Abstract
A high level of transcription has been associated with elevated spontaneous mutation and recombination rates in eukaryotic organisms. To determine whether the transcription level is directly correlated with the degree of genomic instability, we have developed a tetracycline-regulated LYS2 reporter system to modulate the transcription level over a broad range in Saccharomyces cerevisiae. We find that spontaneous mutation rate is directly proportional to the transcription level, suggesting that movement of RNA polymerase through the target initiates a mutagenic process(es). Using this system, we also investigated two hypotheses that have been proposed to explain transcription-associated mutagenesis (TAM): 1) transcription impairs replication fork progression in a directional manner and 2) DNA lesions accumulate under high-transcription conditions. The effect of replication fork progression was probed by comparing the mutational rates and spectra in yeast strains with the reporter gene placed in two different orientations near a well-characterized replication origin. The effect of endogenous DNA damage accumulation was investigated by studying TAM in strains defective in nucleotide excision repair or in lesion bypass by the translesion polymerase Polζ. Our results suggest that both replication orientation and endogenous lesion accumulation play significant roles in TAM, particularly in terms of mutation spectra.
Keywords: spontaneous mutagenesis, transcription, replication direction, polymerase zeta, mutation spectrum
1. INTRODUCTION
Mutagenesis is an important contributor to both evolutionary processes and carcinogenesis. While the primary DNA sequence (e.g., homopolymer tracts, di- or tri-nucleotide repeats, palindromes) accounts for the presence of certain mutational hotspots within the genome, transcription is also among the factors that can lead to increased mutagenesis [1]. An effect of transcription on genetic stability in a eukaryotic system was first observed using a segment of the RNA polymerase I transcribed ribosomal DNA locus – denoted HOT1 - that functioned as a cis-acting enhancer of recombination in Saccharomyces cerevisiae [2]. A similar increase in mitotic recombination and an additional elevation of spontaneous mutation rates in yeast has been associated with high levels of transcription carried out by RNA polymerase II [3-7]. The effect of such transcription-associated mutagenesis (TAM) and recombination (TAR) would be expected to be generally deleterious (e.g., the loss of a tumor suppressor), but may also be advantageous, promoting processes such as in immunoglobulin somatic hypermutation [8].
TAM and TAR in yeast have been attributed both to indirect and direct effects of transcription ([6]; reviewed in [1]). Evidence for a direct role of transcription in stimulating recombination has come from the analysis of HPR1-deficient yeast strains, where up to 1000-fold increases in recombination between direct repeats have been observed [9]. This hyper-recombination phenotype in HPR1-deficient strains has been shown to be directly related to the movement of RNA polymerase II (RNAP) complexes through the recombination substrate; when transcription elongation was inhibited by the insertion of a transcription termination sequence between the two direct repeats, the hyper-recombination phenotype was abolished [10]. Subsequently, Hpr1 was shown to be a component of the THO/TREX complex, inactivation of which leads to the elongation and stabilization of RNA:DNA hybrids (R-loops) formed during transcription [11]. Such persistent R-loops can induce frequent arrest of transcription complexes and, therefore, impede the processive movement of RNAP holoenzymes.
Based on the observed arrest of RNAP complexes in HPR1-deficient strains, three possible explanations for the initiating steps of TAR and/or TAM have been suggested. First, replication blockage arising from the collision between RNAP and the replication fork may result in collapse of the fork and its subsequent rescue by recombination. Second, arrested or paused RNAP itself might recruit the DNA repair machinery, triggering a process similar to transcription-coupled DNA repair [11,12]. Finally, the extended single-stranded character of the non-transcribed strand of DNA during highly-activated transcription might enhance the accessibility of DNA to endogenous damaging agents, leading to an increase in mutation- or recombination-initiating lesions. Evidence consistent with increased lesions arising from the enhanced accessibility of the highly transcribed region to DNA damaging agents has been reported [13]. Also, at least for TAR, there is evidence that chromatin remodeling associated with the activation of transcription may be a contributing factor [14]. Finally, the stress resulting from transcription-associated changes in DNA topology/superhelicity may lead to nicks or breaks in DNA [10,15,16].
With regard to a possible link between transcription and replication in genome instability, differences in the accumulation of mutagenic or recombinogenic DNA damage might lead to a disparity in mutagenesis when highly-activated transcription is oriented toward versus away from an approaching replication fork. A recent report has demonstrated that the relative orientation of the replication fork with respect to the direction of transcription indeed plays a significant role in transcription-associated recombination in a plasmid-based assay system [17]. This effect has been attributed to an enhanced replication block when the transcription machinery is directed toward an oncoming replication fork. Such transcription-dependent replication pause/stall sites have been observed in yeast [17,18] as well as in bacteria [19]. In addition, in E. coli, it has been observed that the manner in which a given DNA lesion is dealt with depends on the direction of replication [20]. When a plasmid containing a defined DNA lesion was transformed into E. coli cells, the resulting mutation frequency was distinctly different depending on whether the lesion was located on the leading-strand template versus the lagging-strand template [20].
Previously, we have studied the role of transcription in mutation and recombination in yeast using a chromosomal LYS2 reporter system in which the low-level LYS2 promoter (pLYS) was replaced with the highly inducible GAL1-10 promoter (pGAL) [5-7]. When pGAL was highly induced, the rate of spontaneous frameshift mutation was elevated 10- to 20- fold relative to that observed when pGAL was not induced [7]. We have observed a similar stimulatory effect of transcription when examining LYS2 forward mutations [21] or homologous recombination between two lys2 alleles positioned either on non-homologous chromosomes or as direct repeats [5,6,12]. The question remains, however, whether there is a direct and quantitative relationship between the level of transcription and the degree of genome instability. The pGAL-mediated LYS2 reporter system so far used to study TAM allows the reliable comparison of only the two extremes of transcription (very high in Gal80- strains and very low in Gal80+ strains), and is not suitable for the question posed above.
In the current study, we employed a tetracycline-regulated system that allows the level of transcription of a chromosomal mutational target to be varied over a broad range. Because our previous analysis of LYS2 forward mutations revealed that 2-nt deletion events are a distinctive signature of TAM [21], we examined reversion of the lys2ΔA746 +1 frameshift allele, a target that can specifically detect −2 events. We found that there was a linear and proportional relationship between the level of transcription and the rate of reversion, suggesting a direct involvement of transcription in the induction of mutagenesis. In addition, the question of whether convergence between the RNAP machinery and the replication fork contribute to TAM was addressed by assaying mutation rates and examining mutation spectra when the reporter gene was placed in both orientations relative to a defined replication origin on chromosome III. Although the overall mutation rate was not affected by the direction of fork movement through the highly-transcribed region, there were clear effects on the mutation spectrum.
2. MATERIALS AND METHODS
2.1 Construction of yeast strains
All yeast strains used in this study were derived from YPH45 (MATα ura3-52 ade2-101oc trp1Δ1). Plasmids pCM225 and pCM244 used in the construction of the pTET reporter system were acquired from Euroscarf and are described in Belli et al. [22]. The strains with the doxycycline (Dox)-regulated lys2ΔA746 allele (pTET-lys2ΔA746) in both orientations relative to an origin of replication on Chromosome III, ARS306 [23], were constructed by the following steps (specific details are available upon request). First, the endogenous LYS2 gene on chromosome II was replaced with a PCR-generated fragment conferring hygromycin resistance (lys2Δ∷hyg). Next, the expression cassette for the TetR’-SSN6 repressor was integrated at the LEU2 locus by transformation with EcoRV-digested pCM244. A fragment of the LYS2 gene including 300 nt of upstream untranslated region (UTR) and 75 nt of the downstream UTR was inserted at the HIS4 locus on chromosome III, replacing the entire HIS4 ORF along with 30 nt of the upstream UTR. For the strains denoted SAME orientation, the insertion of the fragment was oriented so that the LYS2 gene was transcribed in the same orientation as the endogenous HIS4 gene; for the strains denoted OPPOSITE orientation, the LYS2 gene was transcribed in the opposite direction as that of HIS4 (Figure 1). Substitution of the endogenous LYS2 promoter (pLYS) with a tetracycline-regulated promoter was carried out using a PCR-generated cassette from pCM225 that includes seven repeats of the tetO sequence motif (pTET), the tTA-VP16 transcriptional activator gene, and the KanMX4 selectable marker [22]. Finally, the wildtype pTET-LYS2 allele was replaced with the pTET-lys2ΔA746 allele using a two-step allele replacement strategy [24].
Figure 1.
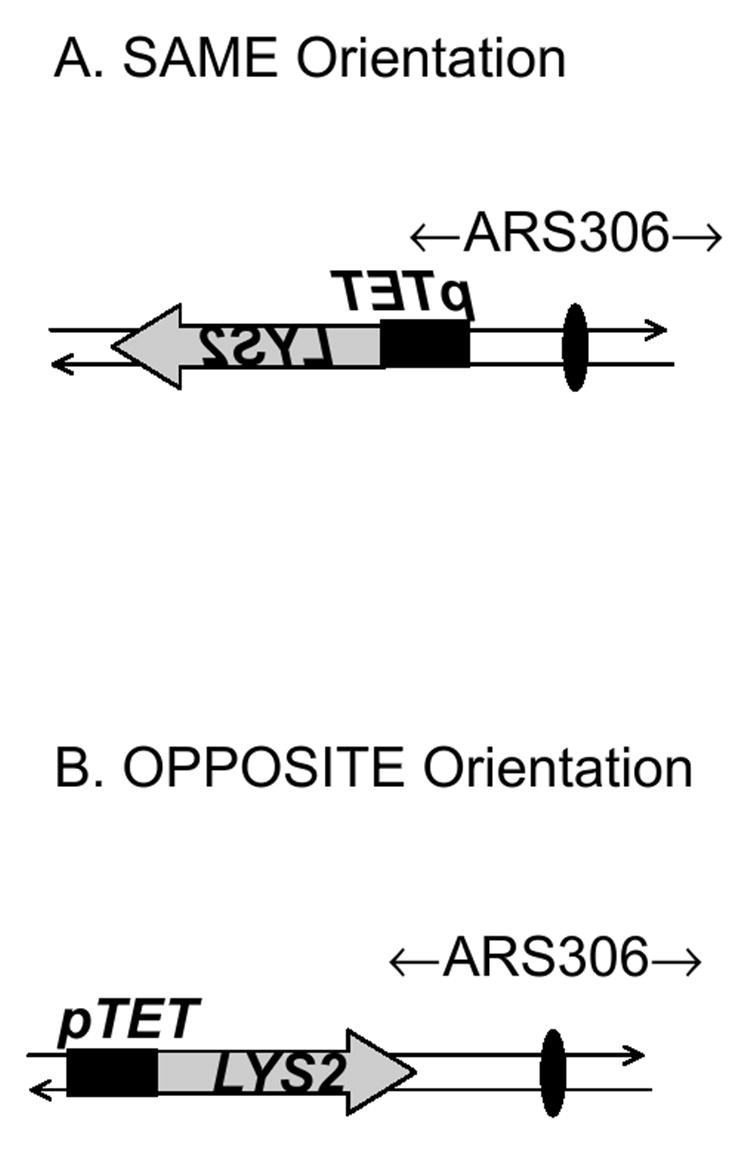
Schematic drawings of a portion of the right arm of chromosome III with LYS2 integrated in (A) SAME orientation or (B) OPPOSITE orientation.
The black oval indicates the replication origin ARS306, the black box indicates the pTET promoter cassette and the shaded arrow represents the LYS2 gene and its direction of transcription. The drawing is not to scale.
The construction of the yeast strains containing the pLYS-lys2ΔA746 allele was as follows. Following the deletion of the endogenous LYS2 gene as described above, a PCR fragment containing the complete LYS2 ORF and the upstream and the downstream UTR was integrated in both orientations at the HIS4 locus. Subsequently, the lys2ΔA746 allele was introduced using the delitto perfetto method [25].
The rev3 null strains were constructed by transformation with a PCR-generated fragment containing the TRP1 marker and short REV3 homology at each end [26]. Trp+ transformants were selected and deletion of the REV3 ORF was confirmed by PCR. The rad14 null strains were similarly constructed by transformation with a PCR-generated fragment containing the HygromycinR marker and short RAD14 homology at each end.
2.2 Mutation rates and spectra
For mutation rate determinations, 5 ml cultures were inoculated with single colonies and grown for two days at 30°C in non-selective YEP medium (1% yeast extract, 2% Bacto-peptone, 250 mg/l adenine; 2% agar for plates) supplemented with either 2% w/v dextrose (YEPD) or 2% glycerol plus 2% ethanol (YEPGE). Where applicable, the liquid medium was supplemented with the indicated concentration of Dox (Doxycycline hyclate; obtained from Sigma). For the measurement of CAN1 forward mutation rates, cells were plated on synthetic complete dextrose medium lacking arginine (SCD-Arg) and supplemented with 60 μg/ml L-canavanine (Sigma). Cells were plated on SCD-Lys plates for the measurement of lys2ΔA746 reversion rates and on YPD medium to determine the total number of cells in each culture. Colonies were counted after 2 days of growth at 30°C. Mutation rates were determined using the method of the median [27] and 95% confidence intervals were calculated as described previously [28]. At least 12 cultures were used for each rate determination.
2.3 Real-time RT-PCR
Total RNA was extracted from 5 ml of exponentially growing YEP-GE cultures (2−2.3 × 107 cells/ml) containing varying concentrations of Dox. The extraction was as described in Gasch et al. [29] with the following modifications. Cells were spun down and resuspended in 0.5 ml of lysis buffer (10 mM Tris-Cl pH7.4, 10 mM EDTA, 0.5% SDS), quickly frozen in liquid nitrogen and then stored at −80°C for up to one week. An equal volume of chilled acidic phenol (Fisher Scientific) was added to the frozen cell suspension and the mix was immediately heated to 70°C and incubated for 30-45 min with intermittent vortexing. After centrifugation at 4°C for 20 min, the aqueous phase was extracted one more time with phenol and then with chloroform. RNA was precipitated by adding NaOAc (pH 7) to 0.3 M final concentration and ethanol to 75%. After washing with 75% ethanol, the RNA pellet was resuspended in reaction buffer containing 10 units of RQ1 DNAse (Promega) and incubated at 37°C for 1 hour to remove any contaminating DNA. RNA was further purified using the Qiagen RNAeasy kit according to the manufacturer’s instructions. Real-time RT-PCR was carried out with the Quantitect One Step SYBR Green RT-PCR kit (Qiagen) and the amount of double-stranded DNA at each amplification cycle was measured using an iCycler iQ (BioRad). To control for the starting RNA amount, quantitative RT-PCR also was carried out in parallel for RFC1 (replication factor C) transcripts. LYS2-specific primers were MO18 (5’ GTAACCGGTGACGATGAT 3’) and LYS615R (5’ TTGAGCTTGTTCAACTCATTG 3’) and RFC1-specific primers were RFC2134F (5’ CGCTTCTGATGTTCGCTC 3’) and RFC2295R (5’ CCTCCGCTCATACCATCAAC 3’).
3. RESULTS
In order to examine the relationship between the level of gene transcription and mutagenesis, the lys2ΔA746 allele was placed under pTET transcriptional control. In the “dual tet-off” system used in this study, the level of transcription is regulated by two tetO-specific DNA binding proteins [22,30]. The tTA’-VP16 activator protein binds the tetO motif in the absence of tetracycline, or tetracycline analogs such as doxycycline (Dox), and activates transcription. The tTA-SSN6 repressor protein binds to tetO in the presence of Dox and represses transcription. Consequently, the addition of Dox to the growth medium incrementally decreases the transcription of a reporter gene.
In order to determine whether the direction of replication fork approach has an effect on transcription-associated mutagenesis, we deleted the entire LYS2 coding sequence from its endogenous location on chromosome II and placed the lys2ΔA746 allele in both orientations at the HIS4 locus on the right arm of chromosome III, approximately 6 kb from the ARS306 replication origin. Along with other replication origins on chromosome III, ARS306 has been extensively studied and is known to be a strongly-firing origin [31]. In the SAME orientation, LYS2 is transcribed in the same direction as the endogenous HIS4 locus while, in the OPPOSITE orientation, LYS2 transcription occurs in the opposite direction as HIS4. Since ARS306 is located upstream of the HIS4 gene, transcription complex progression and replication fork movement occur in the same direction when the lys2ΔA746 allele is in the SAME orientation (Figure 1A). When in the OPPOSITE orientation, transcription and replication fork movement are directed toward each other (Figure 1B). During replication, the coding strand (non-transcribed strand) of the lys2ΔA746 allele is the template for lagging-strand synthesis in the SAME orientation and for the leading-strand synthesis in the OPPOSITE orientation. 2D gel analysis was used to confirm the direction of replication in these strains (See Figure S1).
The steady-state LYS2 transcript level was measured at Dox concentrations varying from 0 to 1000 ng/ml using real-time quantitative RT-PCR with primers designed to amplify a region from +205 to +329 of the LYS2 coding sequence (Table 1). When transcription was fully activated (0 ng/ml Dox), the transcript levels were comparable when the lys2ΔA746 allele was in the SAME or OPPOSITE orientation. As the concentration of Dox increased transcription gradually decreased in both strains, although with the Dox concentration between 50 ng/ml and 200 ng/ml, the LYS2 transcript level in the SAME orientation showed a sharper decline. At the lowest level of transcription analyzed (1000 ng/ml Dox), the transcript levels in both orientations were indistinguishable (Table 1). Compared to the highest level of transcription (0 ng/ml Dox), transcript levels were reduced approximately 1000-fold in both orientations by the addition of 1000 ng/ml Dox to the growth medium (Table 1).
Table 1.
Transcript levels and pTET-lys2ΔA746 reversion rates at varying Dox concentrations
| SAME orientation | OPPOSITE orientation | |||
|---|---|---|---|---|
| Doxycycline (ng/ml) | Relative
Transcript (%) |
Reversion Rate
(×10-10) |
Relative
Transcript (%) |
Reversion Rate
(×10-10) |
| 0 | 100a | 266
(237 – 284) |
100b | 226
(193 – 273) |
| 10 | 55.5 | 237
(205 – 269) |
58.8 | 216
(170 – 1246) |
| 25 | 57.4 | 211
(153 – 224) |
63.7 | 179
(138 – 232) |
| 50 | 20.7 | 163
(131 – 212) |
56.4 | 156
(127 – 190) |
| 75 | 6.6 | 96
(74 – 117) |
44.5 | 135
(117 – 183) |
| 100 | 2.1 | 72.3
(50.3 – 94.3) |
27.3 | 105
(88 – 145) |
| 200 | 0.4 | 37.5
(23 – 47) |
9.0 | 60.4
(40.0 – 73.0) |
| 1000 | 0.1 | 14.4
(12.0 – 16.0) |
0.1 | 21.1
(18.1 – 22.1) |
Relative transcript amounts are in % and are the average of two independent measurements. The 95% confidence interval for each rate is in parentheses.
9.8-fold higher than RFC1 transcript level (ΔCT = CT LYS2 − CTRFC1 = 3.3).
9.1-fold higher than RFC1 transcript level (ΔCT = 3.2) and 93.3% of a.
CT (Threshold cycle number) was determined by iCycler iQ detection system (Biorad) using the “PCR baseline subtracted” curve fit option.
3.1 Direct proportionality between transcription level and mutation rate
The effect of transcription on mutagenesis was examined using the lys2ΔA746 reversion assay. The lys2ΔA746 allele contains a 1-bp deletion and can be reverted to lysine prototrophy by acquiring a compensatory, net +1 frameshift within an approximately 150 bp reversion window [24]. In order to determine the relationship between mutation rate and transcription, the lys2ΔA746 reversion rate was measured at Dox concentrations ranging from 0 to 1000 ng/ml (Table 1). Comparing the highest Dox concentration (low transcription) to the lowest (high transcription), the overall lys2ΔA746 reversion rate was increased by 18.5-fold or 10.7-fold for the strains with LYS2 integrated in the SAME or OPPOSITE orientation, respectively (Table 1). In both strains, there was a gradual decrease in the reversion rate as the concentration of Dox in the medium increased. As illustrated in Figure 2, there was a direct proportionality between the relative LYS2 transcript amount and the reversion rate of the lys2ΔA746 allele with both the SAME and OPPOSITE constructs. In addition, no striking effects of the target orientation on mutation rates were evident.
Figure 2.
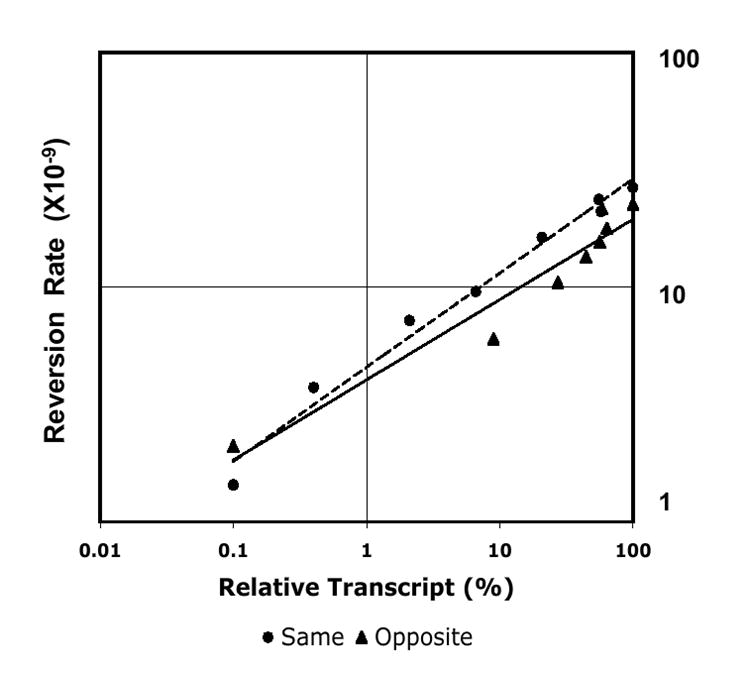
Relationship between transcription and mutagenesis.
Reversion rates and relative transcription levels of pTET-lys2ΔA746 SAME and pTET-lys2ΔA746 OPPOSITE strains at the indicated Dox levels are plotted on a log-log scale (data presented in Table 1). The trend lines were added using Microsoft Excel.
To confirm that the effect of Dox was specific to the pTET-controlled LYS2 gene, CAN1 forward mutation rates were determined at each Dox concentration. The CAN1 gene in all strains was under the control of its endogenous promoter and the transcription of the CAN1 gene would not be expected to be affected by the addition of Dox. As predicted, the concentration of Dox did not affect the CAN1 forward mutation rate, which varied from 3.3 × 10-8 to 4.8 × 10-8 (data not shown).
3.2 Orientation effects on the lys2ΔA746 reversion spectrum under low-transcription conditions
The lys2ΔA746 reversion spectrum was determined when the gene was under control of pLYS or pTET in both orientations relative to ARS306 on chromosome III (Figure 3). The reversion spectrum of pLYS-lys2ΔA746 in the SAME orientation was indistinguishable from that found earlier in a different haploid strain background at the endogenous LYS2 locus, where the relative orientations of replication and transcription were comparable to the SAME orientation (Figure 3A and 3B) [24]. In the OPPOSITE orientation, however, there was a striking increase in the accumulation of 95-nt deletions (“large dels”) relative to the SAME orientation (Figure 3B and Figure 3C). 10-nt (5’-TTGACGAGCT) direct repeats are found at the deletion junctions of the large dels, suggesting that the deletions likely result from slippage during DNA replication. Taking into account the overall reversion rates and the proportions of large dels, the rate of large dels was 8-fold higher in the OPPOSITE than in the SAME orientation (Table 2). A similar effect of replication orientation on the large dels was observed with the pTET-lys2ΔA746 allele under low-transcription conditions (Figure 3D and 3E) and when the direction of replication through the pLYS-lys2ΔA746 allele was reversed by inactivation of ARS306 (data not shown). In addition to the strong orientation dependence of large dels, we observed a weak orientation bias for 1-nt insertion events (+1’s) within the run of 6 adenines (6A run) located near the 5’ end of the reversion window. The 6A run represents the longest mononucleotide run present within the reversion window and is subject to frequent polymerase slippage events. Whether under pLYS-regulated transcription or under pTET-regulated transcription, +1 events in the 6A run occurred about two fold more frequently when LYS2 was in the SAME orientation than when it was in the OPPOSITE orientation (Figure 3 and Table 2). This orientation bias could reflect a strand bias in polymerase slippage and/or a strand bias in postreplicative mismatch repair.
Figure 3.
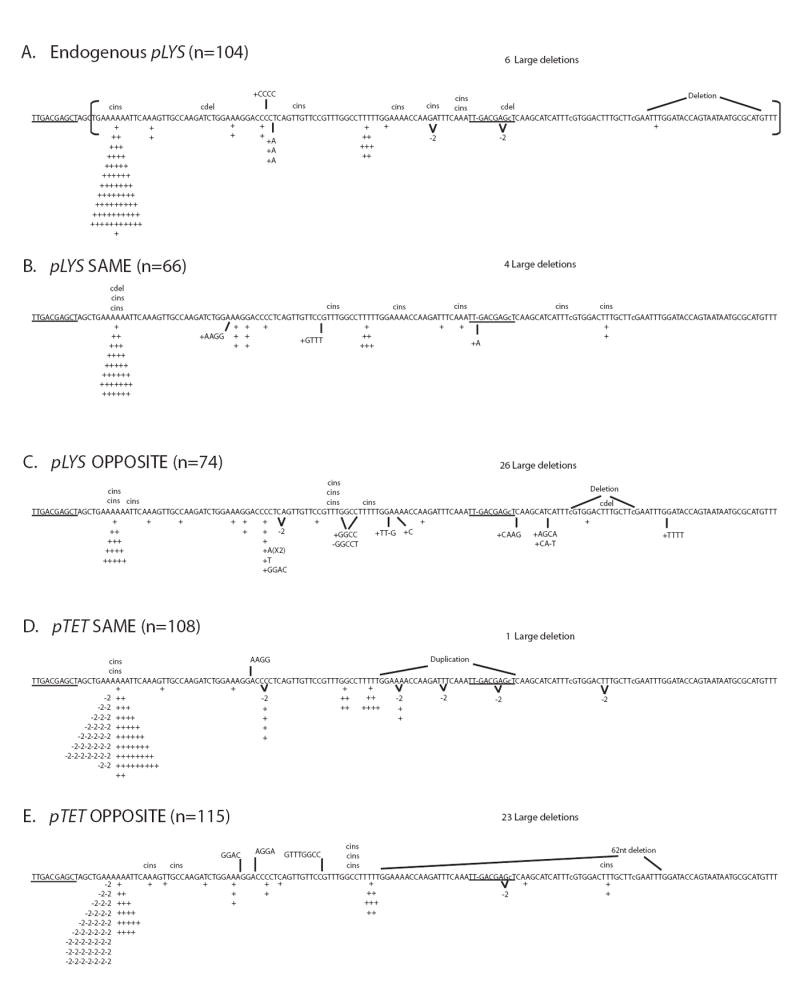
Reversion spectra for lys2ΔA746 allele under low-transcription conditions (no Dox for pLYS alleles and 1000 ng/ml Dox for pTET alleles).
The sequence shown includes the reversion window and the 14 nts upstream (the reversion window begins with 5’TGAAAAAA… and is marked by a bracket); the position of the A nucleotide deleted to create the lys2ΔA746 allele is indicated by a dash and the nucleotides changed to extend the reversion window are indicated by lowercase letters [42]. 1-nt insertions and 2-nt deletions are indicated under the sequence by “+” or “-2”, respectively. Complex insertions (cins) and deletions (cdel) are indicated above the sequence. The number of 95-nt deletion events are indicated as “large dels” above each spectrum. The 10-nt direct repeat sequences at the end points of the large dels are underlined. An abbreviated designation for each allele is used; pLYS same is pLYS-lys2ΔA746 in the SAME orientation, etc. The total number of revertants sequenced (n) for each strain is indicated. The reversion spectrum for pLYS-lys2ΔA746 allele at its endogenous location was previously published [24].
Table 2.
Rates of specific classes of frameshift mutations under low-transcription conditions
| Allele
Information |
pLYS-lys2ΔA746 (Endoa) |
pLYS-lys2ΔA746 SAME |
pLYS-lys2ΔA746 OPPOSITE |
pTET-lys2ΔA746 SAME |
pTET-lys2ΔA746 OPPOSITE |
|---|---|---|---|---|---|
| Total | 14
(0 – 16) |
6.8
(4.9 – 7.7) |
9.4
(8.4 – 12.4) |
14.4
(12.0 – 16.0) |
21.1
(18.1 – 22.1) |
| +1 at 6A | 8.8 | 3.5 | 1.9 | 6.3 | 3.6 |
| -2 at 6A | <0.1* | <0.1* | <0.1* | 4.0 | 7.8 |
| Other -2 | 0.3 | <0.1* | 0.1* | 0.7 | 0.2* |
| Cins | 0.8 | 0.7 | 0.9 | 0.3 | 1.1 |
| Cdel | 0.3 | 0.1* | 0.1* | <0.1* | <0.2* |
| Large del | 0.8 | 0.4 | 3.3 | 0.1* | 4.2 |
| Others | 2.9 | 2.1 | 3.0 | 2.9 | 4.4 |
Reversion rates (× 10-10) were measured at 1000 ng/ml Dox.
Rates are considered unreliable as they are based on ≤1 event.
Endogenous location on chromosome II.
3.3 Novel 2-nt deletions associated with the pTET-lys2ΔA746 allele under low-transcription conditions
Previous studies examining transcription-associated LYS2 forward mutations revealed that 2-nt deletion events (-2’s) are a distinctive mutational signature of TAM [21]. It should be noted that the lys2ΔBgl allele used in an earlier study of TAM is a +1 frameshift allele that reverts to lysine prototrophy by acquiring a net −1 frameshift mutation [7,32] and thus was unable to detect −2 frameshifts. In the current study, the effect of activated transcription on the reversion spectrum of the lys2ΔA746 -1 frameshift allele was analyzed because this assay can specifically detect −2 events. At the endogenous LYS2 locus in the context of the endogenous promoter, -2 events typically account for less than 5% of lys2ΔA746 reversion mutations, and these events occur entirely outside of the 6A run ([24], Figure 3A). A similar pattern was evident with the pLYS-lys2ΔA746 allele on chromosome III, with no −2 events observed in the 6A run (Figure 3B and 3C). Unexpectedly, there were a large number of −2 events within the 6A run of the pTET-lys2ΔA746 allele under low-transcription conditions (Figure 3D and 3E). The −2 events within the 6A run comprised 28% and 36% of total mutations in the SAME and OPPOSITE orientations, respectively.
There are several possible explanations for the novel 2-nt deletions at the 6A run associated with the pTET promoter. For example, they could be templated from the promoter cassette sequence itself. Other possibilities include an effect of Dox treatment, or other differences between strains such as the expression of the activator and/or repressor proteins. Further analysis excluded the latter possibilities; neither the addition of Dox to the growth medium nor the trans expression the tTA-SSN6/tTA’-VP16 regulatory proteins in a strain containing the pLYS-lys2ΔA746 allele was associated with an accumulation of −2 events in the 6A run (data not shown). It is important to note that the increase in −2 mutations in pTET-lys2ΔA746 allele under low-transcription conditions was confined to the 6A run, allowing us to still examine the effects of transcription on -2 events outside the run. We speculate that the mechanism that generates the -2 events in the 6A run may reflect either a sequence-specific templating event or a polymerase slippage error related to the presence of pTET.
3.4 Complex insertion/deletion mutations are associated with high levels of transcription
In order to determine how activated transcription affects mutagenesis at the molecular level and whether there are orientation-specific effects, pTET-lys2ΔA746 reversion spectra were obtained during maximal levels of transcription. These spectra are presented in Figure 4A and 4B and the rates of specific classes of events are given in Table 3. Although there was a general increase in all types of events (with the exception of the large deletion class in the opposite orientation), specific classes were over-represented and there were some clear orientation-specific effects. To facilitate the analysis of TAM, the ratio of the mutation rate under high- versus low- transcription was calculated for each frameshift class (Table 4). If a given event is over-represented in a high-transcription spectrum, the ratio will be greater than the total mutation rate ratio (18.5 or 10.7 for the SAME or OPPOSITE orientation, respectively).
Figure 4.

Reversion spectra for lys2ΔA746 allele under high-transcription conditions (0 ng/ml Dox). See Figure 3 legend for details.
Table 3.
Rates of specific classes of frameshift mutations under high-transcription conditions
| Allele
Information |
pTET-lys2ΔA746 SAME |
pTET-lys2ΔA746 OPPOSITE |
pTET-lys2ΔA746 SAME rev3 |
pTET-lys2ΔA746 OPPOSITE rev3 |
pTET-lys2ΔA746 SAME rad14 |
pTET-lys2ΔA746 OPPOSITE rad14 |
|---|---|---|---|---|---|---|
| Total | 266
(237 – 284) |
226
(193 – 273) |
74.0
(49.8 – 112) |
76.4
(56.2 – 105) |
549
(488 – 601) |
566
(499 – 594) |
| +1 at 6A | 100 | 27.1 | 49.7 | 18.4 | 115 | 49.8 |
| -2 at 6A | 39.9 | 29.4 | 16.6 | 41.6 | 44.9 | 45.3 |
| Other -2 | 23.9 | 40.7 | 0.6* | 5.4 | 9.9 | 41.3 |
| Cins | 18.6 | 56.5 | <0.6* | <0.7* | 95 | 250.7 |
| Cdel | 8.0 | 20.3 | <0.6* | <0.7* | 14.8 | 36.8 |
| Large del | 2.7 | <2.0* | 1.9 | 4.1 | 4.9 | 31.7 |
| Others | 71.8 | 49.7 | 5.1 | 6.8 | 265 | 109.8 |
Reversion rates (× 10-10) measured at 0 ng/ml Dox.
Rates are considered unreliable as they are based on ≤1 event.
Table 4.
Transcription- and orientation-specific effects of high levels of transcription in the lys2ΔA746 reversion assay
| Relative rates of specific frameshift classes (× 10-10) | ||||||||
|---|---|---|---|---|---|---|---|---|
| +1 at 6A | -2 at 6A | Other -2 | Cins | Cdel | Large del | Others | Total | |
| High/low txn with pTET SAME | 15.9 | 10.0 | 34.1 | 62.0 | >80.0 | 27.0 | 24.8 | 18.5 |
| High/low txn with pTET OPPOSITE | 7.5 | 3.8 | 204 | 51.4 | >102 | <0.5 | 11.3 | 10.7 |
| OPPOSITE/SAME with pTET high txn | 0.27 | 0.74 | 1.7 | 3.0 | 2.5 | <0.74 | 0.69 | 0.85 |
For both orientations, the most striking transcription-associated change was the disproportionate increase in complex insertion (cins) and deletion (cdel) events. Cins and cdels are frameshift mutations (either +1 or -2, respectively) accompanied by a base substitution within 5 nt of the selected event. While we typically have observed ~5% cins in lys2ΔA746 reversion spectra [24], cdels are very rare, and none were observed with the pTet allele under low-transcription conditions. In addition to the complex events, the “other -2” class (i.e., -2 events not in the 6A run) also was disproportionately elevated under high-transcription conditions (Figure 4A and 4B). The disproportionate increases in the cdel and “other -2” classes are consistent with the transcription-associated appearance of -2 events among LYS2 forward mutations reported previously [21].
In addition to the above transcription-associated changes in reversion spectra, orientation-specific effects on TAM were evident if one takes the ratio of the rates of individual frameshift classes when the lys2ΔA746 reporter allele was in the OPPOSITE versus SAME orientation relative to ARS306 (Table 4). In the OPPOSITE orientation, there were several-fold more of the complex and “other -2” events than in the SAME orientation (Figure 5A). While there appeared to be a deficit of +1 events in the 6A run in the OPPOSITE orientation, this deficit is a general property of OPPOSITE orientation spectra, and thus is unrelated to transcriptional status.
Figure 5.
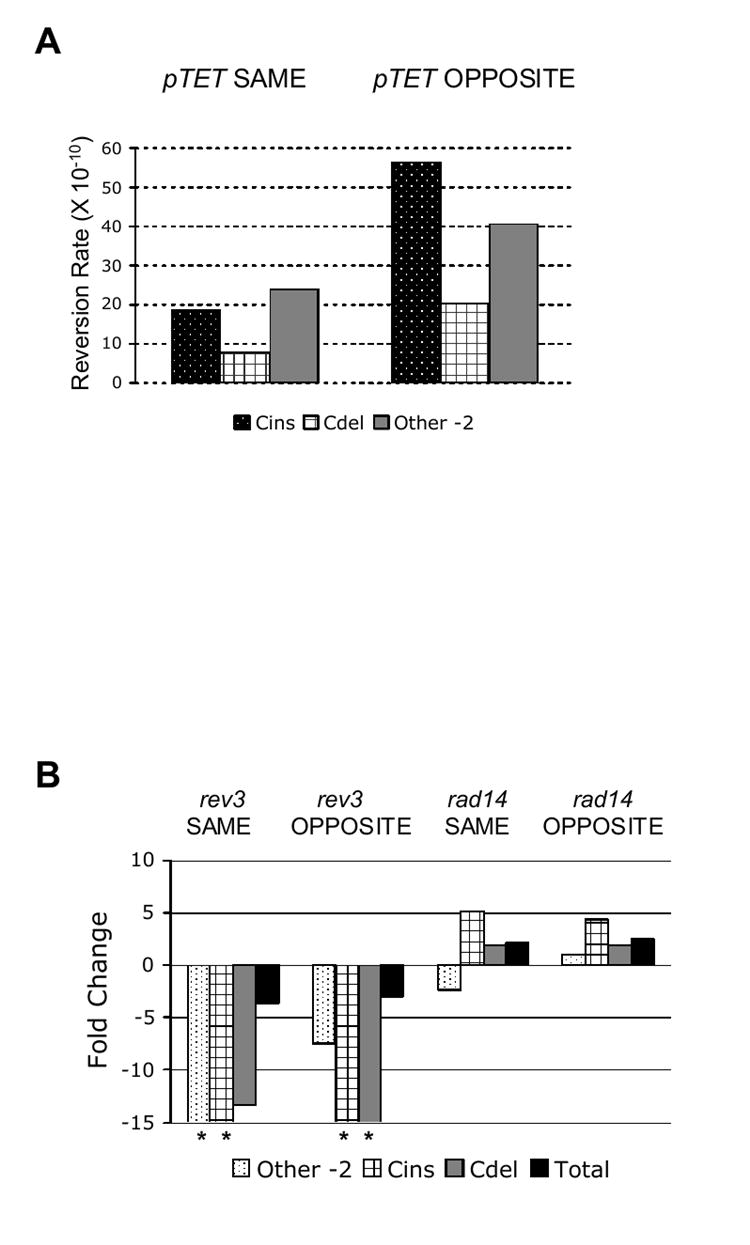
(A) Orientation bias of mutation distribution under high-transcription conditions (0 ng/ml Dox). (B) Change in mutation distribution in rev3 or rad14 background under high-transcription conditions. Reversion rates for strains indicated were divided by the corresponding rates in the wildtype background. Fold decreases in rates are represented as negative numbers and fold increases in rates are represented as positive numbers. Data for (A) and (B) are presented in Table 3. (*Values are greater than 15).
3.5 Transcription-associated complex mutations dependent on Rev3 are enhanced in the absence of Rad14
The complex mutations evident in the pTET-lys2ΔA746 reversion spectra under high-transcription conditions were reminiscent of the cins found for the pLYS-lys2ΔA746 allele in strains with defective nucleotide excision repair (NER) [24]. While the majority of the cins found in an NER-deficient background (62%) were clustered at two distinct hotspots, there was relatively minor clustering of cins in the pTET-lys2ΔA746 reversion spectra under high-transcription conditions (Figure 4A and 4B). Our previous studies have shown that cins associated with an NER defect or due to ultraviolet irradiation are dependent on the TLS polymerase Polζ, encoded by the REV3 and REV7 genes [24]. In order to determine whether the complex mutations occurring during highly-activated transcription are also dependent on Polζ, we deleted the REV3 gene in strains with the pTET-lys2ΔA746 allele. When compared to the wildtype strain, there was approximately a 3-fold reduction in reversion rate under high-transcription conditions in rev3 strains (Table 3 and Figure 5B). This is consistent with previous observations made with the pGAL-lys2ΔBgl system, where loss of REV3 led to a 3.2-fold reduction in reversion rate under high-transcription conditions [7]. The effect of inactivating REV3 was especially pronounced when the mutation spectra from rev3 strains were compared to those from wildtype strains. Under high-transcription conditions, no cins or cdel events were observed in rev3 strains in either orientation (Figure 4C and 4D). In addition, there was a clear reduction of −2 events occurring outside of the 6A run in the rev3 strains (Table 3).
We generated NER-deficient strains by deleting the RAD14 gene in strains with the pTET-lys2ΔA746 allele to gain further insight into the underlying cause of TAM-associated complex mutations. RAD14 encodes a yeast homolog of the human XPA protein, which is required in the damage recognition step of the NER pathway ([33]; reviewed in [34]). Under high-transcription conditions, the overall reversion rates were elevated by approximately 2.5 fold in NER-deficient strains when compared to the reversion rates in the wildtype backgrounds (Table 3). In our earlier investigation, a similar effect on the reversion rate was observed for the pGAL-lys2ΔBgl allele in strains where the RAD2 gene, which encodes a yeast homolog of human XPG protein, was deleted [32]. XPG is an endonuclease required for the incision of the damage-containing strand during NER ([35]; reviewed in [34]). Mutation spectra obtained under high-transcription conditions showed that cins were elevated approximately 5-fold in both the NER-deficient SAME and OPPOSITE strains whereas cdels and “other −2’s” were relatively unaffected (Figure 4E and 4F, Figure 5).
While there was no clear orientation-dependence of cins, cdel or “other -2” events in the high-transcription rad14 strains, there was a very striking orientation-dependence of two novel types of +1 event. In the SAME orientation, 40% (43/110) of the reversion events were insertion of a C or T immediately downstream of the 6A run. The +C mutation was not evident in any of the spectra presented in Figures 3 and 4, and the +T event was only seen twice in the rad14 OPPOSITE spectrum. These events provide a very clear example of a transcription-related lesion/structure that is generated in an orientation-specific manner. The molecular mechanism for generating the novel +C versus the +T may be similar, but one could also consider the +T as a simple addition to a 2T run. Regardless of the precise molecular nature(s) of the initiating event, however, it is a substrate specifically for the NER machinery.
4. DISCUSSION
In order to gain insight into the mechanism(s) involved in transcription-associated mutatgenesis (TAM), we established an assay system in which the transcription of the lys2ΔA746 reporter gene was modulated by the addition of the antibiotic Dox to the growth medium. In addition, we inserted the Dox-regulated reporter in both orientations relative to a defined origin of replication in order to examine whether the direction of replication fork movement affects TAM. In the SAME orientation, transcription occurs in the same direction as replication fork movement; in the OPPOSITE orientation, they converge. For both orientations, a similar proportional relationship between transcription level and reversion rate was observed over an approximately 1000-fold range of transcription, suggesting that the density of the transcription machinery along the mutation target has a direct effect on mutation rate. A threshold effect, which we might expect to see if transcription-associated chromatin structure was the major cause of enhanced mutagenesis, was not observed.
The change in the mutation spectra due to activated transcription was also examined in both orientations at the two transcriptional extremes. Comparing the lys2ΔA746 reversion spectra from the low- and high- transcription conditions, we verified that −2 frameshifts are an unique signature of TAM as was first noted by Lippert et al. [21]. This was most evident when examining the -2 events outside of the 6A run (“other -2’s”). In addition, we identified complex insertions and deletions (cins and cdels, respectively), where a +1 or −2 frameshift is accompanied by a base substitution(s) in close proximity, as a new signature of TAM. All three types of events occurred at higher rates when the lys2ΔA746 allele was in the OPPOSITE orientation, indicating replication-associated effects that were not evident when only reversion rates were measured (Figure 5). Whereas the transcription-associated cins were reminiscent of the spontaneous, Polζ-dependent cins found earlier in NER-deficient strains [24], the cdels have only been very rarely observed under low-transcription conditions. Polζ was required for approximately 70% of transcription-associated mutagenesis and was absolutely required for the cdels, as well as the cins, found under these conditions. Finally, Polζ was required for the majority of the “other -2” events.
As with the spontaneous cins found in the NER-deficient yeast cells, we speculate that DNA lesions are bypassed by a Polζ-dependent misincorporation-slippage mechanism resulting in the transcription-associated cins [36]. In this model, the incorporation of an incorrect nucleotide opposite a lesion (base substitution) results in an unstable 3’ terminus that increases the likelihood of a subsequent slippage event (frameshift). However, the transcription-associated cins identified in the current study have features distinct from the cins studied previously. In the earlier analyses done with the pLYS2-lys2ΔA746 allele in NER-deficient backgrounds, the majority of cins clustered at two distinct hotspots, HS1 and HS2 [24,37]. We did not observe such a distinctive clustering of transcription-associated cins among the pTET-lys2ΔA746 revertants; in addition to HS1 and HS2, additional hotspots were evident under high-transcription conditions, especially in the NER-defective (rad14) mutants (See Figure S2). A second distinguishing feature of the transcription-associated cins was the position/nature of linked base substitutions. The spontaneous cins described previously were largely homogeneous, with the changes CCGTTTGG→CCGTTTTGT (HS1) and ACTTTGC→ACTTTTTC (HS2) making up the majority of the complex events [24]. Although a similar pattern was evident with the pTET-lys2ΔA746 allele in NER-deficient background under low-transcription conditions, the transcription-associated cins at a given location, even at HS1 and HS2, did not show clear preference for one particular type of base substitution. The discrepancies between the distributions and the characteristics of the cins found under high- versus low-transcription conditions suggest that source and/or types of initiating lesions may be different. At least under low-transcription conditions, the cins at HS1 and HS2, but not at other positions, appear to result from spontaneous oxidative damage [36]. Whether the transcription-associated cins also result from oxidative damage is not known.
As noted previously, the transcription-associated cins, cdels and “other -2’s” were either significantly reduced or completely abolished in rev3 strains (Table 3), implicating the Polζ-dependent lesion bypass pathway in the generation of these novel mutation types. However, further analysis suggests that they can be separated into two distinct classes that likely arise via different mechanisms. First, the frameshifts associated with cins occurred almost exclusively at mononucleotide runs ≥3 nt in length (33 of 37 events), whereas “other −2’s” and cdels did not occur preferentially at runs (only 4 of 15 events). In addition, there appeared to be a possible hotspot for cdels and “other −2” events for the OPPOSITE orientation in the NER-proficient background. Specifically, four out of 11 cdels as well as 5 out of the 21 “other −2’s” clustered at a sequence (5’-GACGAGCTCAA) that coincides with the second direct repeat involved in the 95-nt deletion events. It should be noted that the first direct repeat is located outside of the reversion window, preventing the detection of events at this position. Finally, only the cins were significantly elevated in an NER-defective background, suggesting a distinct type of initiating event. One novel mechanism that might be involved in the generation of cdels and “other −2’s” under high-transcription conditions involves the repair of spontaneous or topoisomerase-mediated double-strand breaks. As observed, such breaks might be expected to be more prevalent when the directions of transcription and replication fork movement converge, generating positive supercoiling ahead of each, and might be expected to occur at hotspots. A dependence of cdel and/or “other -2” events on the non-homologous end-joining pathway would lend support to a double-strand break as an initiating event.
The observation that the overall pTET-lys2ΔA746 reversion rate under high-transcription conditions increased in rad14 background, along with our previous report that the pGAL-lys2ΔBgl reversion rate increased when NER was eliminated (rad1 or rad2 mutants) [32], suggest that the types of lesions normally recognized and removed by NER are a major contributing cause of TAM. This concurs with models of TAM and TAR that assume an enhanced accumulation of spontaneous damage in the transiently single-stranded regions exposed during transcription [1,13]. Such damage would be expected to accumulate to a greater extent on the nontranscribed strand; because the entire lys2ΔA746 allele was inverted in our experiments, the identity of the nontranscribed strand is the same in both orientations examined. However, a “strandedness” could be detected by inverting some or all of the reversion window. With regard to a greater accessibility to DNA damage, activated transcription and treatment with DNA-damaging agents have been previously reported to have synergistic effects on TAR, suggesting that enhanced accessibility to DNA damaging agents may be a major cause of all types of transcription-related genomic instability [13]. The effect of such exogenous damage on TAM has not yet been explored.
As noted previously, the transcription-associated cins were found far more frequently when the lys2ΔA746 allele was in the OPPOSITE orientation than in the SAME orientation. The fact that these events increased in the absence of NER and were completely dependent on the presence of Polζ suggests a greater accumulation of some types of lesions in the OPPOSITE orientation when the transcription machinery and replication fork converge. This difference could reflect the introduction of a disparate amount of damage, perhaps related to torsional stress. The appearance of the novel +C/T events immediately downstream of the 6A run in only the SAME rad14 strain illustrates an even more striking orientation-specific accumulation of transcription-associated damage. One possible way for these +C/T events (AAAAAA to AAAAAAC or AAAAAAT) to occur is through the expansion of 5A run to 6A run accompanied by the base change of the 3’ A to C or T; therefore these events could be interpreted as complex insertions. This interpretation is supported by the absence of the +C/T events in rad14 rev3 double mutants. (data not shown). The transcription-related orientation effects observed here may reflect a fundamental difference in how the lys2ΔA746 allele sequence is replicated, with the coding strand being the template for lagging-strand synthesis in the SAME orientation, but the template for the leading-strand synthesis in the OPPOSITE orientation. A given sequence, for example, might be particularly susceptible to damage during lagging-strand synthesis but not during leading-strand synthesis. Alternatively, a similar amount of damage may be introduced in either orientation, but an asymmetry in DNA damage repair [38] and/or bypass efficiency might lead to a net asymmetry in mutation accumulation. However, there is unlikely to be an underlying asymmetry in NER in our system since the relative increases in TAM that accompanied the loss of NER were similar for both orientations.
As discussed above, analysis of the reversion spectra of the lys2ΔA746 allele in both orientations relative to a well-defined replication origin revealed a biased accumulation of cins, cdel and “other” -2 events under high-transcription conditions. In addition, analyses under low-transcription conditions uncovered a strong, orientation-related bias in the accumulation of 95-bp deletions (large dels) with endpoints in 10-bp direct repeats. Previously, an increase in the same large del class was observed at the endogenous LYS2 locus in a pol32 null background [37]. Pol32 is a subunit of DNA Polδ and the deletion of the POL32 gene results in reduced Polδ processivity and a compromised DNA damage response [39]. Similarly, in a strain with mutation in the major catalytic subunit of the Polδ (pol3-t mutant), a large orientation-independent increase in the deletion frequency of sequences flanked by 6-7 bp direct repeats has been reported [40]. It has been suggested that large dels reflect the inherent single-stranded character of the lagging-strand template, which would be expected to be further enhanced if polymerization is compromised, allowing the formation of misaligned slippage intermediates between nontandem direct repeats [41]. In our experimental design, the large dels occurred much more frequently when the lys2ΔA746 allele was in the OPPOSITE orientation, with the transcribed (non-coding) strand as lagging-strand template. Assuming an analogous “slippage” model for the large dels in our assay, the initiating event is likely to be a replication-blocking lesion or secondary structure at or near the first direct repeat (see Figure S2).
In summary, the work presented here demonstrates a direct proportionality between mutagenesis and transcription in the lys2ΔA746 reversion assay. Although the orientation of the mutational target with respect to a defined origin of replication did not affect overall reversion rates, there were distinctive differences in mutation spectra. Of particular significance, use of a reversion assay allowed not only confirmation of -2 events as a signature of TAM, but also revealed a dramatic increase in complex insertions and deletions. Finally the increase and decrease in TAM upon loss of the NER pathway and Polζ, respectively, clearly implicate DNA damage as a causative factor. The experiments reported here underscore the importance of factors other than primary DNA sequence in establishing patterns and rates of mutagenesis, and demonstrate that both expression level and the manner in which a sequence is replicated are relevant. Further studies into the genetic requirements of the transcription-associated complex mutations and -2 events, and of their strand bias, will broaden our knowledge of how activated gene expression can lead to an enhancement of genetic instability.
Supplementary Material
Acknowledgments
We would like to thank Raj Gondalia for technical help and Brian Harfe for the permission to adapt a figure from earlier publication. This work was supported by grant R01 GM38464 to S. J.-R. from the National Institutes of Health. A.L.A. was partially supported by Graduate Division of Biological and Biomedical Sciences at Emory University and NIH Training Grant T32 GM08367. M.J.L. was supported by the Vermont Genetics Network through grant P20 RR16462 from INBRE program of the National Center for Research Resources, a component of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguilera A. The connection between transcription and genomic instability. EMBO J. 2002;21:195–201. doi: 10.1093/emboj/21.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voelkel-Meiman K, Keil RL, Roeder GS. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987;48:1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- 3.Nevo-Caspi Y, Kupiec M. Transcriptional induction of Ty recombination in yeast. Proc Natl Acad Sci USA. 1994;91:12711–12715. doi: 10.1073/pnas.91.26.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas BJ, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 5.Saxe D, Datta A, Jinks-Robertson S. Stimulation of mitotic recombination events by high levels of RNA polymerase II transcription in yeast. Mol Cell Biol. 2000;20:5404–5414. doi: 10.1128/mcb.20.15.5404-5414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman JA, Jinks-Robertson S. Genetic requirements for spontaneous and transcription-stimulated mitotic recombination in Saccharomyces cerevisiae. Genetics. 2002;162:15–27. doi: 10.1093/genetics/162.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta A, Jinks-Robertson S. Association of increased spontaneous mutation rates with high levels of transcription in yeast. Science. 1995;268:1616–1619. doi: 10.1126/science.7777859. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Fulop Z, Zhong Y, Evinger AJ, 3rd, Zan H, Casali P. DNA lesions and repair in immunoglobulin class switch recombination and somatic hypermutation. Ann N Y Acad Sci. 2005;1050:146–162. doi: 10.1196/annals.1313.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguilera A, Klein HL. Genetic control of intrachromosomal recombination in Saccharomyces cerevisiae. I. Isolation and genetic characterization of hyper-recombination mutations. Genetics. 1988;119:779–790. doi: 10.1093/genetics/119.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prado F, Piruat JI, Aguilera A. Recombination between DNA repeats in yeast hpr1Δ cells is linked to transcription elongation. EMBO J. 1997;16:2826–2835. doi: 10.1093/emboj/16.10.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell. 2003;12:711–721. doi: 10.1016/j.molcel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Freedman JA, Jinks-Robertson S. Effects of mismatch repair and Hpr1 on transcription-stimulated mitotic recombination in the yeast Saccharomyces cerevisiae. DNA Repair. 2004;3:1437–1446. doi: 10.1016/j.dnarep.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Rubio M, Huertas P, Gonzalez-Barrera S, Aguilera A. Recombinogenic effects of DNA-damaging agents are synergistically increased by transcription in Saccharomyces cerevisiae. New insights into transcription-associated recombination. Genetics. 2003;165:457–466. doi: 10.1093/genetics/165.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malagon F, Aguilera A. Yeast spt6-140 mutation, affecting chromatin and transcription, preferentially increases recombination in which Rad51p-mediated strand exchange is dispensable. Genetics. 2001;158:597–611. doi: 10.1093/genetics/158.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Barrera S, Garcia-Rubio M, Aguilera A. Transcription and double-strand breaks induce similar mitotic recombination events in Saccharomyces cerevisiae. Genetics. 2002;162:603–614. doi: 10.1093/genetics/162.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangloff S, Lieber MR, Rothstein R. Transcription, topoisomerases and recombination. Experientia. 1994;50:261–269. doi: 10.1007/BF01924009. [DOI] [PubMed] [Google Scholar]
- 17.Prado F, Aguilera A. Impairment of replication fork progression mediates RNA polII transcription-associated recombination. EMBO J. 2005;24:1267–1276. doi: 10.1038/sj.emboj.7600602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshpande AM, Newlon CS. DNA replication fork pause sites dependent on transcription. Science. 1996;272:1030–1033. doi: 10.1126/science.272.5264.1030. [DOI] [PubMed] [Google Scholar]
- 19.Mirkin EV, Mirkin SM. Mechanisms of transcription-replication collisions in bacteria. Mol Cell Biol. 2005;25:888–895. doi: 10.1128/MCB.25.3.888-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veaute X, Fuchs RP. Greater susceptibility to mutations in lagging strand of DNA replication in Escherichia coli than in leading strand. Science. 1993;261:598–600. doi: 10.1126/science.8342022. [DOI] [PubMed] [Google Scholar]
- 21.Lippert MJ, Freedman JA, Barber MA, Jinks-Robertson S. Identification of a distinctive mutation spectrum associated with high levels of transcription in yeast. Mol Cell Biol. 2004;24:4801–4809. doi: 10.1128/MCB.24.11.4801-4809.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belli G, Gari E, Aldea M, Herrero E. Functional analysis of yeast essential genes using a promoter-substitution cassette and the tetracycline-regulatable dual expression system. Yeast. 1998;14:1127–1138. doi: 10.1002/(SICI)1097-0061(19980915)14:12<1127::AID-YEA300>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Newlon CS, Collins I, Dershowitz A, Deshpande AM, Greenfeder SA, Ong LY, Theis JF. Analysis of replication origin function on chromosome III of Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 1993;58:415–423. doi: 10.1101/sqb.1993.058.01.048. [DOI] [PubMed] [Google Scholar]
- 24.Harfe BD, Jinks-Robertson S. DNA polymerase ζ introduces multiple mutations when bypassing spontaneous DNA damage in Saccharomyces cerevisiae. Mol Cell. 2000;6:1491–1499. doi: 10.1016/s1097-2765(00)00145-3. [DOI] [PubMed] [Google Scholar]
- 25.Storici F, Lewis LK, Resnick MA. In vivo site-directed mutagenesis using oligonucleotides. Nat Biotechnol. 2001;19:773–776. doi: 10.1038/90837. [DOI] [PubMed] [Google Scholar]
- 26.Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 27.Lea DE, Coulson CA. The distribution of the numbers of mutants in bacterial populations. J Genet. 1949;49:264–284. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 28.Spell RM, Jinks-Robertson S. Determination of mitotic recombination rates by fluctuation analysis in Saccharomyces cerevisiae. Methods Mol Biol. 2004;262:3–12. doi: 10.1385/1-59259-761-0:003. [DOI] [PubMed] [Google Scholar]
- 29.Gasch AP. Yeast genomic expression studies using DNA microarrays. Methods Enzymol. 2002;350:393–414. doi: 10.1016/s0076-6879(02)50976-9. [DOI] [PubMed] [Google Scholar]
- 30.Belli G, Gari E, Piedrafita L, Aldea M, Herrero E. An activator/repressor dual system allows tight tetracycline-regulated gene expression in budding yeast. Nucl Acids Res. 1998;26:942–947. doi: 10.1093/nar/26.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newlon CS, Lipchitz LR, Collins I, Deshpande A, Devenish RJ, Green RP, Klein HL, Palzkill TG, Ren RB, Synn S et al. Analysis of a circular derivative of Saccharomyces cerevisiae chromosome III: a physical map and identification and location of ARS elements. Genetics. 1991;129:343–357. doi: 10.1093/genetics/129.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morey NJ, Greene CN, Jinks-Robertson S. Genetic analysis of transcription-associated mutation in Saccharomyces cerevisiae. Genetics. 2000;154:109–120. doi: 10.1093/genetics/154.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bankmann M, Prakash L, Prakash S. Yeast RAD14 and human xeroderma pigmentosum group A DNA-repair genes encode homologous proteins. Nature. 1992;355:555–558. doi: 10.1038/355555a0. [DOI] [PubMed] [Google Scholar]
- 34.Prakash S, Prakash L. Nucleotide excision repair in yeast. Mutat Res. 2000;451:13–24. doi: 10.1016/s0027-5107(00)00037-3. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds RJ, Friedberg EC. Molecular mechanisms of pyrimidine dimer excision in Saccharomyces cerevisiae: incision of ultraviolet-irradiated deoxyribonucleic acid in vivo. J Bacteriol. 1981;146:692–704. doi: 10.1128/jb.146.2.692-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minesinger BK, Abdulovic AL, Ou TM, Jinks-Robertson S. The effect of oxidative metabolism on spontaneous Pol ζ-dependent translesion synthesis in Saccharomyces cerevisiae. DNA Repair. 2005;5:226–234. doi: 10.1016/j.dnarep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Minesinger BK, Jinks-Robertson S. Roles of RAD6 epistasis group members in spontaneous pol ζ-dependent translesion synthesis in Saccharomyces cerevisiae. Genetics. 2005;169:1939–1955. doi: 10.1534/genetics.104.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong JD, Kunz BA. Excision repair and gene orientation modulate the strand specificity of UV mutagenesis in a plasmid-borne yeast tRNA gene. Environ Mol Mutagen. 1995;25:12–22. doi: 10.1002/em.2850250104. [DOI] [PubMed] [Google Scholar]
- 39.Huang ME, Rio AG, Galibert MD, Galibert F. Pol32, a subunit of Saccharomyces cerevisiae DNA polymerase delta, suppresses genomic deletions and is involved in the mutagenic bypass pathway. Genetics. 2002;160:1409–1422. doi: 10.1093/genetics/160.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran HT, Degtyareva NP, Koloteva NN, Sugino A, Masumoto H, Gordenin DA, Resnick MA. Replication slippage between distant short repeats in Saccharomyces cerevisiae depends on the direction of replication and the RAD50 and RAD52 genes. Mol Cell Biol. 1995;15:5607–5617. doi: 10.1128/mcb.15.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trinh TQ, Sinden RR. Preferential DNA secondary structure mutagenesis in the lagging strand of replication in E. coli. Nature. 1991;352:544–547. doi: 10.1038/352544a0. [DOI] [PubMed] [Google Scholar]
- 42.Harfe BD, Jinks-Robertson S. Removal of frameshift intermediates by mismatch repair proteins in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4766–4773. doi: 10.1128/mcb.19.7.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


