Abstract
The regulation of transporters by nutrient-responsive signaling pathways allows cells to tailor nutrient uptake to environmental conditions. We investigated the role of feedback generated by transporter regulation in the budding yeast phosphate-responsive signal transduction (PHO) pathway. Cells starved for phosphate activate feedback loops that regulate high- and low-affinity phosphate transport. We determined that positive feedback is generated by PHO pathway-dependent up-regulation of Spl2, a negative regulator of low-affinity phosphate uptake. The interplay of positive and negative feedback loops leads to bistability in phosphate transporter usage – individual cells express predominantly either low- or high-affinity transporters, both of which can yield similar phosphate uptake capacity. Cells lacking the high-affinity transporter, and associated negative feedback, exhibit phenotypes that arise from hysteresis due to unopposed positive feedback. In wild-type cells, population heterogeneity generated by feedback loops may provide a strategy for anticipating changes in environmental phosphate levels.
Introduction
The availability of nutrients often limits the growth of microbes (Abelson, 1999; Gray et al., 2004). Organisms employ nutrient-responsive regulatory networks to monitor nutrient levels and adjust cellular processes accordingly (Gray et al., 2004; Oshima, 1997). Nutrient homeostasis, the ability to maintain a relatively constant internal level of the limiting nutrient, is achieved by controlling the balance of nutrient uptake and utilization.
Homeostasis is a systems property, arising from interactions between components of a signaling network. Several networks responsible for nutrient sensing in budding yeast share a common overall architecture, but it is unclear what aspects of this network structure are important for homeostasis. Many networks are responsive to internal levels of nutrient (Barnes and Zierath, 2005; Waters and Eide, 2002; Wisnicka et al., 1997; Wykoff and O’Shea, 2001). Additionally, for most nutrients, there exist high- and low-affinity transporters that have different uptake properties (Auesukaree et al., 2003; BunYa et al., 1991; Harris et al., 2001; Tomas-Cobos et al., 2004); regulation of these transporters allows nutrient uptake to be optimized over a wide range of extracellular nutrient concentrations. High-affinity transport systems are transcriptionally up-regulated in response to nutrient limitation to allow nutrient uptake the nutrient in low nutrient environments (Bun-Ya et al., 1991; Waters and Eide, 2002). During starvation, some low-affinity nutrient transporters are selectively down-regulated, although the functional role of this down-regulation and its effect on systems properties are unclear (Bird et al., 2004; Harris et al., 2001; Jensen and Culotta, 2002; Tomas-Cobos et al., 2004; Waters and Eide, 2002).
Alteration of the activity of high- or low-affinity transporters has the potential to generate feedback in nutrient homeostatic systems; transporters can influence the internal level of nutrient, which in turn influences the signaling pathway, which can feed back to control the transporters. For nutrient-responsive signaling pathways that are activated by decreases in nutrient availability, up-regulation of transporters could lead to increased nutrient uptake and negative feedback, or the tendency to reduce the activity of the signal transduction pathway. Conversely, down-regulation of transporters can generate positive feedback by reducing internal levels of nutrient, leading to further activation of the signaling pathway. In other systems, feedback has been demonstrated to confer system properties such as increased sensitivity, dampening of noise or oscillations, hysteresis, multistability, and a rapid approach to steady-state values (Acar et al., 2005; Brandman et al., 2005; Ferrell, 2002).
The S. cerevisiae phosphate-responsive signaling pathway (PHO pathway) is thought to monitor cytoplasmic levels of inorganic phosphate, allowing cells to sense and respond to changes in phosphate availability through transcriptional control of genes required for uptake and scavenging of phosphate and phosphate containing compounds, and mobilization of internal phosphate stores (Auesukaree et al., 2004; Lenburg and O’Shea, 1996; Wykoff and O’Shea, 2001). There are five known phosphate transporters: three uptake phosphate with low-affinity (Pho87, Pho90, Pho91), one is the major high-affinity phosphate transporter (Pho84), and another is utilized under specialized conditions (Pho89) (Persson et al., 1998). Pho89 and Pho84 are transcriptionally up-regulated during phosphate limitation (Persson et al., 1998; Bun-Ya et al., 1991) and this up-regulation requires Pho4, a transcription factor regulated by a kinase complex composed of the cyclin Pho80, cyclin-dependent kinase (CDK) Pho85, and CDK inhibitor Pho81 (Oshima, 1997; Schneider et al., 1994). In high phosphate conditions, this complex phosphorylates Pho4, causing it to be localized to the cytoplasm (O’Neill et al., 1996). When cells are limited for phosphate, Pho81 inhibits the kinase complex and unphosphorylated Pho4 accumulates in the nucleus where it activates transcription of phosphate-responsive genes (O’Neill et al., 1996; Schneider et al., 1994). When the extracellular concentration of phosphate is between these two extremes the kinase complex is partially active, leading to accumulation of a nuclear, partially phosphorylated form of Pho4 that is capable of activating transcription of a subset of phosphate-responsive genes, including the high-affinity phosphate transporter Pho84 (Springer et al., 2003).
In this study, we investigate regulation of low-affinity phosphate transport, the feedback generated by this regulation, and the effect that feedback has on systems properties of the phosphate-responsive regulatory network. We find that yeast cells regulate low-affinity transport activity in response to phosphate availability and demonstrate that induction of the phosphate-responsive gene, SPL2, is necessary and sufficient for PHO pathway-dependent down-regulation of low-affinity transport. This down-regulation creates positive feedback that generates bistability, causing individual cells to express either low-or high-affinity transporters. These findings also provide insight into the origins of the phenotype of cells lacking the high-affinity phosphate transporter, which can now be understood to arise from hysteresis caused by positive feedback.
Results
Low-affinity phosphate transport is down-regulated by the PHO pathway
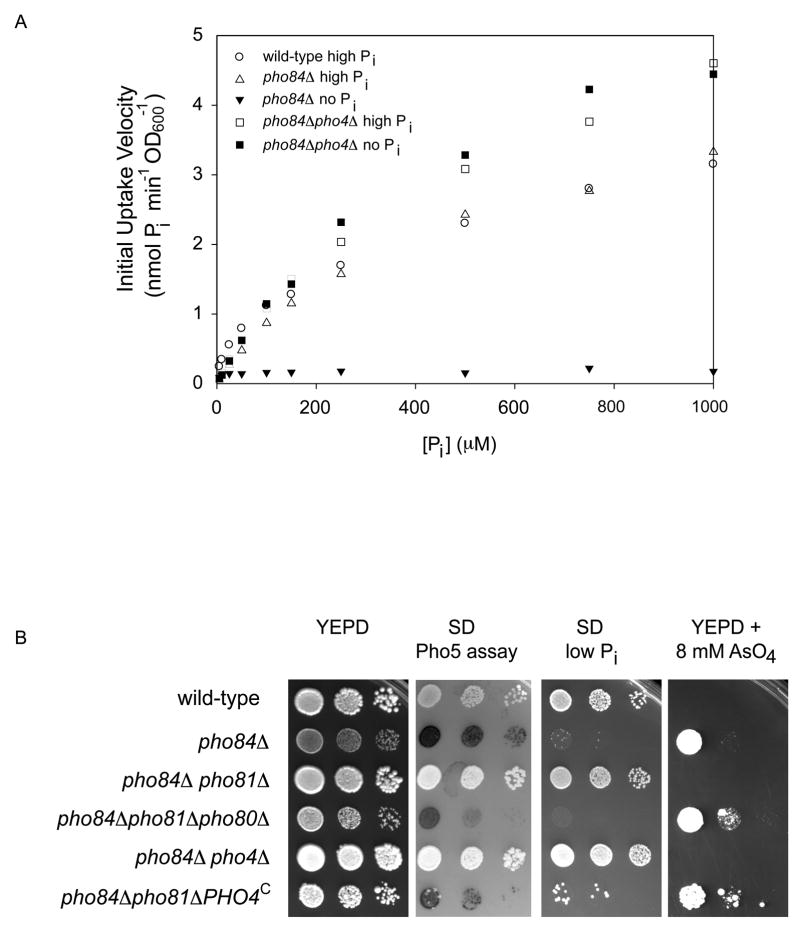
Because precedent exists for down-regulation of some low-affinity transporters in response to nutrient limitation (Eide, 2003; Waters and Eide, 2002), we examined whether low-affinity phosphate transport is regulated in response to phosphate availability. Transcription of the three low-affinity proton/phosphate transporters (PHO87, PHO90, and PHO91) is not regulated in response to external phosphate availability (Auesukaree et al., 2003). To determine if low-affinity phosphate transport is regulated post-transcriptionally, we measured phosphate uptake in a strain lacking the high-affinity transporter Pho84 (in which phosphate transport is primarily low-affinity (Km ~ 200 μM phosphate) (Wykoff and O’Shea, 2001), pre-grown either for 24–48 hours in high phosphate conditions, or grown in no phosphate conditions (for details of growth conditions, see Figure 1A legend). When pre-grown in high phosphate conditions, pho84Δ cells take up phosphate with a velocity similar to that of wild-type cells. In contrast, when pre-grown in phosphate limiting conditions, pho84Δcells lose almost all phosphate transport, indicating that low-affinity transport is down-regulated in response to phosphate limitation (Figure 1A). Inactivation of the downstream transcription factor Pho4 prevents this down-regulation; the pho84Δpho4Δ strain displays little change in phosphate uptake in response to phosphate starvation (Figure 1A). We conclude that a functional PHO pathway is required for down-regulation of low-affinity phosphate transport in response to phosphate limitation.
Figure 1.
Phosphate uptake and growth of wild-type and mutant strains. (A) EY57 (wild-type), EY105 (pho84Δ) and EY329 (pho84Δpho4Δ) were grown in logarithmic phase for 24–48 hours in high phosphate medium (SD medium containing 15 mM phosphate), transferred to SD or SD medium lacking phosphate for 4 hours at 30°C, and 32P uptake measurements were performed as described previously (Wykoff and O’Shea, 2001). The initial uptake velocity of wild-type cells grown in no phosphate conditions is not displayed; the uptake velocity values approach 20 nmol phosphate min−1 OD600−1. EY105 exhibited a 50% defect in phosphate uptake when inoculated from a plate and grown for 8–10 hours in SD medium (Wykoff and O’Shea, 2001), but this defect was suppressible by growth for 24–48 hours in SD medium containing 15 mM phosphate. (B) Strains EY57, EY105, EY152 (pho84Δpho81Δ), EY334 (pho84Δpho81Δpho80Δ), EY329, and EY1880 (pho84Δpho81Δ PHO4SA1-4PA6) were grown overnight in SD medium, diluted to OD600~0.3 and plated in 10-fold dilutions. The first three panels of photographs are of plates that were incubated for two days at 30°C. The last panel is a photograph of a plate that was incubated for 5 days at 30°C. In the second panel, the plate was overlaid with a substrate to detect Pho5 acid phosphatase activity (Bun-Ya et al., 1991).
Phenotypes of the pho84Δ strain result from feedback in the PHO pathway
Deletion of PHO84 results in constitutive activation of the PHO pathway, which can be measured by monitoring the expression of the secreted acid phosphatase, Pho5 (Bun-Ya et al., 1991) (Figure 1B). pho84Δ strains also exhibit other phenotypes – they are unable to grow on low phosphate medium and exhibit resistance to the toxic phosphate analog arsenate (Figure 1B) (Bun-Ya et al., 1991). Although it has been thought that these phenotypes result from a reduction in phosphate uptake due to loss of the phosphate transporter Pho84, it is unlikely that these defects arise solely from loss of Pho84 function since, unexpectedly, mutations that inactivate the PHO pathway (e.g. pho81Δ or pho4Δ; Figure 1B) suppress the growth phenotypes of the pho84Δ strain. Since a mutation that inactivates the PHO pathway can also prevent down-regulation of low-affinity phosphate transport (Figure 1A), we speculate that positive feedback in the PHO pathway (through PHO pathway-dependent down-regulation of low-affinity transporters in response to phosphate limitation) is the cause of the phenotypes associated with loss of Pho84 function. We hypothesize that in cells lacking Pho84, activation of the PHO pathway triggers down-regulation of low-affinity transport, causing cells to uptake less phosphate and further activate the PHO pathway, ultimately driving them to a state where the PHO pathway is constitutively on and transport capacity is minimal. This state results in a defect in low phosphate growth and resistance to the toxic phosphate analog arsenate. Consistent with this model, activation of Pho4 is sufficient for down-regulation of low-affinity phosphate transport; introduction of a constitutively activated allele of PHO4 (Komeili and O’Shea, 1999) into the pho84Δ pho81Δ strain restores the arsenate resistance phenotype, presumably by restoring the ability to down-regulate low-affinity transport and therefore uptake less arsenate (Figure 1B).
Spl2 is necessary and overexpression is sufficient to down-regulate low-affinity phosphate transport
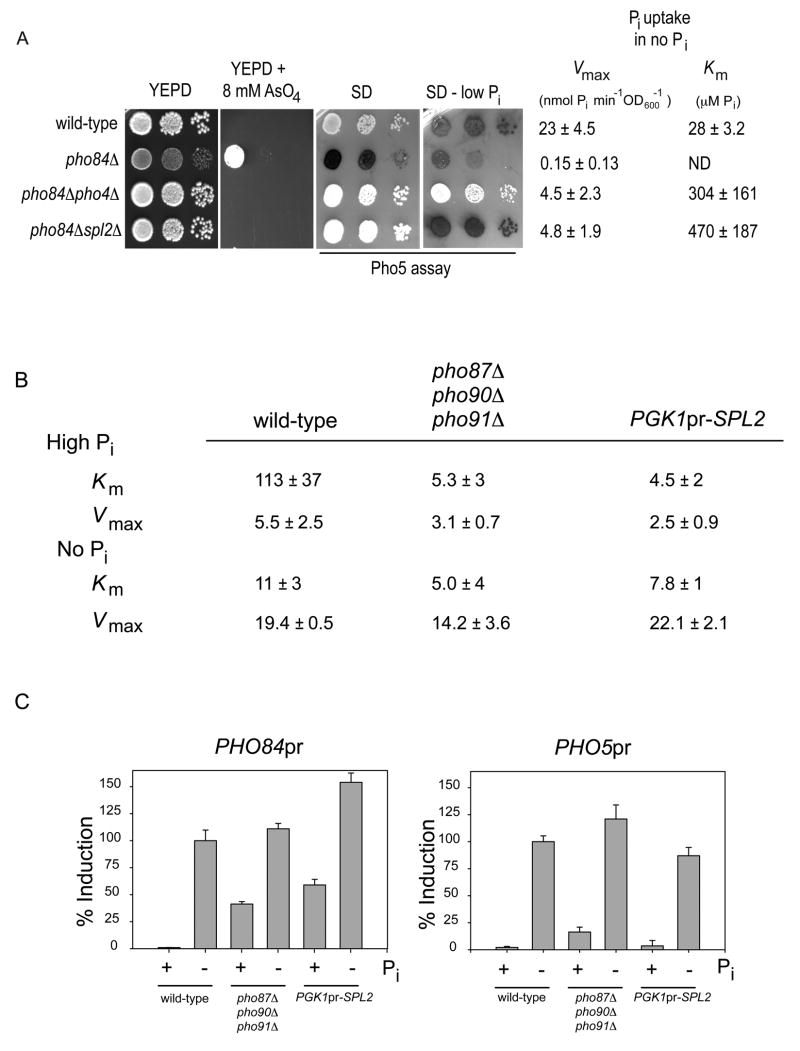
Because Pho4 is a known activator of transcription (Barbaric et al., 1998), we hypothesized that Pho4 activates transcription of a gene whose protein product then down-regulates low-affinity phosphate transport. To identify this gene, we inactivated 21 Pho4-dependent genes (Carroll et al., 2001; Springer et al., 2003) in a pho84Δ background and assayed these double mutant strains for the ability to grow in medium containing arsenate (Figure S1). We identified only one Pho4-induced gene, other than PHO81, which suppressed the arsenate resistance phenotype of the pho84Δ strain when inactivated: SPL2 (Figure 2A). Like deletion of PHO4 (and PHO81), deletion of SPL2 in the pho84Δ strain restores arsenate sensitivity and low-affinity phosphate transport in low phosphate conditions (Figure 2A). However, in contrast to the pho84Δ pho4Δ strain, the PHO pathway is otherwise regulated appropriately in the pho84Δ spl2Δ mutant, as evidenced by the ability to control expression of the phosphate-responsive gene PHO5 (Figure 2A). spl2Δ mutants exhibit no obvious defect in PHO5 induction ((Flick and Thorner, 1998) and data not shown), explaining why SPL2 was not identified in previous screens for mutants defective in PHO pathway regulation. These observations strongly suggest that Spl2 is required for down-regulation of low-affinity phosphate transport in conditions of phosphate limitation. In support of these results, our genetic evidence indicates that Spl2 down-regulates the low-affinity transporters Pho87 and Pho90 (Figure S2).
Figure 2.
Spl2 is necessary and overexpression is sufficient to down-regulate low-affinity phosphate transport. (A) Strains EY57 (wild-type), EY105 (pho84Δ), EY329 (pho84Δpho4Δ), and EY1718 (pho84Δ spl2Δ) were grown and plated as described in Figure 1, except that the YEPD + 8 mM AsO4 plate was incubated for 3.5 days at 30°C. The aggregate Vmax and Km was calculated from phosphate uptake data of three independently grown cultures and the error is the standard deviation. Because there is negligible phosphate uptake in the pho84Δ strain, the Km was not determined. (B) Strains EY57 (wild-type), EY1982 (pho87Δpho90Δpho91Δ), and EY1959 (PGK1pr-SPL2) were grown in high or no phosphate medium for 4 hours. Phosphate uptake measurements were performed and the aggregate kinetic constants were derived. The error is the standard deviation of three independent experiments. Vmax and Km values are reported in units of nmol Pi min−1 OD600−1 and μM Pi, respectively (C) Strains containing integrated versions of the PHO84 and PHO5 promoters controlling GFP expression (the PHO84pr-GFP reporter is integrated at the URA3 locus so that the wild-type copy of PHO84 is intact) were grown in high and no phosphate medium and assayed by flow cytometry. The fluorescence was background subtracted and normalized to total induction of wild-type cells. The errors are the standard deviation between the mean fluorescence of three independent cultures. The strains are EY2094 (PHO5pr-GFP), EY1981 (pho87Δpho90Δpho91ΔPHO84pr-GFP), EY1958 (PGK1pr-SPL2 PHO84pr-GFP), EY1995 (PHO84pr-GFP), EY2044 (pho87Δpho90Δpho91ΔPHO5pr-GFP), and EY2045 (PGK1pr-SPL2 PHO5pr-GFP).
If it is true that positive feedback resulting from Spl2-dependent down-regulation of low-affinity transport causes the pho84Δ phenotypes, deletion of SPL2 in a pho84Δ strain should increase internal phosphate levels, and in turn increase Pho80-Pho85 kinase activity, in high phosphate conditions. To test if this prediction is correct, we measured Pho80–Pho85 kinase activity in pho84Δ and pho84Δspl2Δ strains grown in high phosphate conditions using Pho4-YFP localization as a reporter. We find that Pho80–Pho85 is inactive in the pho84Δ strain, and is active in the pho84Δspl2Δ strain (Figure S3). Thus, the phenotypes of the pho84Δstrain are a consequence of inappropriate activation of a positive feedback loop involving Pho4-dependent activation of SPL2, leading to down-regulation of low-affinity transport, less internal phosphate, and further PHO pathway activation. In the absence of PHO84 induction, positive feedback causes cells to become trapped in a state where the PHO pathway is constitutively activated.
To determine whether SPL2 expression is sufficient to down-regulate low-affinity phosphate transport, we compared the phosphate uptake kinetics and gene expression phenotypes caused by overexpression of SPL2 in high phosphate conditions to those resulting from deletion of the low-affinity phosphate transporters. When SPL2 is overexpressed (Figure S4) in high phosphate conditions, we observe a 20-fold decline in the Km of phosphate uptake (Figure 2B), consistent with loss of low-affinity phosphate uptake and a compensatory increase in Pho84 activity. As expected, if Spl2 overexpression inactivates low-affinity transport, the Km we observe in cells overexpressing SPL2 is similar to the Km observed in cells lacking the three low-affinity phosphate transporters (Pho87, Pho90, Pho91). Inactivation of low-affinity transporters also causes changes in the activity of the PHO signaling pathway that can be quantified by monitoring expression of transcriptional reporters for the phosphate-responsive genes PHO84 and PHO5. SPL2 overexpression and deletion of low-affinity transporters induce similar levels of PHO84 (Figure 2C, left panel), but little induction of PHO5 (Figure 2C, right panel). The differential effect on PHO5 and PHO84 induction can be explained by the higher sensitivity of PHO84 to changes in the PHO pathway; low-affinity transporter deletion only partially activates the PHO pathway and PHO84 is induced at a higher phosphate concentration and lower level of pathway activation than is PHO5 (Thomas and O’Shea, 2005). We conclude that overexpression of SPL2 (in high phosphate conditions) phenocopies deletion of the low-affinity transporters, indicating that it is sufficient to down-regulate low-affinity transport.
Spl2 alters systems properties of the PHO pathway
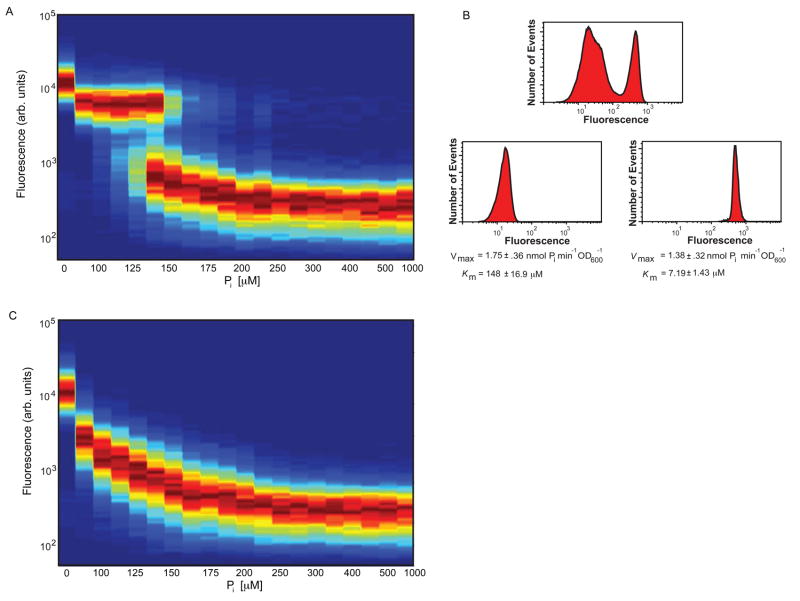
In high phosphate conditions neither SPL2 nor PHO84 is significantly expressed (BunYa et al., 1991; Lau et al., 2000 and Figure S4), and cells use low-affinity transporters to uptake phosphate into the cell. SPL2 and PHO84 induction in conditions of phosphate starvation causes cells to switch their complement of phosphate transporters from low to high-affinity, allowing cells to uptake phosphate under conditions where it is limiting. Our previous studies revealed that in intermediate phosphate conditions (between these two extremes of phosphate-replete and phosphate-limiting) PHO84 expression is induced to ~50% of maximal levels (Springer et al., 2003). This sub-maximal induction is at least partly a consequence of population heterogeneity (Thomas and O’Shea, 2005) that is readily observed when PHO84 expression is monitored by flow cytometric analysis of single cells containing a transcriptional reporter in which GFP expression is under the control of the PHO84 promoter (PHO84pr-GFP; integrated into the yeast genome at an ectopic locus so as to maintain a wild-type copy of PHO84). When cells expressing PHO84pr-GFP are grown in high or no phosphate, most cells exhibit either low or very high GFP expression, respectively ((Thomas and O’Shea, 2005) and Figure 3A). In contrast, over a range of phosphate concentrations from ~100–200 μM phosphate (intermediate phosphate) there are two populations of cells at steady state – one that expresses little PHO84, and another that highly expresses PHO84 (Figure 3A). These observations suggest that in intermediate phosphate conditions, part of the population uses Pho84 to uptake phosphate, and the remainder uses a different transport system.
Figure 3.
Positive feedback through induction of Spl2 generates complex population dynamics. (A) Phosphate titration contour maps demonstrate the bistable response of wild-type cells in intermediate phosphate conditions. Strain EY1995 expressing PHO84pr-GFP was grown in no phosphate medium supplemented with the indicated concentrations of inorganic phosphate. Cells were grown at low density (104–105 cells per ml) to prevent phosphate depletion. After 18 hours of growth (at which time the levels of GFP expression had reached steady-state), cells were harvested and subjected to flow cytometry. Titration contour plots were generated for each phosphate concentration condition by normalizing to maximum peak height and creating a color map for all concentration measurements. (B) Phosphate uptake assays were conducted on subpopulations of wild-type EY2095 (PHO84pr-GFP) cells grown in intermediate phosphate (150 μM phosphate) and sorted into populations expressing high and low levels of GFP by flow cytometry. The Km values of the two populations suggest that low-affinity phosphate transporters dominate the un-induced population and high-affinity transporters predominate in the induced population. (C) Strain EY2096 (PHO84pr-GFP spl2Δ) was grown and analyzed as in (A) and a contour map was generated from flow cytometry data.
To determine if the two populations of cells observed in intermediate phosphate use different transport systems to uptake phosphate, we sorted wild-type cells from intermediate phosphate conditions based on their expression of PHO84pr-GFP and measured phosphate uptake kinetics and Spl2 expression (Figure 3B). Cells expressing high levels of PHO84 have a Km consistent with high-affinity phosphate transport (~7 μM), whereas cells expressing little PHO84 use predominantly low-affinity phosphate transporters for uptake (Km ~ 150 μM). The velocity of phosphate uptake in each population grown in 150 μM phosphate is similar (1.75 ± 0.36 vs. 1.38 ± 0.32 nmol phosphate min−1 OD600−1, respectively), suggesting that cells have approximately the same uptake rate, but utilize different uptake systems to achieve this. As assayed by immunoblot analysis (Figure S5), cells that express low levels of PHO84 also express little Spl2, whereas cells that induce PHO84 express a high level of Spl2. Therefore, the two populations of cells in intermediate phosphate differ in the phosphate transporters they use to obtain phosphate from the environment.
Since positive feedback can cause bimodal expression and bistability (Ferrell, 2002) and Spl2 is postulated to be part of a positive feedback loop in the PHO pathway, we tested if bimodal expression of PHO84 was dependent on Spl2 (Figure 3C). Cells lacking SPL2 express PHO84pr-GFP in a graded fashion, suggesting that SPL2 induction is a positive feedback (or double-negative feedback) element in the PHO pathway required for bimodal expression of PHO84 in intermediate phosphate conditions. Although we observe a striking effect of Spl2 on PHO84 expression, we do not find a difference in growth of wild-type and spl2Δ mutant cells in high or no phosphate conditions (data not shown).
Discussion
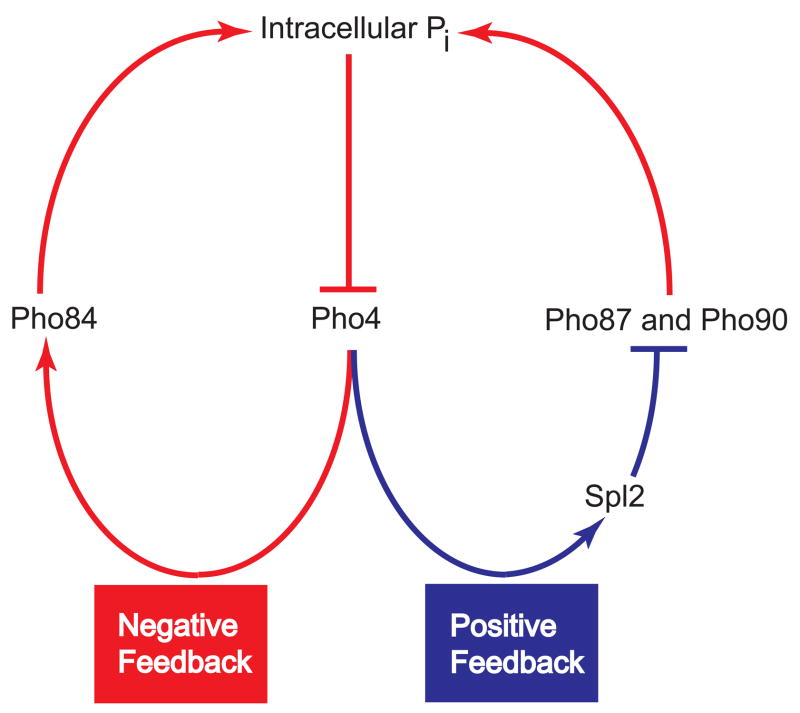
We have demonstrated that feedback exists in the PHO pathway that allows yeast cells to switch their complement of phosphate transporters in response to phosphate availability. In response to a decrease in internal phosphate levels, cells activate the PHO pathway, triggering two feedback elements: a negative feedback loop consisting of Pho4-dependent induction of PHO84, which helps to bring phosphate into the cell and inactivate the PHO pathway; and a positive feedback loop consisting of Pho4-dependent up-regulation of SPL2, which tends to reduce phosphate uptake, leading to further pathway activation (Figure 4). Because the feedback loops are both controlled by the PHO pathway, they create mutually exclusive states in which wild-type cells either activate the PHO pathway, express high-affinity transporters, and down-regulate the low-affinity transport system or, instead, keep the PHO pathway turned off and utilize low-affinity transporters for phosphate uptake. As the concentration of phosphate in the medium approaches the Km for transport by the low-affinity transport system, phosphate uptake decreases and can no longer keep up with cellular usage, resulting in a drop in intracellular phosphate levels and activation of the PHO pathway. We speculate that the bimodality in PHO84 expression arises under these conditions (intermediate phosphate) because cell-cell variability leads some cells to activate the PHO pathway and use high affinity transporters, whereas others keep the pathway repressed and instead take up phosphate using low-affinity transporters. In intermediate phosphate conditions, these two states appear to be equivalent solutions to obtaining phosphate, as their uptake rates are not distinguishable. The origin of this cell-cell variability in genetically identical cells is unclear – it may arise from noise in the signaling pathway (Maheshri, 2007), or from pre-existing differences between cells that bias them towards one fate or another. spl2Δ cells lacking the positive feedback loop cannot down-regulate low-affinity transport and, when phosphate levels decrease near the Km of the low-affinity transport system, individual cells in the population respond similarly and produce enough Pho84 to allow them to balance phosphate uptake with usage. A graded response in PHO84 results because, as phosphate levels drop further, more Pho84 is required to balance uptake with usage until eventually Pho84 is produced at maximal levels.
Figure 4.
Phenotypic switching of the complement of phosphate transporters is mediated by counteracting positive and negative feedback loops. Pho4, which is regulated by internal phosphate concentrations, controls the transcription of PHO84 and SPL2. Pho84, because of its ability to transport inorganic phosphate, serves as a negative feedback element in the network. Spl2 is a positive feedback element because it down-regulates the activity low-affinity phosphate transporters, reducing internal phosphate levels and leading to further activation of Pho4.
It is unclear why S. cerevisiae cells exhibit such a complex response to phosphate starvation; in particular, what benefit cells derive from generating two phenotypic states in intermediate phosphate and why low-affinity phosphate transport is down-regulated in response to phosphate limitation. One rationalization is that the existence of two states may confer an advantage in an environment that includes conditions of phosphate limitation. It is also possible that the two states generated by feedback ensure that some fraction of the cells is optimally positioned to respond to a change in phosphate availability. For example, cells that already express Pho84 should respond faster to a change to phosphate starvation conditions than those utilizing low-affinity transporters. Therefore, feedback may allow the cells to carry out a form of “bet hedging” (Kussell and Leibler, 2005; Wolf et al., 2005). Yet another possibility is that transporter switching is merely a consequence of a system design utilized for a different property.
This work points to the difficulty in interpreting mutant phenotypes when genes are embedded in pathways containing feedback loops. The phenotypes of the pho84Δ strain have been difficult to rationalize, given that little Pho84 protein is present under the conditions where striking phenotypes are observed (Bun-Ya et al., 1991; Lau et al., 2000). With knowledge of feedback in the PHO pathway, these phenotypes can now be understood to arise from hysteresis in the pho84Δstrain – fluctuations that might otherwise lead to transient activation of the PHO pathway (e.g. in the pho84Δ strain which lacks polyphosphate stores (Ogawa et al., 2000), activation of the PHO pathway may result from phosphate demands that fluctuate during cell growth and division; (Neef and Kladde, 2003) likely trigger positive feedback through induction of SPL2 and down-regulation of low-affinity transport, which further reduces internal phosphate and drives the system to full activation of the PHO pathway. In contrast, wild-type cells balance SPL2 induction with PHO84 up-regulation so that phosphate uptake is maintained. Therefore, the constitutive PHO pathway activation observed in the pho84Δ strain results from unconstrained positive feedback, not from a direct role for Pho84 in phosphate uptake in high phosphate conditions.
SPL2 is a Pho4-regulated gene, and was originally identified in a screen for genes which, when overexpressed, suppress the temperature sensitivity of a plc1Δ strain (Flick and Thorner, 1998). This study revealed that several perturbations and conditions that cause activation of the PHO pathway suppress the plc1Δ temperature sensitivity, suggesting that SPL2 was isolated because it activates the PHO pathway and bypasses the Plc1 requirement at elevated temperatures. Spl2 may directly or indirectly down-regulate low-affinity transporters; if it acts directly it may target the low-affinity phosphate transporters during their trafficking or at the plasma membrane.
It is striking that other nutrient signal transduction pathways, such as the galactose utilization (GAL) pathway in budding yeast and the lac operon in E. coli (Acar et al., 2005; Ozbudak et al., 2004), contain embedded positive and negative feedback loops and respond in a similar switch-like manner. Additionally, nutrient-responsive signaling systems in budding yeast that share no sequence homology at the protein level have a similar pathway architecture consisting of regulated high-affinity transport and a low-affinity transport system (Bird et al., 2004; Harris et al., 2001; Jensen and Culotta, 2002; Tomas-Cobos et al., 2004; Waters and Eide, 2002). Feedback generated by this design may be an important feature of the ability to maintain nutrient homeostasis in the face of variable conditions.
Experimental Procedures
Strain Construction
All yeast strains utilized in this study are listed in Table S1. Yeast media has been previously described (Wykoff and O’Shea, 2001). Methodologies for gene disruption have been detailed in the supplement.
Phosphate uptake and derivation of kinetics
Uptake assays were performed (Wykoff and O’Shea, 2001) with final concentrations of phosphate assayed for initial velocity in a three minute period being 5 μM, 10 μM, 25 μM, 50 μM, 100 μM, 150 μM, 250 μM, 500 μM, 750 μM, 1 mM, 2.5 mM, and 5 mM. For high phosphate measurements, pho84Δ strains were grown in mid-log phase for 24 hours, minimizing the down-regulation of phosphate uptake observed previously (Wykoff and O’Shea, 2001). Aggregate Vmax and Km measurements were determined by reciprocal plots of at least 8 data points. Reported means and standard deviations are from at least three independent experiments.
Assay for growth on plates
Cultures were grown to stationary phase overnight at 30°C in SD medium (or galactose containing medium when required), diluted to OD~0.3 and serially diluted 10-fold. Approximately, 3 microliters was spotted onto media containing agar with a pinning tool and allowed to grow at 30°C. Plates were photographed with a FlourChem 880 (Alpha Innotech, CA) and digital images were scaled with Adobe Photoshop 6.0 (Adobe Systems, CA) so that colony details could be readily visualized.
Flow cytometry
Overnight cultures were diluted and grown to an OD600 of 0.1. The culture was then diluted to 1×104 cells/ml, into SD supplemented with inorganic phosphate concentrations ranging from 0–1mM. Cultures were grown for 12–18 hours, diluting every 4 hours to ensure cells do not alter the phosphate concentration in the medium. Cells were sonicated to avoid aggregation and studied via flow cytometry using a LSRII (BD Biosciences, Franklin Lakes, NJ). Titration contour plots were generated for each phosphate concentration. To do so, semi-log distributions of cell counts as a function of fluorescence intensity were 1D interpolated and normalized by the maximum cell count. These data were then concatenated and steady state fluorescence intensity was plotted as a function of phosphate concentration to create a FACS density series. All analysis was conducted using MATLAB. (Mathworks, Natick, MA).
Cell Sorting
Strains were sorted using a cytomation MoFlow cell sorter (Dako, CA) in 10 mM NaCl and 150 μM KH2PO4 solution and sorted into SD medium containing 150 μM KH2PO4. Post sorted cells were recovered in SD with 150 μM phosphate medium for 5 hours, then concentrated 1000-fold and subjected to 32P uptake (Wykoff and O’Shea, 2001) or immunoblot analysis. To ensure that PHO84 expression was not altered by the sorting and recovery, cells were reanalyzed by flow cytometry.
Supplementary Material
Acknowledgments
The authors thank the O’Shea laboratory, Narendra Maheshri, Naama Barkai, and Bodo Stern for helpful discussions. This work was supported by the National Institutes of Health (GM51377) and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelson PH. A potential phosphate crisis. Science. 1999;283:2015. doi: 10.1126/science.283.5410.2015. [DOI] [PubMed] [Google Scholar]
- Acar M, Becskei A, van Oudenaarden A. Enhancement of cellular memory by reducing stochastic transitions. Nature. 2005;435:228–232. doi: 10.1038/nature03524. [DOI] [PubMed] [Google Scholar]
- Auesukaree C, Homma T, Kaneko Y, Harashima S. Transcriptional regulation of phosphate-responsive genes in low-affinity phosphate-transporter-defective mutants in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2003;306:843–850. doi: 10.1016/s0006-291x(03)01068-4. [DOI] [PubMed] [Google Scholar]
- Barbaric S, Munsterkotter M, Goding C, Horz W. Cooperative Pho2-Pho4 interactions at the PHO5 promoter are critical for binding of Pho4 to UASp1 and for efficient transactivation by Pho4 at UASp2. Mol Cell Biol. 1998;18:2629–2639. doi: 10.1128/mcb.18.5.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BR, Zierath JR. Role of AMP--activated protein kinase in the control of glucose homeostasis. Curr Mol Med. 2005;5:341–348. doi: 10.2174/1566524053766103. [DOI] [PubMed] [Google Scholar]
- Bird AJ, Blankman E, Stillman DJ, Eide DJ, Winge DR. The Zap1 transcriptional activator also acts as a repressor by binding downstream of the TATA box in ZRT2. Embo J. 2004;23:1123–1132. doi: 10.1038/sj.emboj.7600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Ferrell JE, Jr, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–498. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-Ya M, Nishimura M, Harashima S, Oshima Y. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol. 1991;11:3229–3238. doi: 10.1128/mcb.11.6.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AS, Bishop AC, DeRisi JL, Shokat KM, O’Shea EK. Chemical inhibition of the Pho85 cyclin-dependent kinase reveals a role in the environmental stress response. Proc Natl Acad Sci U S A. 2001;98:12578–12583. doi: 10.1073/pnas.211195798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide DJ. Multiple regulatory mechanisms maintain zinc homeostasis in Saccharomyces cerevisiae. J Nutr. 2003;133:1532S–1535S. doi: 10.1093/jn/133.5.1532S. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Flick JS, Thorner J. An essential function of a phosphoinositide-specific phospholipase C is relieved by inhibition of a cyclin-dependent protein kinase in the yeast Saccharomyces cerevisiae. Genetics. 1998;148:33–47. doi: 10.1093/genetics/148.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JV, Petsko GA, Johnston GC, Ringe D, Singer RA, Werner-Washburne M. Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2004;68:187–206. doi: 10.1128/MMBR.68.2.187-206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RM, Webb DC, Howitt SM, Cox GB. Characterization of PitA and PitB from Escherichia coli. J Bacteriol. 2001;183:5008–5014. doi: 10.1128/JB.183.17.5008-5014.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LT, Culotta VC. Regulation of Saccharomyces cerevisiae FET4 by oxygen and iron. J Mol Biol. 2002;318:251–260. doi: 10.1016/S0022-2836(02)00093-1. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Herskowitz I, Tjian R, O’Shea EK. Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80–PHO85. Science. 1994;263:1153–1156. doi: 10.1126/science.8108735. [DOI] [PubMed] [Google Scholar]
- Komeili A, O’Shea EK. Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science. 1999;284:977–980. doi: 10.1126/science.284.5416.977. [DOI] [PubMed] [Google Scholar]
- Kussell E, Leibler S. Phenotypic diversity, population growth, and information in fluctuating environments. Science. 2005;309:2075–2078. doi: 10.1126/science.1114383. [DOI] [PubMed] [Google Scholar]
- Lau WT, Howson RW, Malkus P, Schekman R, O’Shea EK. Pho86p, an endoplasmic reticulum (ER) resident protein in Saccharomyces cerevisiae, is required for ER exit of the high-affinity phosphate transporter Pho84p. Proc Natl Acad Sci U S A. 2000;97:1107–1112. doi: 10.1073/pnas.97.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenburg ME, O’Shea EK. Signaling phosphate starvation. Trends Biochem Sci. 1996;21:383–387. [PubMed] [Google Scholar]
- Neef DW, Kladde MP. Polyphosphate loss promotes SNF/SWI- and Gcn5-dependent mitotic induction of PHO5. Mol Cell Biol. 2003;23:3788–3797. doi: 10.1128/MCB.23.11.3788-3797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N, DeRisi J, Brown PO. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell. 2000;11:4309–4321. doi: 10.1091/mbc.11.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill EM, Kaffman A, Jolly ER, O’Shea EK. Regulation of PHO4 nuclear localization by the PHO80-PHO85 cyclin-CDK complex. Science. 1996;271:209–212. doi: 10.1126/science.271.5246.209. [DOI] [PubMed] [Google Scholar]
- Oshima Y. The phosphatase system in Saccharomyces cerevisiae. Genes Genet Syst. 1997;72:323–334. doi: 10.1266/ggs.72.323. [DOI] [PubMed] [Google Scholar]
- Ozbudak EM, Thattai M, Lim HN, Shraiman BI, Van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427:737–740. doi: 10.1038/nature02298. [DOI] [PubMed] [Google Scholar]
- Maheshri N, O’Shea EK. Living with noisy genes: how cells function reliably with inherent variability in gene expression. Ann Rev Biophys Biomol Struc. 2007;36:413–434. doi: 10.1146/annurev.biophys.36.040306.132705. [DOI] [PubMed] [Google Scholar]
- Persson BL, Berhe A, Fristedt U, Martinez P, Pattison J, Petersson J, Weinander R. Phosphate permeases of Saccharomyces cerevisiae. Biochim Biophys Acta. 1998;1365:23–30. doi: 10.1016/s0005-2728(98)00037-1. [DOI] [PubMed] [Google Scholar]
- Schneider KR, Smith RL, O’Shea EK. Phosphate-regulated inactivation of the kinase PHO80-PHO85 by the CDK inhibitor PHO81. Science. 1994;266:122–126. doi: 10.1126/science.7939631. [DOI] [PubMed] [Google Scholar]
- Springer M, Wykoff DD, Miller N, O’Shea EK. Partially phosphorylated Pho4 activates transcription of a subset of phosphate-responsive genes. PLoS Biol. 2003;1:E28. doi: 10.1371/journal.pbio.0000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MR, O’Shea EK. An intracellular phosphate buffer filters transient fluctuations in extracellular phosphate levels. Proc Natl Acad Sci U S A. 2005;102:9565–9570. doi: 10.1073/pnas.0501122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas-Cobos L, Casadome L, Mas G, Sanz P, Posas F. Expression of the HXT1 Low Affinity Glucose Transporter Requires the Coordinated Activities of the HOG and Glucose Signalling Pathways. J Biol Chem. 2004;279:22010–22019. doi: 10.1074/jbc.M400609200. [DOI] [PubMed] [Google Scholar]
- Waters BM, Eide DJ. Combinatorial control of yeast FET4 gene expression by iron, zinc, and oxygen. J Biol Chem. 2002;277:33749–33757. doi: 10.1074/jbc.M206214200. [DOI] [PubMed] [Google Scholar]
- Wisnicka R, Krzepilko A, Wawryn J, Bilinski T. Iron toxicity in yeast. Acta Microbiol Pol. 1997;46:339–347. [PubMed] [Google Scholar]
- Wolf DM, Vazirani VV, Arkin AP. Diversity in times of adversity: probabilistic strategies in microbial survival games. Journal of Theoretical Biology. 2005;234:227–253. doi: 10.1016/j.jtbi.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Wykoff DD, O’Shea EK. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics. 2001;159:1491–1499. doi: 10.1093/genetics/159.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.