Abstract
Acute lung injury affects close to 200,000 people in the U.S. annually and leads to death in 40–50% of affected patients. Chronic ethanol abuse is thought to contribute to up to 40–50% of subjects who develop acute lung injury. We previously demonstrated in a rat model that chronic ethanol ingestion promoted acute lung injury and associated with chronic oxidant stress, activated matrix metalloproteinases, increased release of transforming growth factor-β, as well as increased expression and deposition of fibronectin, a matrix glycoprotein implicated in lung injury and repair. Since fibronectin can activate monocytes to increase proinflammatory cytokine expression, we hypothesized that generation of fibronectin-enriched matrices during chronic ethanol ingestion might contribute to the development of acute lung injury by stimulating unopposed inflammation. To test this hypothesis, we harvested alveolar type II cells from rats fed the Lieber DiCarli diet (6 wk; 36% of calories from ethanol). After 96 hours of culture, the matrices deposited ex vivo by the type II cells derived from ethanol-fed rats showed increased amounts of fibronectin protein as demonstrated by ELISA. When monocytic U937 cells were plated atop these matrices, there was increased expression of interleukin-1β. This stimulation was inhibited by antibodies against α5β1, a receptor that mediates many of the biological effects of fibronectin. We then tested whether antioxidants ameliorated these effects. Dietary supplements of the antioxidants N-acetylcysteine and Procysteine normalized matrix production by type II cells. Furthermore, the newly derived matrices did not stimulate interleukin-1β expression over control cells. These studies suggest that chronic ethanol exposure induces oxidant stress and activates lung tissue remodeling characterized by increased expression of fibronectin by alveolar type II cells. The newly deposited fibronectin-enriched matrices may stimulate the expression of proinflammatory cytokines in monocytic cells recruited to the lung after injury thereby explaining the priming effects of ethanol.
Keywords: extracellular matrix, lung injury, oxidant stress, tissue remodeling
INTRODUCTION
The Acute Respiratory Distress Syndrome (ARDS) is a common and devastating form of acute lung injury that occurs in response to a variety of pulmonary and extra-pulmonary insults (Sloane et al., 1992). Epidemiological studies have demonstrated that alcohol abuse is an independent co-morbid variable that significantly increases the risk of developing ARDS (Moss et al., 1996). In a multi-center prospective study of 220 septic shock patients, 70% of the alcoholic patients developed ARDS compared to 31% in those who did not abuse alcohol (Moss et al., 2003). After controlling for potential confounding variables, the relative risk of ARDS in patients who abused alcohol versus patients who had no history of alcohol abuse was 3.7 to 1.
In an attempt to gain insight into the mechanisms involved in ethanol-induced susceptibility to acute lung injury, we conducted studies in a rat model of chronic ethanol ingestion and these revealed evidence of chronic oxidant stress in alveolar epithelial II cells (Brown et al., 2001a; Brown et al., 2001b). This ethanol-induced chronic oxidant stress resulted in altered type II cell function characterized by barrier dysfunction (Guidot et al., 2000), compromised surfactant synthesis and secretion (Guidot and Brown, 2000; Velasquez et al., 2002), and increased apoptosis in response to diverse pro-inflammatory mediators (Brown et al., 2001a; Brown et al., 2001b) In addition, chronic ethanol ingestion resulted in activation of lung tissue remodeling as highlighted by the increased expression at baseline of the pro-fibrotic growth factor Transforming Growth Factor-β (TGF-β) and increased release of activated TGF-β during endotoxemia (Bechara et al., 2003). Ethanol-induced oxidant stress was also associated with increased activation of Matrix Metalloproteinase (MMP) 9 and MMP-2 as well as collagen degradation during endotoxemia (Lois et al., 1999).
In more recent studies, we showed that chronic ethanol ingestion triggers increased expression of the matrix glycoprotein fibronectin in both in vitro and in vivo models via a nicotinic acetylcholine receptor but independent of the α7 subunit (Roman et al., 2005). The latter was considered important since fibronectin expression represents a sensitive marker of activation of tissue remodeling in the lung. Furthermore, fibronectin might participate in the processes that prime the lung to injury in the setting of chronic ethanol abuse since it is able to stimulate fibroblast proliferation (Bitterman et al., 1983). Depending on the receptor repertoire on resident cells and cells recruited to the lung, fibronectin may also promote cell adhesion, migration, chemotaxis, proliferation, and the differentiation of immune cells, among other cells, and processes associated with the pathogenesis of acute lung injury and repair (Bitterman et al., 1983; Cordes and van Beuningen, 2003; Engel, 1996; Prieto et al., 1994; Rabinovitch, 2001; Roman, 1996; Wadsworth et al., 2004). Because chronic oxidant stress has been associated with progressive accumulation of extracellular matrices and fibrosis, including fibronectin deposition (Lee et al., 1004; Park et al., 2001; Vesey et al., 2005), we postulated that ethanol-induced chronic oxidant stress would promote the generation of a fibronectin-enriched matrix capable of disrupting the function of resident and recruited lung cells, and sought to test this hypothesis in cells harvested from ethanol-treated animals.
In addition to fibroblasts, alveolar type II cells also produce an extracellular matrix containing fibronectin (Dunsmore et al., 1996a; Rannels et al., 1987), and this process is stimulated by TGF-β (Lee and Rannels, 1998). Given that chronic ethanol ingestion causes so many derangements in alveolar type II cells, we examined the possibility that it might also promote type II cell synthesis of fibronectin thereby generating a fibronectin-enriched matrix capable of affecting the function of incoming monocytic cells and their expression of proinflammatory cytokines. Herein, we provide support for this hypothesis and show that these mechanisms could be mediated through fibronectin-binding integrin receptors and are prevented by the concomitant treatment of animals with anti-oxidants.
MATERIALS AND METHODS
Rat model of chronic ethanol ingestion
Weanling male Sprague-Dawley rats (175–250 gm, Harlan, St. Louis, MO) were fed the Lieber-DeCarli liquid diet (Research Diets, New Brunswick, NJ) containing ethanol ad libitum for 6 weeks (Brown et al., 2001a). The ethanol content of the Lieber-DeCarli diet was 36% of the calories, comparable to the calories consumed by otherwise healthy alcoholics (Lieber, 1993) and resulted in a blood alcohol of 0.08 ± 0.2%, a level considered legally intoxicated in the state of Georgia. Pair-fed controls were fed an isocaloric mixture of liquid diet without ethanol. Where appropriate, GSH precursors N-acetylcysteine (NAC) (0.163 mg/ml; Sigma, St. Louis, MO) or (−)-2-oxo-4-thiazolidinecarboxylic acid, procysteine (PRO) (0.35%; Sigma, St. Louis, MO) were added to the diet as previously described (Brown et al., 2001a; Brown et al., 2001b). Animal protocols were reviewed and approved in advance by the Emory University Institutional Animal Care Committee, and all animals were used in accordance with the NIH Guidelines (Guide for the Care and Use of Laboratory Animals).
Reagents
The anti-α2 antibody (P1E6; directed to the α2 subunit of the α2β1 integrin), the anti-α5 antibody (PID6; directed to the α5 subunit of the α5β1 integrin), and the anti-β1 antibody (P4C10; directed to the β1 integrin subunit) were purchased from Life Technologies, Inc. Gaithersburg, MD. The anti-β2 antibody (MAB1962) was purchased from Chemicon, Temecula, CA. All other reagents were purchased from Sigma Chemical Company (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) unless otherwise specified.
Primary alveolar type cell isolation and culture
Alveolar type II cells were isolated from control and ethanol-fed animals using a standard protocol (Dobbs et al., 1986) and were cultured as we described previously (Brown et al., 2001a; Brown et al., 2001b). Briefly, rats were anesthetized, a tracheostomy was placed and the lung removed en bloc. After perfusion to remove red blood cells, elastase was then instilled into the lung via the trachea to dissociate the cells from the lung tissue. After filtration through 100- and 20- μm nylon mesh, the cell suspension was then plated on plastic plates coated with rat IgG. After 1 h, the nonadherent type II cells were removed from the plate and immediately seeded at a density of 2 × 106/ml onto tissue culture plastic. The cells obtained by this method contained approximately 90% type II cells as assessed by positive staining for surfactant apoprotein C and chronic ethanol ingestion did not alter this type II cell profile. Type II cells were maintained in DMEM-F12 media (Sigma) supplemented with 10% fetal bovine serum plus an antibiotic/antimycotic solution and incubated in a humidified 10% CO2 incubator at 37 °C. Cells were judged to be >95% viable by trypan blue exclusion. After 48 hours in culture, the alveolar type II cells from control and ethanol-fed rats were washed and treated with a solution containing 0.25 M ammonium hydroxide and 1 mM EDTA to selectively remove the cells. After three washes, the extracellular matrix deposited by the type II cells was used as a substratum for U937 cells (discussed below). After the extraction of the cytosol, the dishes were treated with hematoxylin to stain for nuclei. Visual assessment by microscopy indicated that no cells remained on the dish after the extraction procedure for any treatment group. In parallel experiments, the deposited extracellular matrix was solubilized with a solution containing 1% sodium dodecyl sulfate, 150 mM NaCl, and 5 M EDTA (Dunsmore et al., 1996a; Dunsmore et al., 1996b).
U937 cell culture and treatment
Human monocytic/macrophage cells from a histiocytic lymphoma, U937 (ATCC CRL #1593.2), were maintained in RPMI-1640 (MediaTech, Herdon, Virginia) supplemented with 10% heat inactivated fetal bovine serum, 1% antibiotic-antimycotic solution (100 units/ml penicillin G sodium, 100 units/ml streptomycin sulfate, 0.25 μg/ml amphotericin B), and incubated in a humidified 5% CO2 incubator at 37°C. Cells were judged to be >95% viable by trypan blue exclusion.
Removal of LPS
LPS was removed from samples by an endotoxin affinity resin (Associates of Cape Cod, Woods Hole, MA) following the manufacturers instructions. All samples were tested after LPS removal as described (Perez et al., 1994) and determined to contain less than 0.06 ng/ml of LPS which is within the accepted background levels of other endotoxin assays.
Detection of fibronectin
After obtaining the cytosol and the extracellular matrix of cultured alveolar type II cells, the fibronectin content was measured using a quantitative sandwich enzyme immunoassay technique. A polyclonal antibody specific for fibronectin (Sigma) was pre-coated onto a microplate. The fibronectin in standards and samples was sandwiched by the immobilized antibody and a biotinylated polyclonal antibody specific for fibronectin. After unbound material was removed by washing, the wells were treated with a streptavidin-peroxidase conjugate and then a peroxidase enzyme substrate. Absorbance was determined at a wavelength of 450 nm. Each sample was analyzed in triplicate and the data expressed relative to the number of type II cells attached to the dish before extraction. Similar results were obtained by densitometry analysis of western blots using polyclonal antibodies directed against fibronectin.
Electroporation and Luciferase Assays
Electroporation of U937 cells was used to introduce the IL-1β promoter construct, pIL-1(−4.0kb)LUC. Briefly, cells were washed with PBS and added to serum free media supplemented with 10 mM dextrose and 0.1 mM DTT to a final concentration of 6 × 107 cells/ml. U937 cells (4.8 × 107 cells) were added to electroporation cuvettes (0.4 cm electrode gap) along with 40 μg of promoter construct plasmid DNA, 20 μg of the β-galactosidase reporter plasmid DNA, and subjected to 400 v and 1075 μF (Gene Pulser II Electroporation System, Bio Rad, Hercules, CA). Electroporated cells were pooled, aliquoted into 24 well plates, and incubated on non-coated or matrix-coated plates for 24–48 h at 37° C and 5% CO2. In antibody studies, cells were preincubated for 1 h at 37°C and 5% CO2 with anti-α5 (PID6, 100 μg/ml), anti-β1 (P4C10, 100 μg/ml) or control IgG antibodies. Cells were harvested, washed, resuspended in 100 μl cell lysis buffer and a 20 μl aliquot was tested for luciferase activity by adding 50 μl Luciferase Assay Reagent (Promega, Madison, WI). Light intensity was measured using a ThermoLabsystems Luminoskan Ascent microtiter plate luminometer. Results were recorded as relative luciferase units and normalized for transfection efficiency using β-galactosidase activity.
Electrophoresis Mobility Shift Assay (EMSA)
U937 cells (1 × 108) were cultured on the extracellular matrix from type II cells derived from control or ethanol-fed rats at 37°C in a 5% CO2 incubator for 20 h. The U937 cells were washed with ice cold PBS, and nuclear binding proteins were extracted by a published method (Dignam et al., 1983). Protein concentrations were determined by the Bradford method using BioRad protein assay reagent (Bradford, 1976). Double-stranded NF-κB consensus oligonucleotide was radiolabeled with 32P gamma ATP using T4 polynucleotide kinase enzyme. Nuclear protein (5 μg) was incubated with radiolabeled NF-κB (50–100,000 cpm/ng) for 30 min at room temperature as described previously (Michaelson et al., 2002). For competition reactions, 50 fold molar excess of double-stranded NF-κB (5′ AGTTGAGGGGACTTTCCCAGGC) consensus oligonucleotide or double stranded mutated NF-κB oligonucleotide (5′ AGTTGAGGCGACTTTCCCAGGC) was added to the reaction. DNA-protein complexes were separated on 6% native polyacrylamide gel (20:1 acrylamide/bis ratio) in low ionic strength buffer (22.25 mM Tris borate, 22.25 mM boric acid, 500 mM EDTA) for 2–3 h at 4°C at 10 V/cm. Gels were fixed in a 10% acid acid/10% methanol solution for 10 min, dried under vacuum and exposed to X-ray film.
Statistical evaluation
Means plus standard deviation of the mean were calculated for all experimental values. Significance was assessed by ANOVA followed by a Student Newman Keul’s test for post-hoc analysis with a p ≤ 0.05 accepted as statistical significance. All experiments were repeated 4–9 times.
RESULTS
Alveolar type II cells harvested from ethanol-fed rats produce a fibronectin-enriched matrix
Type II cells were harvested from rats fed with the Lieber DiCarli diet for 6 weeks. After 96 h of culture, >95% were type II cells as confirmed by immunohistochemistry for surfactant apoprotein C (data not shown). When compared to controls, the fibronectin content of the type II cellular extract (minus the matrix component) was increased 4.3-fold when the cells were derived from ethanol-fed rats (Figure 1A). Similarly, the fibronectin content of the extracellular matrix deposited by type II cells was increased by 3.9-fold when the cells were derived from ethanol-fed rats (Figure 1B).
Figure 1. Fibronectin content is increased in the cellular extract (A) and in the extracellular matrix (B) of alveolar type II cells harvested from ethanol-fed animals.

Alveolar epithelial type II cells were harvested from ethanol-fed and control mice. After 48 h of culture on plastic, the cellular contents of the type II cells were first extracted and the extracellular matrix deposited onto the plastic was extracted. The fibronectin content of the cellular extract and the extracellular matrix was determined by a sandwich ELISA and normalized to the cell number obtained at the time of extraction. Normalized data are expressed as means ± SD of 11 independent experiments. For statistical analysis, an a denotes p ≤ 0.05 when compared to the control.
Matrices derived from alveolar epithelial type II cells harvested from ethanol-fed rats promote the activation of U937 cells
The above findings suggested that extracellular matrices derived from type II cells harvested from ethanol-fed rats contained increased amounts of fibronectin. Based on this, we speculated that immune cells exposed to this matrix would respond differently than when exposed to control matrices. To test this, type II cells harvested from ethanol-fed and control-fed animals were allowed to deposit a matrix for 96 hours and then the cells extracted. U937 cells were then applied atop the extracellular matrix deposited by type II cells and cultured for another 24–48 hours. In these experiments, the U937 cells were transfected with a DNA construct containing the human IL-1β promoter fused to a luciferase reporter gene. After culture on the type II cell matrix, the U937 cells were harvested and tested for luciferase expression as a surrogate marker of IL-1β expression. When cultured on a matrix derived from type II cells obtained from ethanol-fed rats, IL-1β reporter activity by U937 was increased over 2-fold when compared to the matrix from control type II cells (Figure 2A). The capacity of the matrix from alcoholic type II cells to enhance IL-1β reporter activity in U937 cells was associated with an increase in DNA binding by NF-κB, a transcription factor known for its ability to stimulate IL-1β expression (Figure 2B).
Figure 2. Effects of type II cell matrix on U937 production of IL-1β (A) and NF-κB activation (B).

A, U937 cells were transfected with a DNA construct containing the human IL-1β promoter fused to a luciferase reporter gene. These U937 cells were then cultured onto the extracellular matrix deposited by type II cells derived from rats fed the control or ethanol diet. The luciferase units were determined and normalized for transfection efficiency using β-galactosidase activity. The bars represent the mean ± SD. The a denotes p ≤ 0.05 when compared to the U937 cells plated onto a matrix from control type II cells. B, After culturing on the matrix of type II cells from control or ethanol-fed rats, the nuclear proteins of the U937 cells were extracted for Electrophoresis Mobility Shift Assay to determine DNA binding by NF-κB. Non-radiolabeled consensus and mutated NF-κB double-stranded oligonucleotides were added to the reaction mixture at 50x molar concentration for competition reactions. DNA-protein complexes were separated on 6% native polyacrylamide gel for 2–3 h at 4°C at 10 V/cm. Gels were fixed, dried under vacuum and exposed to X-ray film.
In other work (Roman et al., 2000), we showed that fibronectin stimulated IL-1β expression in U937 cells through signals transduced by the integrin α5β1, a fibronectin receptor responsible for many of the biological effects of fibronectin (Midwood et al., 2006). To test whether the effect observed was mediated through fibronectin and α5β1 interactions, U937 cells were pretreated with antibodies against the α5 and β1 subunits of the integrin receptor or control IgG for 1 hour before plating onto the type II cell matrices. The IL-1β reporter activity after 48 hours showed that, as expected, the anti-α5 integrin antibody significantly reduced the IL-1β upregulation induced by the matrix derived from alcoholic type II cells (Figure 3). In parallel experiments, the U937 cells were pretreated with an antibody directed against the β1 integrin subunit before they were plated onto the type II cell matrices. Preincubation with a control IgG antibody was not able to diminish the effects of the matrix derived from alcoholic type II cells. These results suggested that the capacity of the alcoholic matrix to activate U937 cells were at least partially mediated via fibronectin-α5 interactions on the monocytic cells.
Figure 3. The role of the α5β1 integrin in matrix activation of U937 cells.
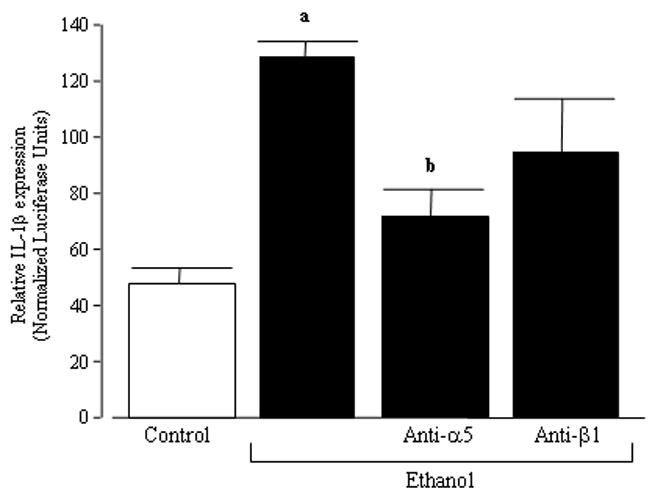
U937 cells were transfected with a DNA construct containing the human IL-1β promoter fused to a luciferase reporter gene. Transfected U937 cells were then pretreated with a control IgG antibody, an antibody directed against the α5 integrin subunit, or an antibody directed against the β1 integrin subunit. The U937 cells were then cultured onto the extracellular matrix deposited by type II cells derived from rats fed the control or ethanol diet. The luciferase units were determined and normalized for transfection efficiency using β-galactosidase activity. The bars represent the mean ± SD. An a denotes p ≤ 0.05 when compared to the U937 cells plated onto a matrix derived from control type II cells. A b denotes p ≤ 0.05 when compared to the U937 cells plated onto a matrix deposited by type II cells derived from ethanol-fed rats and pretreated with control IgG.
Antioxidants inhibit the effects of ethanol on the alveolar epithelial type II cell matrix
In previous studies, we demonstrated that addition of antioxidants like NAC and PRO to the ethanol diet not only attenuated chronic oxidant stress, but also inhibited many of the effects of ethanol on type II cells in vitro and on acute lung injury in vivo (Holguin et al., 1998) (Guidot et al., 1999; Guidot and Brown, 2000; Guidot et al., 2000; Lois et al., 1999). Thus, we postulated that ethanol-induced oxidant stress could be responsible for the altered matrix deposition by type II cells and that antioxidant therapy would inhibit or, at least, attenuate the aberrant matrices. To test this, type II cells were harvested from rats fed the ethanol diet supplemented with NAC or PRO for 6 weeks. Addition of NAC or PRO to the ethanol diet significantly attenuated the cellular fibronectin content of type II cells (Figure 4A). Furthermore, dietary supplements of NAC or PRO normalized the ethanol-induced exaggerated fibronectin deposition (Figure 4B). We next examined whether the matrix derived from animals fed ethanol plus antioxidants altered the reporter activity of IL-1β. In this series of experiments, IL-1β reporter activity was at control values if the U937 cells were plated onto matrices derived from type II cells harvested from rats fed ethanol plus the antioxidant NAC or PRO (Figure 5).
Figure 4. Antioxidants diminish fibronectin deposition into the cellular extract (A) and extracellular matrix (B) of alveolar type II cells harvested from ethanol-fed animals.

Rats were fed control, ethanol, ethanol + NAC, or ethanol + PRO diets for 6 wk. Type II cells were isolated and cultured on tissue culture plastic for 48 h. After the cellular contents of the type II cells were first eradicated, the extracellular matrix deposited onto the plastic was extracted. The fibronectin content of the cellular extract and the extracellular matrix was determined by a sandwich ELISA and normalized to the cell number obtained at the time of extraction. Normalized data are expressed as means ± SD and represent 11 independent experiments. For statistical analysis, an a denotes p ≤ 0.05 when compared to the control and b denotes p ≤ 0.05 when compared to the ethanol alone group.
Figure 5. Antioxidants attenuate the effects of type II cell matrix on U937 production of IL-1β.

U937 cells were transfected with a DNA construct containing the human IL-1β promoter fused to a luciferase reporter gene. These U937 cells were then cultured onto the extracellular matrix deposited by type II cells derived from rats fed the control, ethanol, ethanol + NAC, or ethanol + PRO diets. The luciferase units were determined and normalized for transfection efficiency using β-galactosidase activity. The bars represent the mean ± SD. The a denotes p ≤ 0.05 when compared to the U937 cells plated onto a matrix from control type II cells and b denotes p ≤ 0.05 when compared to the U937 cells plated into type II cells from the ethanol alone group.
DISCUSSION
Previous studies by this research group demonstrated in a rodent model that chronic ethanol ingestion increased fibronectin expression and deposition in lung tissue (Roman et al., 2005). In that study, physiologically relevant concentrations of ethanol or its metabolites stimulated lung fibroblasts to produce fibronectin via an as yet unidentified nicotinic acetylcholine receptor. Intense staining of the alveolar septae at the corners suggested that chronic ethanol ingestion also promoted fibronectin synthesis by alveolar type II cells. That observation was verified in the current study. When type II cells were derived from ethanol-fed rats, there was approximately a 5-fold increase in fibronectin content of both the cells as well as the extracellular matrix when compared to cells from pair-fed rats. The mechanism by which chronic ethanol increases fibronectin synthesis and deposition by type II cells are unclear. However, previous studies by this research group have demonstrated that chronic ethanol ingestion increases oxidant stress in type II cells (Brown et al., 2001a; Brown et al., 2001b) as well as TGF-β (Bechara et al., 2004). Both oxidant stress (Maniscalco et al., 1998) and TGF-β (Maniscalco et al., 1994) have been shown to increase fibronectin synthesis by type II cells; therefore, this might explain the observed increase in susceptibility to acute lung injury in the setting of chronic ethanol exposure. Thus, chronic ethanol ingestion altered matrix formation by alveolar epithelial type II cells to include enhanced synthesis and incorporation of fibronectin into the extracellular matrix.
Fibronectin is chemotactic to monocytes and stimulates the expression of pro-inflammatory cytokines. Thus, given that cell-substratum interactions can alter cell functions, we next examined whether increased incorporation of fibronectin as a constituent of the type II cell substratum could also activate other cell types. In order to address this issue, the cellular contents of the type II cells were extracted to leave the cell-derived matrix attached to the plastic. When U937 cells were plated onto that extracellular matrix generated by alcoholic type II cells, IL-1β promoter activity was increased 2.5-fold over that expressed by U937 cells plated onto a matrix from control cells. As expected, the matrix derived from alcoholic cells promoted IL-1β promoter activity by U937 cells and this coincided with increased DNA binding by NF-κB. Therefore, the fibronectin-enriched extracellular matrix generated by alcoholic type II cells is primed to activate monocytes that come in contact with it.
The activation of monocytes by matrices is dependent on their interactions with surface integrins, a group of heterodimeric transmembrane adhesion receptors that mediate the attachment of monocytes to matrix proteins such as fibronectin, collagen and laminin. One fibronectin receptor on monocytes is the α5β1 integrin, which mediates many of the biological effects of fibronectin (Roman et al., 2005). In the current study, pretreatment of monocytes with antibodies directed against the α5 subunit attenuated the increase in IL-1β expected when monocytes were plated onto the alcoholic type II cell matrix. In contrast, IL-1β synthesis was also reduced when the monocytes were pretreated with a β1 antibody before plating onto the alcoholic type II cell matrix. The fact that IL-1β activity was not normalized suggests that integrins other than β1 may also play a role in this activation. These results further supported the suggestion that ethanol-induced enrichment of the alveolar epithelial type II cell matrix with fibronectin can translate into monocytic cell activation and facilitate their migration into the alveolar space in the setting of lung injury (Guidot and Roman, 2002). Additional studies are needed to determine whether these effects of ethanol on type II cells are elicited via the nicotinic acetylcholine receptor similar to that observed in lung fibroblasts (Roman et al., 2005).
As discussed above, chronic ethanol ingestion is associated with chronic oxidant stress in alveolar type II cells, a potential mechanism for increased fibronectin synthesis and deposition. In previous studies, addition of glutathione precursors NAC or PRO to the ethanol diet normalized oxidant stress in the type II cells as well as cell functions such as viability and surfactant synthesis (Brown et al., 2001b; Guidot and Brown, 2000). In the current study, dietary supplements of NAC or PRO normalized fibronectin synthesis and deposition. In previous studies, we demonstrated that NAC supplements prevented ethanol-induced cytosolic oxidant stress, but not mitochondrial stress (Brown et al., 2001b). In contrast, PRO supplements protected both the cytosolic and the mitochondrial pools against ethanol-induced oxidant stress (Brown et al., 2001b). The capacity of NAC to prevent ethanol-induced fibronectin synthesis and deposition suggests that these events are modulated by cytosolic rather than mitochondrial oxidant stress. The capacity of NAC or PRO to normalize type II cell fibronectin deposition resulted in normalization of U937 activation and IL-1β promoter activity.
In summary, these studies suggest that type II cells contribute to the exaggerated deposition of fibronectin in the lungs of ethanol-fed rats. This newly deposited fibronectin-containing substratum in likely to prime incoming monocytes and exacerbates proinflammatory cytokine production after injury. This excess production of fibronectin-containing matrices in the setting of chronic ethanol ingestion might contribute to the development of acute lung injury by stimulating unopposed inflammation. Thus, ethanol-induced chronic oxidant stress may promote the generation of a new matrix that, without disrupting the macroscopic lung architecture, affects the function of resident and recruited cells. Further delineation of the factors and conditions that regulate ethanol-induced fibronectin expression by alveolar type II cells and the response of monocytic cells through their interaction with that substratum are required before a full understanding of the true consequences of this process in the lung is possible.
Acknowledgments
This study was funded by NIH NIAAA grant R01 AA12197 (LAB), NIH NHLBI grant R01 HL67399 (LAB), and NIAAA Center Grant P50 AA 135757 (LAB, DMG and JR). This work was also supported by the Department of Defense Grant DAMD17-02-1-0179 (JR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechara RI, Brown LA, Eaton DC, Roman J, Guidot DM. Chronic ethanol ingestion increases expression of the angiotensin II type 2 (AT2) receptor and enhances tumor necrosis factor-alpha- and angiotensin II-induced cytotoxicity via AT2 signaling in rat alveolar epithelial cells. Alcohol Clin Exp Res. 2003;27:1006–1014. doi: 10.1097/01.ALC.0000071932.56932.53. [DOI] [PubMed] [Google Scholar]
- Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med. 2004;170:188–194. doi: 10.1164/rccm.200304-478OC. [DOI] [PubMed] [Google Scholar]
- Bitterman P, Rennard S, Adelberg S, Crystal RG. Role of fibronectin in fibrotic lung disease. A growth factor for human lung fibroblasts. Chest. 1983;83:96S. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72 doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Bechara R, Guidot DM. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alcohol Clin Exp Res. 2001a;25:1078–1085. [PubMed] [Google Scholar]
- Brown LA, Harris FL, Guidot DM. Chronic ethanol ingestion potentiates TNF-alpha-mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol Lung Cell Mol Physiol. 2001b;281:L377–386. doi: 10.1152/ajplung.2001.281.2.L377. [DOI] [PubMed] [Google Scholar]
- Cordes N, van Beuningen D. Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3beta (GSK-3beta) in vitro. Br J Cancer. 2003;88:1470–1479. doi: 10.1038/sj.bjc.6600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucl Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LG, Gonzalez GR, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- Dunsmore SE, Lee YC, Martinez-Williams C, Rannels DE. Synthesis of fibronectin and laminin by type II pulmonary epithelial cells. Am J Physiol. 1996a;270:L215–223. doi: 10.1152/ajplung.1996.270.2.L215. [DOI] [PubMed] [Google Scholar]
- Dunsmore SE, Martinez-Williams C, Goodman RA, Rannels DE. Composition of extracellular matrix of type II pulmonary epithelial cells in primary culture. Am J Physiol. 1996b;269:L754–L765. doi: 10.1152/ajplung.1995.269.6.L754. [DOI] [PubMed] [Google Scholar]
- Engel J. Domain organizations of modular extracellular matrix proteins and their evolution. Matrix Biol. 1996;15:295–299. doi: 10.1016/s0945-053x(96)90130-4. [DOI] [PubMed] [Google Scholar]
- Guidot D, Moss M, Holguin F, Lois M, Brown L. Ethanol ingestion impairs alveolar epithelial glutathione homeostasis and function, and predisposes to endotoxin-mediated acute lung injury. Chest. 1999;116:82S. doi: 10.1378/chest.116.suppl_1.82s. [DOI] [PubMed] [Google Scholar]
- Guidot DM, Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res. 2000;24:1070–1076. [PubMed] [Google Scholar]
- Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol. 2000;279:L127–135. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- Guidot DM, Roman J. Chronic ethanol ingestion increases susceptibility to acute lung injury: role of oxidative stress and tissue remodeling. Chest . 2002;122(6 Suppl):309S–314S. doi: 10.1378/chest.122.6_suppl.309s. [DOI] [PubMed] [Google Scholar]
- Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest. 1998;101:761–768. doi: 10.1172/JCI1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, Yu MR, Song JS, Ha H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int. 1004;65:1170–1179. doi: 10.1111/j.1523-1755.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- Lee YC, Rannels DE. Regulation of extracellular matrix synthesis by TNF-alpha and TGF-beta1 in type II cells exposed to coal dust. Am J Physiol. 1998;275:L637–644. doi: 10.1152/ajplung.1998.275.4.L637. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Biochemical factors in alcoholic liver disease. Sem Liver Dis. 1993;13:136–153. doi: 10.1055/s-2007-1007345. [DOI] [PubMed] [Google Scholar]
- Lois M, Brown LA, Moss IM, Roman J, Guidot DM. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am J Respir Crit Care Med. 1999;160:1354–1360. doi: 10.1164/ajrccm.160.4.9811060. [DOI] [PubMed] [Google Scholar]
- Maniscalco WM, Sinkin RA, Watkins RH, Campbell MH. Transforming growth factor-beta1 modulates type II cell fibronectin and surfactant protein C expression. Am J Physiol. 1994;267:L569–L577. doi: 10.1152/ajplung.1994.267.5.L569. [DOI] [PubMed] [Google Scholar]
- Maniscalco WM, Watkins RH, Chess PR, Sinkin RA, Horowitz S, Toia L. Cell-specific expression of fibronectin and EIIIA and EIIIB splice variants after oxygen injury. Am J Physiol. 1998;274:L599–609. doi: 10.1152/ajplung.1998.274.4.L599. [DOI] [PubMed] [Google Scholar]
- Michaelson J, Ritzenthaler J, Roman J. Regulation of serum-induced fibronectin expression by protein kinases, cytoskeletal integrity, and CREB. Am J Physiol Lung Cell Mol Physiol. 2002;282:L291–310. doi: 10.1152/ajplung.00445.2000. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell-fibronectin matrix interactions during tissue repair. J Invest Dermatol. 2006;126(Suppl):73–78. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of Acute Respiratory Distress Syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- Moss M, Parsons P, Steinberg K, Hudson L, Guidot D, Burnham E, Cotsonis G. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- Park SK, Kim J, Seomun Y, Choi J, Kim DH, Han IO, Lee EH, Chung SK, Joo CK. Hydrogen peroxide is a novel inducer of connective tissue growth factor. Biochem Biophys Res Commun. 2001;284:966–971. doi: 10.1006/bbrc.2001.5058. [DOI] [PubMed] [Google Scholar]
- Perez RL, Roman J, Staton GW, Jr, Hunter RL. Extravascular coagulation and fibrinolysis in murine lung inflammation induced by mycobacterial cord factor trehalose-6,6-dimycolate. Am J Respir Crit Care Med. 1994;149:510–518. doi: 10.1164/ajrccm.149.2.8306054. [DOI] [PubMed] [Google Scholar]
- Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophageS. Cell Immunol. 1994;156:191–211. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin Chest Med. 2001;22:433–449. doi: 10.1016/s0272-5231(05)70282-3. viii. [DOI] [PubMed] [Google Scholar]
- Rannels SR, Fisher CS, Heuser LJ, Rannels DE. Culture of type II pneumocytes on a type II cell-derived fibronectin-rich matrix. Am J Physiol. 1987;253:C759–765. doi: 10.1152/ajpcell.1987.253.6.C759. [DOI] [PubMed] [Google Scholar]
- Roman J. Extracellular matrix and lung inflammation. Immunol Res. 1996;15:163–178. doi: 10.1007/BF02918505. [DOI] [PubMed] [Google Scholar]
- Roman J, Ritzenthaler J, Fenton M. Regulation of IL-1b gene transcription by fibronectin in human monocytic cells. Role of protein kinase C and AP-1. Cytokine. 2000;12:1581–1596. doi: 10.1006/cyto.2000.0759. [DOI] [PubMed] [Google Scholar]
- Roman J, Ritzenthaler JD, Bechara R, Brown LA, Guidot D. Ethanol stimulates the expression of fibronectin in lung fibroblasts via kinase-dependent signals that activate CREB. Am J Physiol Lung Cell Mol Physiol. 2005;288:L975–987. doi: 10.1152/ajplung.00003.2004. [DOI] [PubMed] [Google Scholar]
- Sloane PJ, Gee MH, Gottlieb JE, Albertine KH, Peters SP, Burns JR, Machiedo G, Fish JE. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. Am Rev Respir Dis. 1992;146:419–426. doi: 10.1164/ajrccm/146.2.419. [DOI] [PubMed] [Google Scholar]
- Velasquez A, Bechara RI, Lewis JF, Malloy J, McCaig L, Brown LA, Guidot DM. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- Vesey DA, Cheung C, Endre Z, Gobe G, Johnson DW. Role of protein kinase C and oxidative stress in interleukin-1beta-induced human proximal tubule cell injury and fibrogenesis. Nephrology (Carlton) 2005;20:73–80. doi: 10.1111/j.1440-1797.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, Freyer AM, Corteling RL, Hall IP. Biosynthesized matrix provides a key role for survival signaling in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L596–603. doi: 10.1152/ajplung.00217.2003. [DOI] [PubMed] [Google Scholar]


