Abstract
Introduction
Recent studies suggest that bone marrow adipose tissue (BMAT) might play a role in the pathogenesis of osteoporosis. Previous research using regional magnetic resonance spectroscopy methods to measure BMAT has reported inconsistent findings on the relationship between BMAT and dual-energy absorptiometry (DXA)-measured bone mineral density (BMD).
Methods
In the present study, total body and pelvic BMAT were evaluated in 56 healthy women (age 18–88 yrs, mean±SD, 47.4±17.6 yrs; BMI, 24.3±4.2 kg/m2) with T1-weighted whole-body magnetic resonance imaging (MRI). BMD was measured using the whole-body DXA mode (GE Lunar DPX, software version 4.7).
Results
A strong negative correlation was observed between pelvic BMAT and BMD (total-body BMD, R=− 0.743, P<0.001; pelvic BMD, R=− 0.646, P<0.001), and between total-body BMAT and BMD (total-body BMD, R=− 0.443, P<0.001; pelvic BMD, R=− 0.308, P < 0.001). The inverse association between pelvic BMAT and BMD remained strong after adjusting for age, weight, total body fat, and menopausal status (partial correlation: total-body BMD, R=− 0.553, P< 0.001; pelvic BMD, R=− 0.513, P<0.001). BMAT was also highly correlated with age (pelvic BMAT, R=0.715, P< 0.001; total-body BMAT, R=0.519, P<0.001).
Conclusion
MRI-measured BMAT is thus strongly inversely correlated with DXA-measured BMD independent of other predictor variables. These observations, in the context of DXA technical concerns, support the growing evidence linking BMAT with low bone density.
Keywords: Body composition, Bone marrow, Bone mineral density, Dual-energy absorptiometry, Magnetic resonance imaging
Introduction
The relationship between bone and fat is complex and not yet fully understood [1]. Recent studies suggest that bone marrow adipose tissue (BMAT) might play a role in the pathogenesis of osteoporosis [2–4]. Most previous research using regional magnetic resonance spectroscopy (MRS) methods to measure BMAT reported inconsistent findings on the relationship between BMAT and dual-energy absorptiometry (DXA)-measured bone mineral density (BMD). Although considered sensitive and accurate for detecting bone marrow fat [5], MRS methods are usually limited to measuring BMD in small volumes (1–1.25 cm3 to 8–17 cm3) of bone marrow within the L3 vertebra [3, 6–8]. MRS thus functions as a noninvasive “biopsy” method and the extent to which bone marrow adipose tissue distribution is homogeneous remains unknown [9]. Lack of adipose tissue homogeneity in the cavity of one bone or across the whole body might be one of the reasons why there are inconsistent reports on the relationship between BMAT and BMD. The aim of the present study was to investigate the relationships between BMAT and BMD. To overcome the regional sampling limitations of MRS, we employed MRI to quantify regional and whole-body BMAT. We chose a sample of Caucasian women for this initial study because of the high osteoporosis prevalence in this group [8].
Methods
Protocol and design
Subjects were a convenience sample of healthy Caucasian females, over the age of 18 years, who completed a screening medical history, physical examination, and blood studies. Race was established in each subject by self-report. Subjects weighing more than 136.2 kg (300 pounds) were excluded from the study due to the weight limits of MRI and DXA systems. Weight, height, and body composition were evaluated on the same day as the screening examination.
Dual-energy x-ray absorptiometry
Dual-energy x-ray absorptiometry (DPX GE Lunar, software version 4.7e, Madison, WI) was used to estimate BMD, total body fat, and percent fat with the whole-body scan mode. All scans were acquired and read by trained technologists. In addition to total-body BMD, we report pelvic BMD since this site approximates the pelvic region used to measure BMAT by MRI. We also report total spine BMD since the lumbar spine BMD was not available in this group of subjects. The estimated precision levels for BMD and percent fat are 1.28 and 3.3%, respectively [10]. The system was routinely calibrated, and quality control measures were followed as recommended by the manufacturer.
Magnetic resonance imaging
Whole-body MRI scans were acquired, as previously reported by our group [11, 12], using a 1.5 T General Electric system (6X Horizon, Milwaukee, WI). The protocol involved acquisition of approximately forty 10-mm-thick axial images at 40-mm intervals from fingers to toes. The subject rested in either a prone or supine position during the procedure with the L4–L5 intervertebral disc as the point of origin. Following acquisition, the bone marrow, visceral, and subcutaneous adipose tissue compartments were segmented at the New York Image Reading Center by trained, quality-controlled and cross-validated technicians using image analysis software (SliceOmatic, Tomovision Inc., Montreal, Canada) (Fig. 1). Technicians were blinded to patient demographic information and test results. From a total of 59 subjects, 3 were excluded because of severe inhomogeneity of the MRI adipose tissue signal. The remaining 56 subjects were included in the study. The threshold for BMAT segmentation was set at 201 on the gray scale (the same level as subcutaneous adipose tissue). BMAT in ribs and skull was not included because of the difficulty in differentiating this component from adjacent intramuscular or subcutaneous adipose tissue. The intraclass correlation coefficients for volume rendering of bone marrow, subcutaneous and visceral adipose tissue for the same scan by different analysts were 0.99, 0.99, and 0.95, respectively. Adipose tissue compartment volume was calculated as:
Fig. 1.
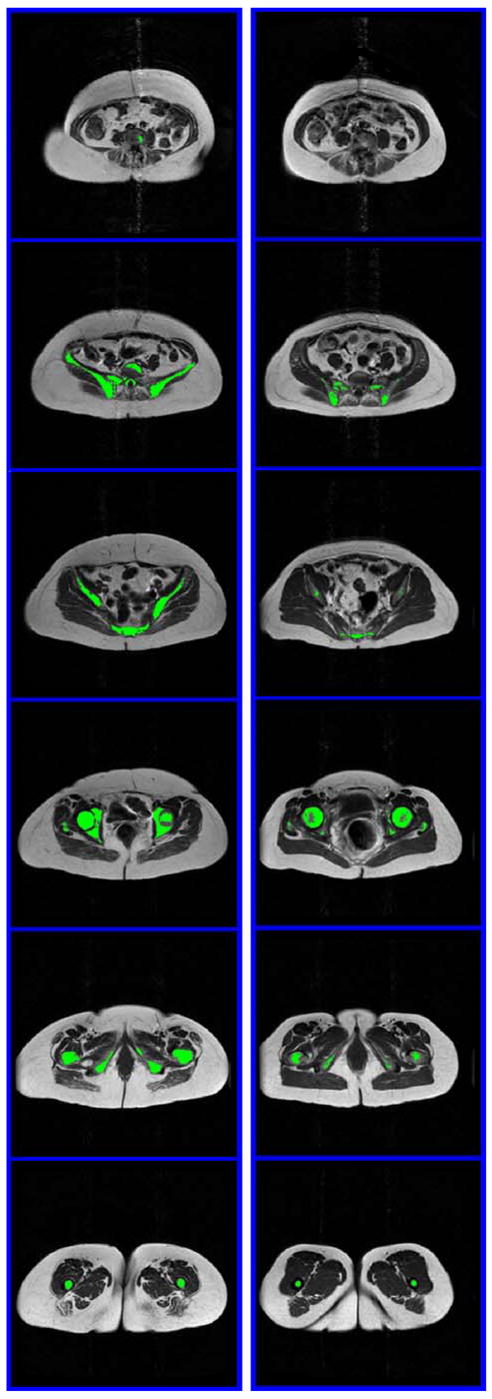
Example of segmented MRI of the pelvic region of a subject with a large volume of BMAT and lower BMD (left, 63 years old, body weight 69.4 kg, BMI 30.1 kg/m2, total–body BMD 0.95 g/cm2) and one with a small amount of BMAT and higher BMD (right, 59 years old, body weight 75.4 kg, BMI 26.9 kg/m2, BMD 1.19 g/cm2). The six images in each subject were acquired every 5 cm and extended from L4–L5 to the separation of legs. BMAT is highlighted in green
where V is volume, Ai is each scan’s cross-sectional area, h is the between-slice interval, t is the thickness of each slice, and N is the number of total slices. Pelvic BMAT volumes were calculated from scans of the pelvic region, defined as all slices between the L4–L5 level and the level of separation of legs from trunk, including the femoral heads. Pelvic BMAT is a part of total-body BMAT and the pelvic region was selected for evaluation because of the large quantifiable cancellous bone volume in this region. Spine BMAT is not reported separately as the small amount of adipose tissue in the spine could not be reliably quantified in some subjects with the current MRI protocol.
Statistical methods
Data are presented as the mean±SD. Pearson correlation coefficients among total-body and pelvic BMAT; total-body, pelvic and spine BMD; visceral and subcutaneous adipose tissue; total body fat; BMI; and age were calculated. Correlation coefficients were also calculated between total-body and pelvic BMAT and total-body, pelvic and spine BMD after adjustment by multiple regression for age, weight, total-body fat, and menopausal status. Regression models were established with total-body BMD, pelvic BMD or spine BMD as the dependent variable and pelvic BMAT, age, weight, total-body fat, visceral adipose tissue, menopause status, and biologically plausible two-way interactions as potential independent variables. Variables and interactions with significant contributions to the model were retained in the regression equation. The intraclass correlation coefficient was calculated with subjects as a random effect and analysts as a fixed effect. All statistical analyses were carried out using SPSS (SPSS for Windows, 11.5, SPSS, Chicago, IL, USA). Two-tailed (α =0.05) tests of significance were used.
Results
Descriptive statistics
The characteristics of the subjects are shown in Table 1. The women ranged in age from 18–88 years with a mean age of 47.4 years. Body weight ranged from 42.3–87.5 kg, and the mean group body weight was 64.9 kg. Body mass index ranged from 15.9–38.9 kg/m2 and the women had a mean BMI of 24.3 kg/m2. Of the 56 total women, 33 were pre-menopausal and 23 were post-menopausal. Total-body BMD in the women ranged from 0.92–1.39 g/cm2 with a mean BMD of 1.14 g/cm2. Total-body BMAT ranged from 0.73–2.33 L with a mean of 1.48 L. The percentage of total adipose tissue as BMAT ranged from 2.5–19.6% with a mean of 6.6%.
Table 1.
Subject characteristics (n=56)
| Descriptive statistics | |
|---|---|
| Age (yrs) | 47.4±17.6 |
| Weight (kg) | 64.9±11.8 |
| BMI (kg/m2) | 24.3±4.2 |
| Total–body bone mineral density (g/cm2) | 1.14±0.12 |
| Pelvic bone mineral density (g/cm2) | 1.08±0.14 |
| Bone marrow adipose tissue (L) | 1.48±0.36 |
| Pelvic bone marrow adipose tissue (L) | 0.25±0.17 |
| Subcutaneous adipose tissue (L) | 22.4±9.0 |
| Visceral adipose tissue (L) | 1.52±1.34 |
| Total body fat (kg) | 21.2±9.7 |
Data are presented as mean±SD. BMI Body mass index
Relationship between BMAT and BMD
A high correlation was observed between pelvic BMAT and BMD (total-body BMD, R=−0.743, P<0.001; pelvic BMD, R=−0.646; spine BMD, R=−0.622, P<0.001) (Fig. 2), and between total-body BMAT and BMD (total-body BMD, R=−0.443, P<0.001; pelvic BMD, R=−0.308; spine BMD, R=−0.334, P<0.001) (Table 2). The association between pelvic BMAT and BMD remained strong even after adjusting for age, weight, total body fat, and menopausal status (total-body BMD, R=−0.553, P<0.001; pelvic BMD, R=−0.513; spine BMD, R=−0.491, P<0.001) (Table 3).
Fig. 2.
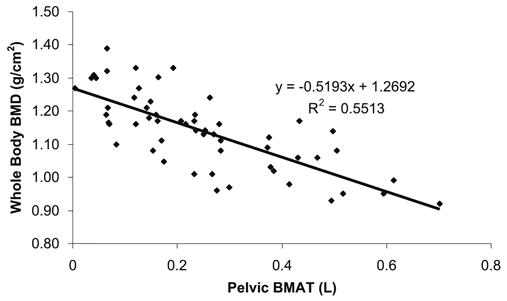
Correlation between total-body BMD and pelvic BMAT
Table 2.
Pearson correlation coefficients among BMD, BMAT, visceral and subcutaneous adipose tissue, total fat, BMI and age (n=56)
| Total-body BMD | Pelvic BMD | Spine BMD | Total-body BMAT | Pelvic BMAT | Age | Weight | BMI | |
|---|---|---|---|---|---|---|---|---|
| Pelvic BMD | 0.927* | – | – | – | – | – | – | – |
| Spine BMD | 0.854* | 0.866* | – | – | – | – | – | – |
| Total-body BMAT | −0.443* | −0.308* | −0.334* | – | – | – | – | – |
| Pelvic BMAT | −0.743* | −0.646* | −0.622* | 0.731* | – | – | – | – |
| Age | −0.604* | −0.462* | −0.420* | 0.519* | 0.715* | – | – | – |
| Weight | 0.273* | 0.390* | 0.156 | 0.123 | −0.015 | 0.054 | – | – |
| BMI | −0.010 | 0.113 | −0.050 | 0.072 | 0.144 | 0.277* | 0.851* | – |
| VAT | −0.360* | −0.171 | −0.257 | 0.429* | 0.483* | 0.603* | 0.542* | 0.721* |
| SAT | −0.002 | 0.118 | −0.039 | 0.150 | 0.212 | 0.275 | 0.879* | 0.926* |
| Total body fat | 0.027 | 0.159 | −0.008 | 0.143 | 0.210 | 0.281* | 0.891* | 0.920* |
BMD Bone mineral density, BMAT bone marrow adipose tissue, BMI body mass index, VAT visceral adipose tissue, SAT subcutaneous adipose tissue.
Asterisk indicates a significant difference from 0, P<0.05.
Table 3.
Partial correlation coefficients between BMD and BMAT, adjusted for age, weight, total body fat and menopausal status (n=56)
| Total–body BMD | Pelvic BMD | Spine BMD | Total BMAT | |
|---|---|---|---|---|
| Pelvic BMD | 0.897* | – | – | – |
| Spine BMD | 0.833* | 0.859* | ||
| Total–body BMAT | −0.343* | −0.243 | −0.222 | – |
| Pelvic BMAT | −0.553* | −0.513* | −0.491* | 0.709* |
BMD Bone mineral density, BMAT bone marrow adipose tissue. Asterisk indicates a statistial difference from 0, P<0.05.
Regression models were developed with total-body BMD, pelvic BMD or spine BMD as dependent variable, and pelvic BMAT, age, weight, total body fat, visceral adipose tissue, menopause status, and their biologically plausible two-way interactions as potential independent variables. The regression models are as follows: total-body BMD=1.007– 0.410(pelvic BMAT) +0.004(weight) −0.027(VAT), adjusted R2=0.647, P<0.001; total-body BMD=1.102–0.517(pelvic BMAT) –0.003(weight), adjusted R2=0.607, P<0.001; pelvic BMD=0.925–0.540(pelvic BMAT) + 0.004(weight), adjusted R2=0.546, P<0.001; spine BMD=1.226–0.612(pelvic BMAT), adjusted R2= 0.376, P<0.001). BMAT, age, weight, and visceral adipose tissue were selected for the regression model because of their relatively high correlations with BMD.
Relationship between BMAT and age and other adipose tissue depots
BMAT was also highly correlated with age (pelvic BMAT, R=0.715, P<0.001; total-body BMAT, R=0.519, P<0.001) (Fig. 3).
Fig. 3.
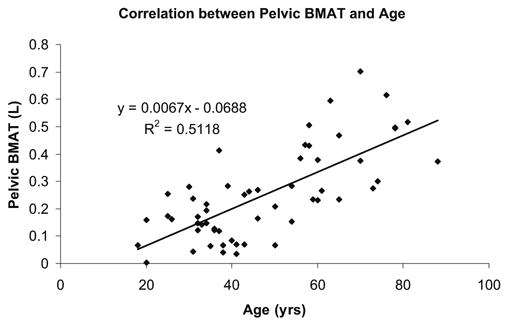
Correlation between pelvic BMAT and age
The correlation between BMAT and total body fat or subcutaneous adipose tissue did not reach significance, although the correlation coefficients were positive (R=0.143–0.255, P=0.058–0.388). BMAT correlated significantly with visceral adipose tissue (total BMAT, R=0.429, P<0.001; pelvic BMAT, R=0.483, P<0.001) without any adjustment for covariates. However, after adjusting for age and menopausal status, the correlation between BMAT and visceral adipose tissue was no longer significant (total BMAT, R=0.221, P=0.11; pelvic BMAT, R=0.066, P=0.63).
Discussion
Based on our review of the literature, the present study is the first to report measurements of total-body and pelvic bone marrow adipose tissue quantified by whole body MRI. Although our method uses a single threshold for BMAT and is thus semiquantitative, we have a preliminary estimate that BMAT accounts for about 7% of total adipose tissue. Compared to previous studies using spectroscopy methods, MRI provides an estimate of total BMAT instead of a small volume measured in a single bone. BMAT cannot be measured by DXA, and DXA may use an estimated BMAT to correct for the calculation of BMD and the amount of other tissue components.
Relationship between BMAT and BMD
Although we did not measure the femoral neck or lumbar spine with DXA, we observed a high correlation between BMAT and BMD. This inverse relationship remained strong for pelvic BMAT after controlling for age, weight, total body fat, and menopausal status. Previous studies reported a larger amount of MRS-measured vertebral BMAT in patients with osteoporosis and osteopenia [7, 8] and by iliac crest biopsy [13]. The correlation we observed between BMD and BMAT is also higher than previously reported in the femoral neck and lumbar vertebrae of men and women (e.g., femoral neck, R=0.32; lumbar vertebrae, R=0.36) [4] and a sample of men [8]. Except for sex-related body composition differences, the higher correlation between BMAT and pelvic BMD in our study may also be explained by our quantification of BMAT in the entire pelvic region using MRI methods rather than BMAT in one bone measured by MRS methods. Some recent studies also suggested a link between bone marrow fat and bone weakening in the absence of an inverse relationship between bone marrow fat and BMD with or without adjustment for age [3, 14]. Future studies need to further investigate the interplay between bone marrow fat, BMD, bone architecture and bone fracture.
There are several hypotheses on the role of BMAT in relation to loss of bone mineral. An increase in BMAT may simply reflect a passive compensation for trabecular bone loss, and fat helps to strengthen mineral-depleted bone [7, 13]. However, there is evidence that replacement of less compressible hemopoietic marrow with the more compressible fatty marrow may cause vertebral weakening [7]. Since osteoblasts and adipocytes share a common precursor in the bone marrow [15], it is hypothesized that an increase in adipogenesis conversely reduces osteoblastogenesis [2, 7, 16]. It is not clear whether there is a causal relationship or a temporal relationship between BMAT and BMD. Longitudinal studies may help to answer this question. We found that pelvic BMAT, but not total BMAT, had a high correlation with BMD. This may be because cortical bones in adults are filled with yellow marrow, and only vertebral, iliac marrow, and marrow at the ends of long bones remains hematopoietically active [17]. Our investigated pelvic bones included ilium, sacrum, ischium, pubis, coccyx and the femoral heads that are hematopoietically active, and an increase in BMAT in these regions may reflect physiological or pathological changes. Including BMAT in the diaphysis of long bones might dilute the changes in BMAT in the cavity formed by cancellous bone.
DXA method concerns
An important concern with the approach applied in present study is that the presence of BMAT may cause an underestimation of the DXA BMD readings. Bolotin et al. reported that in subjects with osteopenia and osteoporosis, an increase in bone marrow fat can cause severe underestimation of BMD, as much as 20–50% in extreme cases when there is a small amount of extraosseous fat [18]. Hangartner et al. reported that a 50% increase in marrow fat content could cause underestimation of the apparent areal bone density by 5–6% [19]. However, the same change in soft-tissue fat content (not in the path of the beam traversing the bone) causes a similar error with the opposite sign, that is, an error leading to overestimation of bone density [4, 18, 19]. Also, due to the longer pathway through soft tissue as compared with bone marrow, the unknown proportion of fat in the soft tissue along the beam path through the bone may influence the accuracy of results to a larger extent than the uncertainty of bone marrow fat content [19]. Considering that subjects in the present study had a mean whole body BMD of 1.14 g/cm2 and a mean subcutaneous fat of 22.4 L, it is unlikely that there was a major underestimation of BMD, if at all.
Relationship between BMAT, age, and total body fat
We did not observe a significant relationship between BMAT and total body fat. This finding is in agreement with the observation that BMAT does not play a role in overall fat metabolism [13]. Marrow adipocytes are not affected by long periods of starvation [20] or plasma insulin levels [21]. It has long been noticed that, with aging, the bones of adults fill with fat [7, 22]. The correlation we observed between pelvic BMAT and age is higher than that reported in a mixed sample of males and females as measured with biopsy methods (0.715 vs. 0.51) [13]. This difference may be accounted for by sex differences between our studies, our group being entirely female. Another possibility is that BMAT may be more variable when measured in small tissue volume biopsies of the iliac crest. The correlation we observed between age and bone marrow fat was also higher than that previously reported in the femoral neck (R=0.51) and L2–L4 lumbar vertebrae (R=0.47) [4]. This difference may be caused by the narrower age range (age 40–80 yrs) in the study of Wehrli et al. versus our subjects who ranged in age from 18–88 yrs.
Limitations and future directions
A limitation of the present study is that we did not have available DXA measurements for the femoral neck and lumbar spine to determine which individuals had osteoporosis [23]. A whole body or pelvic BMD may not reflect the loss of bone in other regions, such as the femoral neck or spine. In addition, the use of quantitative computed tomography (QCT) would have been advantageous since these data can differentiate cortical from cancellous bone [24]. Future studies should therefore include QCT methods as a means to expand our findings, and it would be especially interesting in an osteoporotic population.
A third limitation is that BMAT was semiquantitatively measured using the single threshold MRI methods applied in the current study. However, this aspect of the utililized MRI method should not detract from the overall study conclusions. From a statistical point of view, if a method is less accurate, the observed correlations will be reduced from their potential maximum. It is likely that application of even more accurate BMAT measurement methods would strengthen the observed associations between BMD and BMAT. Methods for quantitatively measuring BMAT are greatly improving and have the potential to expand the current study results. Methods of bone marrow fat measurement were reviewed by Mulkern et al. [25] who also measured average bone marrow fat in three vertebrae using MRS imaging methods. Different magnetic resonance methods were also reviewed by Brix et al. [26] who suggested that multiple spin-echo imaging techniques may be better as a quantitative tool than MRS and chemical shift imaging. Future studies may therefore tailor the applied BMAT magnetic resonance measurement methods to address the specific topic under investigation.
In the future, to better understand the relationship between BMAT and BMD at the whole body level, methods that can accurately quantify BMAT in cancellous bones, both on a regional and total body scale, need to be developed. These future studies ideally would examine if BMAT contributes to bone quality and fracture risk by examining the relationship with the density and architecture of both cortical and cancellous bone. Longitudinal studies may help to answer whether the relationship between BMAT and BMD is causal and the next step would be to test whether inhibiting adipogenesis in bone marrow can prevent osteoporosis. There are many factors influencing the differentiation of bone marrow mesenchymal stem cells through multi-pathways [27]. The application of combined molecular biology techniques would also help to both clarify the physiological role of bone marrow fat and to facilitate the prevention and treatment of osteoporosis [27].
Conclusions
MRI-measured BMAT is strongly correlated with DXA-measured BMD, suggesting a biological relationship between the two and thus a link with osteoporosis. Studies are needed in the future to explore the relationship between osteoblasts and BMAT adipocytes in the pathogenesis of osteoporosis. Future studies also need to establish the extent to which DXA measurement artifacts contribute to the observed BMAT–BMD associations.
Acknowledgments
This study was supported by National Institutes of Health Grants NIDDK R21 DK66360-01, R01 DK42618.
Contributor Information
W. Shen, Obesity Research Center, St. Luke’s-Roosevelt Hospital and Institute of Human Nutrition, Columbia University, 1090 Amsterdam Avenue, 14H, New York, NY 10025, USA
J. Chen, Obesity Research Center, St. Luke’s-Roosevelt Hospital and Institute of Human Nutrition, Columbia University, 1090 Amsterdam Avenue, 14H, New York, NY 10025, USA
M. Punyanitya, Obesity Research Center, St. Luke’s-Roosevelt Hospital and Institute of Human Nutrition, Columbia University, 1090 Amsterdam Avenue, 14H, New York, NY 10025, USA
S. Shapses, Nutritional Sciences Department, Rutgers University, New Brunswick, NJ, USA
S. Heshka, Obesity Research Center, St. Luke’s-Roosevelt Hospital and Institute of Human Nutrition, Columbia University, 1090 Amsterdam Avenue, 14H, New York, NY 10025, USA
S. B. Heymsfield, Merck & Co., Rahway, NJ, USA
References
- 1.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 2.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102:341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 3.Shih TT, Chang CJ, Hsu CY, Wei SY, Su KC, Chung HW. Correlation of bone marrow lipid water content with bone mineral density on the lumbar spine. Spine. 2004;29:2844–2850. doi: 10.1097/01.brs.0000147803.01224.5b. [DOI] [PubMed] [Google Scholar]
- 4.Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217:527–538. doi: 10.1148/radiology.217.2.r00nv20527. [DOI] [PubMed] [Google Scholar]
- 5.Schick F, Duda SH, Lutz O, Claussen CD. Lipids in bone tumors assessed by magnetic resonance: chemical shift imaging and proton spectroscopy in vivo. Anticancer Res. 1996;16:1569–1574. [PubMed] [Google Scholar]
- 6.Schellinger D, Lin CS, Fertikh D, Lee JS, Lauerman WC, Henderson F, Davis B. Normal lumbar vertebrae: anatomic, age, and sex variance in subjects at proton MR spectroscopy-initial experience. Radiology. 2000;215:910–916. doi: 10.1148/radiology.215.3.r00jn42910. [DOI] [PubMed] [Google Scholar]
- 7.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–1627. [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–951. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 9.Vande Berg BC, Malghem J, Lecouvet FE, Maldague B. Magnetic resonance imaging of normal bone marrow. Eur Radiol. 1998;8:1327–1334. doi: 10.1007/s003300050547. [DOI] [PubMed] [Google Scholar]
- 10.Russell-Aulet M, Wang J, Thornton J, Pierson RNJ. Comparison of dual-photon absorptiometry systems for total-body bone and soft tissue measurements: dual-energy X-rays versus gadolinium 153. J Bone Miner Res. 1991;6:411–415. doi: 10.1002/jbmr.5650060413. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 12.Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–E138. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- 13.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 14.Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy X-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am J Roentgenol. 2004;183:1761–1765. doi: 10.2214/ajr.183.6.01831761. [DOI] [PubMed] [Google Scholar]
- 15.Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11:312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 16.Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 17.Feller JF. MRI of bone marrow: advanced MRI from head to toe. 2002 http://mri.cpson.com/pdf/MRI_of_the_Bone_Marrow.pdf Cited 13 Nov 2006.
- 18.Bolotin HH, Sievanen H, Grashuis JL. Patient-specific DXA bone mineral density inaccuracies: quantitative effects of nonuniform extraosseous fat distributions. J Bone Miner Res. 2003;18:1020–1027. doi: 10.1359/jbmr.2003.18.6.1020. [DOI] [PubMed] [Google Scholar]
- 19.Hangartner TN, Johnston CC. Influence of fat on bone measurements with dual energy absorptiometry. Bone Miner. 1990;9:71–81. doi: 10.1016/0169-6009(90)90101-k. [DOI] [PubMed] [Google Scholar]
- 20.Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. 1979;6:191–198. doi: 10.1002/ajh.2830060303. [DOI] [PubMed] [Google Scholar]
- 21.Greenberger JS. Corticosteroid-dependent differentiation of human marrow preadipocytes in vitro. In Vitro. 1979;15:823–828. doi: 10.1007/BF02618309. [DOI] [PubMed] [Google Scholar]
- 22.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H14 spectrum of vertebral bone marrow. J Magn Reson Imaging. 2001;13:263–268. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.WHO Study Group. Technical report series, no. 843. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group. World Health Organization; Geneva: 1994. [PubMed] [Google Scholar]
- 24.Lang T, Augat P, Majumdar S, Ouyang X, Genant HK. Noninvasive assessment of bone density and structure using computed tomography and magnetic resonance. Bone. 1998;22:149S–153S. doi: 10.1016/s8756-3282(98)00005-2. [DOI] [PubMed] [Google Scholar]
- 25.Mulkern RV, Huang J, Vajapeyam S, Packard AB, Oshio K, Grinspoon S. Fat fractions and spectral T2 values in vertebral bone marrow in HIV-and non-HIV-infected men: a 1H spectroscopic imaging study. Magn Reson Med. 2004;52:552–558. doi: 10.1002/mrm.20205. [DOI] [PubMed] [Google Scholar]
- 26.Brix G, Heiland S, Bellemann ME, Koch T, Lorenz WJ. MR imaging of fat-containing tissues: valuation of two quantitative imaging techniques in comparison with localized proton spectroscopy. Magn Reson Imaging. 1993;11:977–991. doi: 10.1016/0730-725x(93)90217-2. [DOI] [PubMed] [Google Scholar]
- 27.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]


