of primary myeloma cells.
Figure 5.
Enhancement of the clonogenicity of bone marrow–derived CD138+ and CD138− cells from myeloma and MGUS patients by Mo-DCs. (a and b) CD138+ and CD138−CD34− cells harvested from bone marrow MNCs were plated with and without autologous/allogeneic Mo-DCs at a ratio of 1:2 in the clonogenic assays. (a) Representative data from an MGUS patient with autologous DCs. (b) Summary of data from 12 patients showing the fold increase in tumor colonies with autologous or allogeneic DCs compared with CD138−CD34− cells alone. (c) Effect of serial replating. Colonies were harvested from clonogenic assays as in panel a and were replated with fresh Mo-DCs at a ratio of 1:2. Data shown are representative of experiments of two myeloma patients. (a–c) The numbers of colonies were enumerated microscopically after an incubation of 3 wk. Error bars represent SD.
Most human tumors recruit diverse immune cells, including DCs, to the tumor bed. However, infiltration of human tumors by DCs has previously been interpreted largely in the context of their immunologic functions (4, 5). Prior studies have shown that both myeloma tumors in patients and mouse plasmacytomas are extensively infiltrated by DCs (19, 20), accounting for up to 10% of all nucleated cells within these lesions. Our data suggest the possibility that tumor-infiltrating DCs may provide a niche to support the clonogenic growth of human myeloma without the need to invoke a viral infection (20). DC-mediated enhancement of tumor clonogenicity may also involve other tumor types, such as lymphoma and breast cancer. Interestingly, a recent study of gene expression profiles of lymphoma has shown that the presence of DC signature in lymphoma portends an adverse outcome, which is consistent with these results (21). However, the involved mechanisms, which are only studied for myeloma here, may differ between different tumor types.
In our studies, blockade of the RANK–RANK-L pathway by OPG or blockade of BAFF–APRIL-mediated interactions via TACI-Fc led to the inhibition of DC-mediated enhancement of the clonogenicity of human myeloma. These data are consistent with prior studies showing the importance of both of these pathways in myeloma biology and point to DCs as an important source of these ligands (11, 13, 15). However, our data does not exclude the possibility that other molecules such as integrins or costimulatory molecules commonly expressed on DCs may also be important in DC–myeloma interactions. Additional mechanisms of the DC-mediated regulation of myeloma growth may include the potential contribution of tumor-associated DCs as precursors to new blood vessels (22) or osteoclasts (23). Indeed, a recent study has shown that osteoclasts can also support the growth of myeloma cells in vitro (24).
The culture of U266 cells with DCs led to an increased proportion of Igλ+ cells lacking CD138, a marker of terminal plasma cell differentiation, as well as induction of BCL6 expression in tumor cells. Suppression of BCL6 is a critical feature of normal plasma cell differentiation. These data are reminiscent of the findings of a previous study that observed the reactivation of the B cell program after exogenous expression of BCL6 in myeloma cell lines (25). Together, these data suggest that the differentiation state of myeloma cells is plastic and can be modified by cues provided by DCs in the tumor bed.
To our knowledge, these data provide the first evidence that DCs can directly impact the clonogenic growth of human tumors. Therefore, the recruitment of DCs into tumors may impact not just the host immune response but also the biology of the tumor itself. In a prior study, we have shown that the effector function of tumor-infiltrating T cells correlates with favorable clinical features in MGUS versus myeloma (26). Thus, the immune system may be a two-edged sword, with distinct components capable of both supporting and inhibiting tumor growth. Identification of tumor-infiltrating DCs as potential contributors to tumor progression also provides the rationale for specifically targeting this interaction as a novel approach for the therapy of human cancer.
MATERIALS AND METHODS
Tumor cell lines and patient samples.
The human MM cell lines ARK (gift from J. Epstein, University of Arkansas, Little Rock, AR) and U266 (American Type Tissue Culture) were cultured in complete medium consisting of RPMI 1640 (Cellgro), 2 mM l-glutamine, 20 μg/ml gentamicin sulfate, and 10% FBS. Other tumor cell lines used were NCEB1 (mantle cell lymphoma; gift of O. O'Connor, Memorial Sloan Kettering Cancer Center [MSKCC], New York, NY), MCF-7 (breast cancer; gift of P. Livingston, MSKCC), and U251 (glioma; gift of R. Puri, Food and Drug Administration, Bethesda, MD). Bone marrow and blood specimens from patients with myeloma and MGUS were obtained after informed consent that was approved by The Rockefeller University Institutional Review Board (IRB).
Generation of DCs.
Peripheral blood samples were obtained from healthy donors after informed consent as approved by The Rockefeller University IRB or were purchased from the New York Blood Center. PBMCs were isolated by density gradient centrifugation (Ficoll-Paque Plus; GE Healthcare). DCs were generated from purified blood monocytes as described previously (27). In brief, monocytes isolated using CD14 microbeads (Miltenyi Biotec) were cultured in the presence of 20 ng/ml GM-CSF (Immunex) and 10 ng/ml IL-4 (R&D Systems). DCs were used on days 5–6 of culture.
Clonogenic assays.
Clonogenic growth of tumor cell lines was evaluated by plating tumor cells (50,000 cells/ml) in methylcellulose containing 5% leukocyte-conditioned media (Methocult; Stem Cell Technologies, Inc.) using a protocol modified from a previous study (12). Cells were plated in 35-mm2 tissue culture dishes in quadruplicates and incubated at 37°C and 5% CO2. Colonies consisting of >40 cells were counted by microscopy 2–3 wk after plating.
DC–myeloma interactions.
To assess the impact of monocytes/DCs on tumor clonogenicity, tumor cells were mixed with purified CD14+ monocytes or monocyte-derived DCs (Mo-DCs) at varying ratios before plating in Methocult. Tumor growth was monitored weekly. For cell contact–dependent assays, DCs were suspended in 2% IMDM and were separated from U266 cells by a Transwell insert. Control inserts had 2% IMDM only. For some experiments, tumor cells and DCs were cultured in the presence or absence of either 1 μg/ml TACI-Fc (R&D Systems) or 0.5 μg/ml OPG (R&D Systems). CD28-Fc protein (R&D Systems) was used as a control.
Clonogenic assays on primary tumor cells.
Bone marrow MNCs were isolated from marrow samples using density gradient centrifugation. For clonogenic assays on primary tumor cells from patients, CD138+ and CD138− fractions were isolated from bone marrow MNCs using CD138 microbeads (Miltenyi Biotec) and AutoMACS (Miltenyi Biotec). The CD138− fraction was further depleted of normal hematopoietic progenitors using CD34 microbeads (Miltenyi Biotec). The resulting fractions, CD138+, and CD138−CD34− cells (5 × 105/ml) were plated with or without Mo-DCs at a ratio of 1:2 in Methocult as described above for cell lines. Tumor colonies were scored at 2 wk of culture. The phenotype of tumor cells was confirmed by immunofluorescence microscopy. For replating assays, cells were harvested from the clonogenic assays, washed, and replated at original cell concentration with or without DCs at a tumor/DC ratio of 1:2. Colonies were scored after 2 wk of culture.
Flow cytometric evaluation of tumor colonies in clonogenic assays.
Tumor colonies harvested from clonogenic assays were analyzed for the cell surface expression of CD138-PE, CD11c-APC, and intracellular κ or λ light chain–FITC (BD Biosciences) by flow cytometry.
Immunofluorescence microscopy.
Cytospins were made on the poly-lysine–coated (Sigma-Aldrich) multiwell slides (Carlson Scientific). Cells were fixed with acetone and stained with primary and secondary antibodies for 30 min at room temperature. Primary antibodies CD138 (PE), Igλ, and Igκ (BD Biosciences) and the secondary antibody AlexaFluor488 goat anti–mouse IgG1 (Invitrogen) were used at 1:30 and 1:200 dilutions, respectively. Acetone-fixed cytospins of tumor cells were also stained for BCL6 mAb (clone P1F6+PG-B6p; Lab Vision Corp.) followed by AlexaFluor488 goat anti–mouse IgG1 (Invitrogen) and CD138 (PE) using the protocol described previously with few modifications (28). Slides were evaluated using an epifluorescence microscope (AX70; Olympus) with a motorized stage to allow 0.5-mm optical sections imaged with a cooled CCD camera (C4742-95; Hamamatsu) and analyzed with MetaMorph software (Universal Imaging Corp.).
Evaluation of BCL6 expression in tumor cells by TaqMan.
RNA was extracted from cells by using the RNeasy Mini Kit (QIAGEN). BCL6 expression was quantified by using Assays-on-Demand primer probes from Applied Biosystems. RT-PCR was performed by using EZ PCR Core Reagents (Applied Biosystems) according to the manufacturer's instructions. A BCL6-expressing plasmid (provided by K. Calame, Columbia University, New York, NY) was used as a positive control. The samples were amplified and quantified on a sequence detection system (PRISM 7700; Applied Biosystems) by using the following thermal cycler conditions: 2 min at 50°C, 30 min at 60°C, 5 min at 95°C, and 40 cycles of 15 s at 95°C followed by 60 s at 60°C. GAPDH, a housekeeping gene, was used to normalize each sample. The data were analyzed, and samples were quantified by the software provided with the Applied Biosystems PRISM 7700.
Statistical analysis.
Data from different experimental groups were compared using the Students' t test, and significance was set at P < 0.05.
Acknowledgments
We thank Drs. Ralph M. Steinman and Kathryn Calame for thoughtful critique and advice regarding this work, Judy Adams for help with figures, Joel Sandler for help with microscopy, Arlene Hurley for help with clinical aspects, and members of the Dhodapkar laboratory for many helpful discussions.
This work is supported, in part, by funds from the National Institutes of Health (grants CA106802, CA109465, and MO1-RR00102), Damon Runyon Cancer Research Fund, Irene Diamond Foundation, Dana Foundation, and Irma T. Hirschl Foundation.
The authors have no conflicting financial interests.
References
- 1.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 2.Steinman, R.M., and M. Dhodapkar. 2001. Active immunization against cancer with dendritic cells: the near future. Int. J. Cancer. 94:459–473. [DOI] [PubMed] [Google Scholar]
- 3.Sandel, M.H., A.R. Dadabayev, A.G. Menon, H. Morreau, C.J. Melief, R. Offringa, S.H. van der Burg, C.M. Janssen-van Rhijn, N.G. Ensink, R.A. Tollenaar, et al. 2005. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin. Cancer Res. 11:2576–2582. [DOI] [PubMed] [Google Scholar]
- 4.Bell, D., P. Chomarat, D. Broyles, G. Netto, G.M. Harb, S. Lebecque, J. Valladeau, J. Davoust, K.A. Palucka, and J. Banchereau. 1999. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med. 190:1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin, E.Y., and J.W. Pollard. 2004. Role of infiltrated leucocytes in tumour growth and spread. Br. J. Cancer. 90:2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehl, W.M., and P.L. Bergsagel. 2002. Multiple myeloma: evolving genetic events and host interactions. Nat. Rev. Cancer. 2:175–187. [DOI] [PubMed] [Google Scholar]
- 7.Caligaris-Cappio, F., L. Bergui, M.G. Gregoretti, G. Gaidano, M. Gaboli, M. Schena, A.Z. Zallone, and P.C. Marchisio. 1991. Role of bone marrow stromal cells in the growth of human multiple myeloma. Blood. 77:2688–2693. [PubMed] [Google Scholar]
- 8.Pollard, J.W. 2004. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer. 4:71–78. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, B., B. Vanbervliet, J. Fayette, C. Massacrier, C. Van Kooten, F. Briere, J. Banchereau, and C. Caux. 1997. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 185:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia De Vinuesa, C., A. Gulbranson-Judge, M. Khan, P. O'Leary, M. Cascalho, M. Wabl, G.G. Klaus, M.J. Owen, and I.C. MacLennan. 1999. Dendritic cells associated with plasmablast survival. Eur. J. Immunol. 29:3712–3721. [DOI] [PubMed] [Google Scholar]
- 11.MacLennan, I., and C. Vinuesa. 2002. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 17:235–238. [DOI] [PubMed] [Google Scholar]
- 12.Matsui, W., C.A. Huff, Q. Wang, M.T. Malehorn, J. Barber, Y. Tanhehco, B.D. Smith, C.I. Civin, and R.J. Jones. 2004. Characterization of clonogenic multiple myeloma cells. Blood. 103:2332–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novak, A.J., J.R. Darce, B.K. Arendt, B. Harder, K. Henderson, W. Kindsvogel, J.A. Gross, P.R. Greipp, and D.F. Jelinek. 2004. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 103:689–694. [DOI] [PubMed] [Google Scholar]
- 14.Moreaux, J., F.W. Cremer, T. Reme, M. Raab, K. Mahtouk, P. Kaukel, V. Pantesco, J. De Vos, E. Jourdan, A. Jauch, et al. 2005. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood. 106:1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreaux, J., E. Legouffe, E. Jourdan, P. Quittet, T. Reme, C. Lugagne, P. Moine, J.F. Rossi, B. Klein, and K. Tarte. 2004. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 103:3148–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearse, R.N., E.M. Sordillo, S. Yaccoby, B.R. Wong, D.F. Liau, N. Colman, J. Michaeli, J. Epstein, and Y. Choi. 2001. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc. Natl. Acad. Sci. USA. 98:11581–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliani, N., R. Bataille, C. Mancini, M. Lazzaretti, and S. Barille. 2001. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 98:3527–3533. [DOI] [PubMed] [Google Scholar]
- 18.Scheeren, F.A., M. Naspetti, S. Diehl, R. Schotte, M. Nagasawa, E. Wijnands, R. Gimeno, F.A. Vyth-Dreese, B. Blom, and H. Spits. 2005. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat. Immunol. 6:303–313. [DOI] [PubMed] [Google Scholar]
- 19.Dembic, Z., K. Schenck, and B. Bogen. 2000. Dendritic cells purified from myeloma are primed with tumor-specific antigen (idiotype) and activate CD4+ T cells. Proc. Natl. Acad. Sci. USA. 97:2697–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said, J.W., M.R. Rettig, K. Heppner, R.A. Vescio, G. Schiller, H.J. Ma, D. Belson, A. Savage, I.P. Shintaku, H.P. Koeffler, et al. 1997. Localization of Kaposi's sarcoma-associated herpesvirus in bone marrow biopsy samples from patients with multiple myeloma. Blood. 90:4278–4282. [PubMed] [Google Scholar]
- 21.Staudt, L.M., and S. Dave. 2005. The biology of human lymphoid malignancies revealed by gene expression profiling. Adv. Immunol. 87:163–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coukos, G., F. Benencia, R.J. Buckanovich, and J.R. Conejo-Garcia. 2005. The role of dendritic cell precursors in tumour vasculogenesis. Br. J. Cancer. 92:1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrlich, L.A., and G.D. Roodman. 2005. The role of immune cells and inflammatory cytokines in Paget's disease and multiple myeloma. Immunol. Rev. 208:252–266. [DOI] [PubMed] [Google Scholar]
- 24.Yaccoby, S., M.J. Wezeman, A. Henderson, M. Cottler-Fox, Q. Yi, B. Barlogie, and J. Epstein. 2004. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 64:2016–2023. [DOI] [PubMed] [Google Scholar]
- 25.Fujita, N., D.L. Jaye, C. Geigerman, A. Akyildiz, M.R. Mooney, J.M. Boss, and P.A. Wade. 2004. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell. 119:75–86. [DOI] [PubMed] [Google Scholar]
- 26.Dhodapkar, M.V., J. Krasovsky, K. Osman, and M.D. Geller. 2003. Vigorous premalignancy specific effector T cell response in the bone marrow of patients with preneoplastic gammopathy. J. Exp. Med. 198:1753–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhodapkar, K.M., J.L. Kaufman, M. Ehlers, D.K. Banerjee, E. Bonvini, S. Koenig, R.M. Steinman, J.V. Ravetch, and M.V. Dhodapkar. 2005. Selective blockade of inhibitory Fc{gamma} receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc. Natl. Acad. Sci. USA. 102:2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cattoretti, G., L. Pasqualucci, G. Ballon, W. Tam, S.V. Nandula, Q. Shen, T. Mo, V.V. Murty, and R. Dalla-Favera. 2005. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 7:445–455. [DOI] [PubMed] [Google Scholar]