Abstract
Background
Randomized trials have largely failed to support an effect of antioxidant vitamins on risk of cardiovascular disease (CVD). Few trials have examined interactions among antioxidants, and no previous trial has examined the individual effect of vitamin C on CVD.
Methods
WACS tested the effects of vitamins C (500 mg daily), E (600 IU every other day), and beta-carotene (50 mg every other day) on the combined outcome of myocardial infarction (MI), stroke, coronary revascularization, or CVD death among 8,171 female health professionals at increased risk in a 2×2×2 factorial design. Participants were 40 years or older with a prior history of CVD or three or more CVD risk factors, and were followed an average 9.4 years, from 1995-96 to 2005.
Results
1,450 women experienced one or more CVD outcomes. There was no overall effect of vitamin C (RR=1.02, 95% CI=0.92-1.13, p=0.71), vitamin E (RR=0.94, 95% CI=0.85-1.04, p=0.23), or beta-carotene (RR=1.02, 95% CI=0.92-1.13, p=0.71) on the primary combined endpoint, or on the individual secondary outcomes of MI, stroke, coronary revascularization, or CVD death. A marginally significant reduction in the primary outcome with active vitamin E was observed among the pre-specified subgroup of women with prior CVD (RR=0.89, 95% CI=0.79-1.00, p=0.04; p-interaction=0.07). There were no significant interactions between agents for the primary endpoint, but those randomized to both active vitamin C and E experienced fewer strokes (p for interaction=0.03).
Conclusion
There were no overall effects of vitamins C, E or beta-carotene on cardiovascular events among women at high risk for CVD.
Trial Registration
clinicaltrials.gov Identifier: NCT00000541
INTRODUCTION
Oxidative damage may play a role in the development of cardiovascular disease, particularly through its effect on lipid peroxidation and DNA damage.1 In addition, free radicals may damage arterial endothelium, encourage thrombosis, and alter vasomotor function.2 Antioxidants scavenge free radicals, and limit the damage they can cause.3 Diets high in fruit and vegetable intake, and thus rich in such antioxidants, have been associated with reduced rates of coronary heart disease and stroke.4
Vitamins C, E and beta-carotene are potential mediators of the apparent protective effect of a plant-based diet on cardiovascular disease (CVD). Observational epidemiologic studies of dietary intake of these vitamins provide some support for this hypothesis, particularly for vitamin E,5-8 although results are not consistent. Some studies found lower event rates among those with higher plasma carotenoids,9, 10 but others found no beneficial effects.11, 12
Several randomized trials have now examined the effects of these antioxidants, and results have generally been disappointing.13 No trials, however, have examined the effect of vitamin C on CVD, except as part of an antioxidant cocktail. The Women’s Antioxidant Cardiovascular Study (WACS) was designed to test the effect of three antioxidant agents, vitamins C, E, and beta-carotene, on prevention of CVD among women at high risk. It used a factorial design, enabling a comparison for each agent alone as well as interactions among them.
METHODS
Study Design and Participants
WACS is a randomized, double-blind, placebo-controlled trial evaluating the effects of vitamin C (500 mg/day synthetic vitamin C [ascorbic acid], provided by BASF Corporation [Mount Olive, NJ]), vitamin E (600 IU natural vitamin E [D-alpha-tocopherol acetate] every other day, provided by Cognis Corporation [La Grange, IL]), and beta-carotene (50 mg Lurotin every other day, provided by BASF) in the prevention of important vascular events in a 2×2×2 factorial design among high risk women with either a history of vascular disease or at least three cardiovascular risk factors. In 1998, approximately 2-3 years following randomization to the antioxidant arms, a folic acid/vitamin B6/B12 component was added to the trial, expanding it to a four-arm factorial trial. This report describes the results of the three antioxidant interventions.
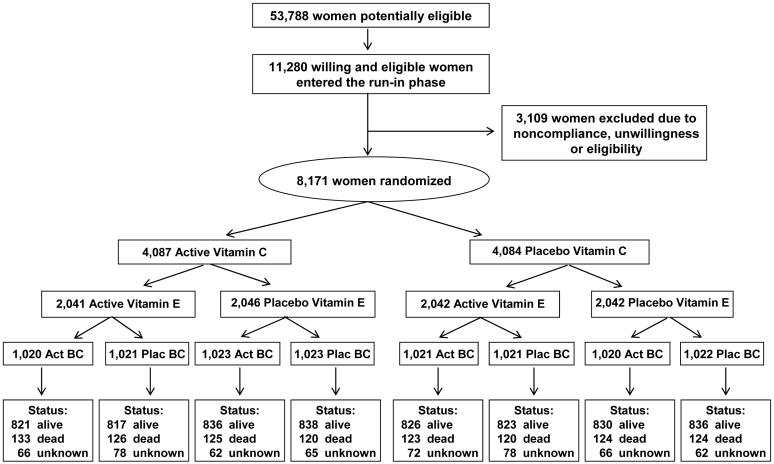
Details of the design have been reported previously.14 Briefly, women were eligible if they were aged 40 years or older, postmenopausal or had no intention of becoming pregnant, had a self-reported history of CVD, or had at least three cardiac risk factors. The cardiac risk factors determining eligibility were self-reported diagnosis of hypertension, high cholesterol, or diabetes mellitus, parental history of premature myocardial infarction (MI) (before age 60), obesity (body mass index (BMI) ≥ 30 kg/m2), current cigarette smoking, and inconsistent report of prior CVD. Women were excluded if they had a self-reported history of cancer (excluding nonmelanoma skin cancer) within the past ten years, any serious non-CVD illness, or were currently using warfarin or other anticoagulants. Potential participants also had to be willing to forgo individual supplements of vitamins A, C, E, and beta-carotene at levels beyond the U.S. recommended daily allowance (RDA) during the trial. 8,171 women were willing and eligible, were compliant during a 12-week run-in, and were randomized into the trial from June, 1995, through October, 1996 (Figure 1). The trial was approved by the institutional review board of the Brigham and Women’s Hospital, Boston, MA, and was monitored by an external data and safety monitoring board.
Figure 1.
Enrollment, randomization and follow-up of participants in the Women’s Antioxidant Cardiovascular Study (WACS).
Following randomization, every six months for the first year then annually, the women were sent monthly calendar packs containing active agents or placebos, along with questionnaires on compliance, side effects, and medical events. Study medications and end point ascertainment were continued in a blinded fashion until the scheduled end of the trial. Pill-taking for women not enrolled in the folic acid component ended as scheduled on January 31, 2005; women in the folic acid component completed pill-taking on July 31, 2005. The average follow-up from randomization to end of study was 9.4 years (range 8.3-10.1 years). Follow-up and validation of reported end points were completed in July, 2006. A search of the National Death Index was conducted for all participants through December, 2003; thus mortality information was virtually complete through 2003. As of the scheduled end of the trial in January or July, 2005, mortality follow-up was 93% complete. In terms of person-time, mortality information was complete for over 99% of person-years of follow-up. Morbidity status was known as of 8 years for 93% of survivors, and response to the final follow-up questionnaire among surviving women was 89%.
Compliance was assessed through self-report and defined as taking at least two-thirds of study pills. Reported compliance was, on average, 76% at 4 years and 68% at 8 years of follow-up for each antioxidant, with no significant difference between active and placebo groups at these times except for vitamin C at 8 years (70% vs. 67% in active vs. placebo, p=0.01). Average compliance over follow-up was approximately 73% for all active and placebo agents. In 1999, blood samples were obtained from 30 local participants to evaluate biomarkers for compliance. Blood levels were elevated in each active vs. placebo group (vitamin C: 1.88 vs. 1.26 mg/dL, p=0.007; vitamin E: 20.19 vs. 12.15 μg/mL, p=0.007; beta-carotene: 54.39 vs. 19.52 μg/mL, p=0.003).
Information was obtained on outside supplements of study medications for at least 4 days per month (“drop-ins”). Use of vitamin C supplements was approximately 10% at both 4 and 8 years, and did not differ by intervention group. Use of vitamin E supplements was 13% at 4 years and 12 % at 8 years, with no difference by randomized group. Use of supplements of beta-carotene or vitamin A averaged 2% at 4 and 8 years, and did not differ by beta-carotene assignment. When use of multivitamins containing more than 100% of the RDA was included in the definition of outside use, rates were approximately 3-5% higher for vitamin C, 4-8% higher for vitamin E, and 1-3% higher for beta-carotene, but again did not differ by randomized assignment.
End Point Definition
The primary outcome was a combined end point of CVD morbidity and mortality, including incident MI, stroke, coronary revascularization procedures (coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA)), and cardiovascular mortality. The individual components of MI, stroke, coronary revascularization, and CVD death were pre-specified secondary end points. Information on transient ischemic attack (TIA) and total mortality was also collected and reviewed.
Women reported relevant endpoints through questionnaire, letter, or phone call. Deaths were reported by family members, postal authorities or through the National Death Index. Written permission for medical records was sought from the participant, or next of kin in case of death. These were reviewed by an endpoints committee of physicians blinded to randomized treatment assignment. An MI was confirmed if symptoms met World Health Organization criteria and cardiac enzymes or diagnostic electrocardiograms were abnormal. Coronary revascularization (CABG or PTCA) was confirmed by medical record review. Confirmed stroke was defined as a new neurologic deficit of sudden onset that persisted for more than 24 hours or until death within 24 hours. Clinical information, computed tomographic scans, and magnetic resonance images were used to distinguish hemorrhagic from ischemic events. A confirmed TIA was defined as a neurologic deficit of sudden onset that lasted less than 24 hours. Death due to cardiovascular cause was confirmed by examination of autopsy reports, death certificates, medical records, and information obtained from the next of kin or other family members. Death from any cause was confirmed by the end points committee or on the basis of a death certificate. Only confirmed end points were included in these analyses, except for total mortality which included 66 reported deaths with no death certificate.
Statistical Analysis
Primary analyses were performed on an intent-to-treat basis, including all randomized women. Baseline characteristics were compared by randomized groups using t-tests, chi-square tests for proportions, and tests for trend for ordinal categories. Kaplan-Meier curves were used to estimate cumulative incidence over time by randomized group, and the log-rank test was used to compare curves. The Cox proportional hazards model was used to estimate the relative risk (RR), expressed as a hazards ratio, along with the 95% confidence interval (CI). Models included main effect terms for each of the three antioxidants along with age. Tests of proportionality of the hazards ratio over timeused an interaction term for treatment times the logarithm of time. To examine the impact of lack of compliance, a post-hoc sensitivity analysis censored women if and when they stopped taking at least two-thirds of their study medication, reported taking outside supplements containing study agents, or were missing compliance information. Analyses were conducted using SAS version 9 (SAS Institute, Cary, NC), using two-sided tests with a significance level of 0.05.
Interaction terms were used to test for additivity of the three antioxidant agents. All two-way interactions as well as a three-way interaction were tested, using multiplicative terms in the Cox model. In addition, we conducted subgroup analyses according to the presence or absence of major cardiovascular risk factors, including age, prior CVD, smoking, alcohol use, BMI, history of hypertension, high cholesterol or diabetes, parental history of MI before age 60, menopausal status and hormone therapy (HT) use, and current multivitamin use. Tests for effect modification by subgroup used interaction terms between subgroup indicators and randomized assignment, with a test for trend for ordinal subgroup categories, or a multi-degree-of-freedom test for unordered categories. Any significant modification of effect is described below.
RESULTS
8,171 women were randomized into the antioxidant arms of the trial, with a mean age of 60.6 years (SD=8.8). Of these, 5,238 (64%) had a prior cardiovascular event, and 2,933 (36%) had three or more CVD risk factors. Mean BMI was 30.3 (SD=6.7), and 1,269 women (16%) were current smokers, 1,564 (19%) had diabetes, 6,137 (75%) had a history of hypertension, and 5,950 (73%) had a history of high cholesterol at baseline. There were no statistically significant differences in baseline characteristics between the randomized groups, except history of high cholesterol by beta-carotene (Table 1).
Table 1.
Comparison of baseline characteristics by randomized groups in the Women’s Antioxidant Cardiovascular Study.*
| Vitamin C |
Vitamin E |
Beta-carotene |
|||||
|---|---|---|---|---|---|---|---|
| Baseline Factor | Total N | Active (N=4087) | Placebo (N=4084) | Active (N=4083) | Placebo (N=4088) | Active (N=4084) | Placebo (N=4087) |
| Age (years), mean ± SD | 8171 | 60.6 ± 8.8 | 60.6 ± 8.8 | 60.6 ± 8.9 | 60.6 ± 8.8 | 60.6 ± 8.9 | 60.6 ± 8.8 |
| Age (years), % | |||||||
| 40-54 | 2400 | 29.4 | 29.4 | 29.3 | 29.4 | 29.4 | 29.4 |
| 55-64 | 2993 | 36.6 | 36.7 | 36.7 | 36.6 | 36.6 | 36.6 |
| 65+ | 2778 | 34.0 | 34.0 | 34.0 | 34.0 | 34.0 | 34.0 |
| Health history, %† | |||||||
| Prior CVD | 5238 | 64.1 | 64.1 | 64.7 | 63.5 | 63.8 | 64.4 |
| 3+ risk factors | 2933 | 35.9 | 35.9 | 35.3 | 36.5 | 36.2 | 35.6 |
| History of hypertension, % | |||||||
| Yes | 6137 | 75.4 | 74.8 | 74.4 | 75.8 | 75.3 | 74.9 |
| No | 2034 | 24.6 | 25.2 | 25.6 | 24.2 | 24.7 | 25.1 |
| History of high cholesterol, % | |||||||
| Yes | 5950 | 72.6 | 73.0 | 72.7 | 73.0 | 71.5 | 74.1§ |
| No | 2221 | 27.4 | 27.0 | 27.3 | 27.0 | 28.5 | 25.9 |
| History of diabetes, % | |||||||
| Yes | 1564 | 19.4 | 18.9 | 18.7 | 19.6 | 19.2 | 19.1 |
| No | 6607 | 80.6 | 81.1 | 81.3 | 80.4 | 80.8 | 80.9 |
| Parental History of MI, %‡ | |||||||
| Yes | 3032 | 38.2 | 36.4 | 36.7 | 37.9 | 37.1 | 37.5 |
| No | 5097 | 61.8 | 63.6 | 63.3 | 62.1 | 62.9 | 62.5 |
| Smoking status, % | |||||||
| Current | 1269 | 15.1 | 15.9 | 15.5 | 15.6 | 15.3 | 15.8 |
| Past | 3405 | 42.0 | 41.4 | 41.7 | 41.6 | 41.6 | 41.8 |
| Never | 3497 | 42.9 | 42.7 | 42.8 | 42.8 | 43.1 | 42.4 |
| Alcohol use in past year, % | |||||||
| Never | 4514 | 56.1 | 54.4 | 54.7 | 55.8 | 55.6 | 54.9 |
| 0 - < 1/week | 1008 | 12.3 | 12.4 | 12.1 | 12.6 | 12.3 | 12.4 |
| 1-6 / week | 1964 | 23.9 | 24.2 | 24.9 | 23.2 | 23.7 | 24.4 |
| Daily | 685 | 7.8 | 9.0 | 8.3 | 8.5 | 8.4 | 8.4 |
| Body mass index (kg/m2), mean ± SD | 8164 | 30.3 ± 6.7 | 30.3 ± 6.7 | 30.3 ± 6.6 | 30.3 ± 6.7 | 30.2 ± 6.7 | 30.4 ± 6.6 |
| Body mass index, % | |||||||
| <25 kg/m2 | 1906 | 23.2 | 23.5 | 23.0 | 23.7 | 24.2 | 22.4 |
| 25-<30 kg/m2 | 2339 | 29.1 | 28.2 | 29.2 | 28.1 | 28.4 | 28.9 |
| 30+ kg/m2 | 3919 | 47.8 | 48.2 | 47.8 | 48.2 | 47.4 | 48.6 |
| Menopause and HT use, % | |||||||
| Premenopausal | 653 | 8.1 | 8.2 | 8.2 | 8.0 | 7.8 | 8.5 |
| Uncertain | 1167 | 14.4 | 14.7 | 14.5 | 14.6 | 14.5 | 14.6 |
| Postmenopausal, current HT | 3083 | 39.2 | 37.7 | 38.8 | 38.1 | 38.7 | 38.2 |
| Postmenopausal, no HT | 3121 | 38.4 | 39.4 | 38.5 | 39.3 | 39.0 | 38.8 |
| Current multivitamin use, % | |||||||
| Yes | 2192 | 26.5 | 27.5 | 27.1 | 26.9 | 26.9 | 27.1 |
| No | 5927 | 73.5 | 72.5 | 72.9 | 73.1 | 73.1 | 72.9 |
Abbreviations: SD = standard deviation; CVD = cardiovascular disease; MI = myocardial infarction; HT = hormone therapy.
Prior CVD is history of MI, stroke, coronary revascularization, angina pectoris, or transient ischemic attack. 3+ risk factors denotes women with no prior CVD but with at least 3 of the following: hypertension, high cholesterol, diabetes mellitus, parental history of premature MI (before age 60), obesity (body mass index (BMI) ≥ 30 kg/m2), current cigarette smoking, and inconsistent report of prior CVD.
Before age 60.
p=0.007 for active vs. placebo.
During the average 9.4 year follow-up, 1,450 women experienced a confirmed CVD event, including 274 MIs, 298 strokes, 889 coronary revascularization procedures, and 395 cardiovascular deaths, with some experiencing more than one event. 995 women died during follow-up.
Vitamin C
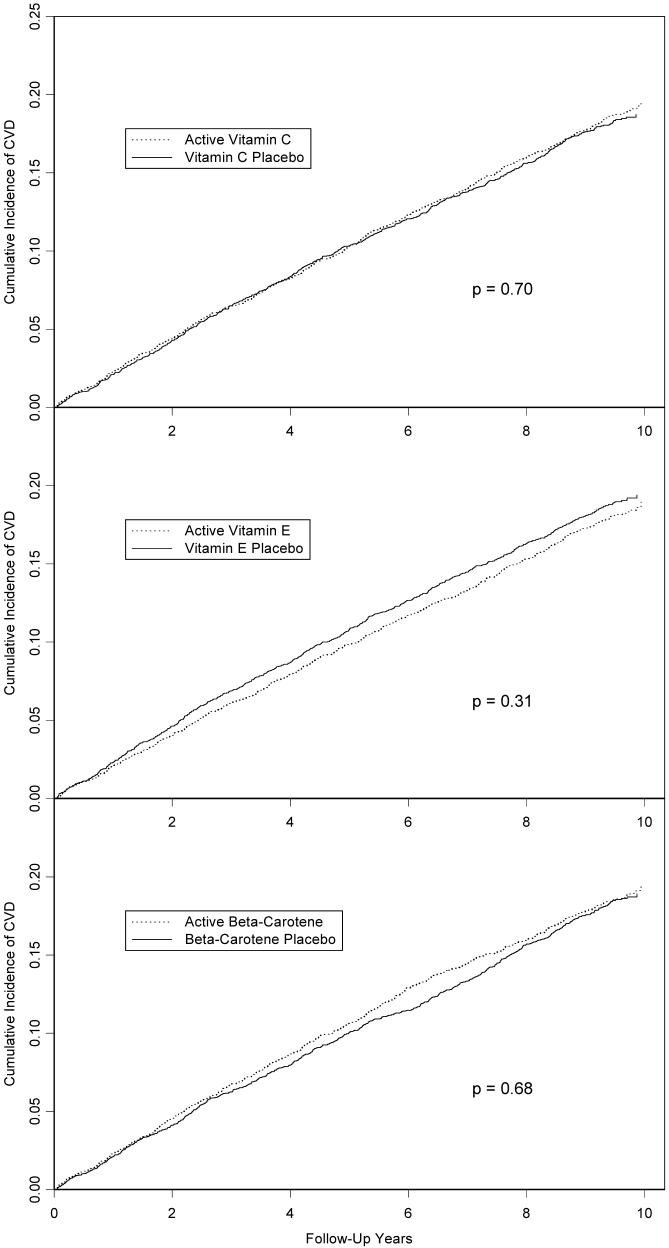
There was no effect of vitamin C on the primary combined endpoint, with a relative risk (RR) of 1.02 (95% confidence interval (CI)=0.92-1.13, p=0.71) (Table 2). Cumulative incidence curves showed no variation of the effect over time (Figure 2), and the test for proportionality of the RR over time was not significant. Individual components of the primary end point also did not differ significantly, although there was some suggestion of a benefit for ischemic stroke. When participants were censored upon noncompliance, results were similar (RR=0.95, 95% CI=0.83-1.09, p=0.47). There were no significant effects of randomized vitamin C on the primary end point in any cardiovascular risk factor subgroup considered (Table 3).
Table 2.
Relative risk of cardiovascular outcomes by randomized antioxidant intervention group in the Women’s Antioxidant Cardiovascular Study.*
| Vitamin C |
Vitamin E |
Beta-carotene |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | N Active | N Plac | RR (95% CI) | p | N Active | N Plac | RR (95% CI) | p | N Active | N Plac | RR (95% CI) | p |
| Major CVD† | 731 | 719 | 1.02 (0.92-1.13) | 0.71 | 708 | 742 | 0.94 (0.85-1.04) | 0.23 | 731 | 719 | 1.02 (0.92-1.13) | 0.71 |
| MI, stroke, CVD death | 419 | 415 | 1.01 (0.88-1.16) | 0.87 | 399 | 435 | 0.90 (0.78-1.03) | 0.12 | 435 | 399 | 1.09 (0.95-1.25) | 0.21 |
| MI‡ | 140 | 134 | 1.05 (0.83-1.33) | 0.70 | 131 | 143 | 0.91 (0.72-1.15) | 0.44 | 135 | 139 | 0.97 (0.77-1.23) | 0.82 |
| Fatal | 15 | 19 | 0.79 (0.40-1.55) | 0.49 | 18 | 16 | 1.11 (0.57-2.18) | 0.76 | 10 | 24 | 0.42 (0.20-0.87) | 0.02 |
| Nonfatal | 125 | 115 | 1.09 (0.85-1.41) | 0.50 | 113 | 127 | 0.88 (0.69-1.14) | 0.34 | 125 | 115 | 1.09 (0.85-1.40) | 0.51 |
| Revascularization‡ | 446 | 443 | 1.01 (0.89-1.15) | 0.88 | 438 | 451 | 0.96 (0.85-1.10) | 0.59 | 438 | 451 | 0.98 (0.86-1.11) | 0.71 |
| Total CHD | 510 | 489 | 1.05 (0.93-1.19) | 0.46 | 491 | 508 | 0.96 (0.85-1.09) | 0.52 | 500 | 499 | 1.01 (0.89-1.14) | 0.92 |
| Stroke‡ | 138 | 160 | 0.86 (0.69-1.08) | 0.21 | 137 | 161 | 0.84 (0.67-1.05) | 0.12 | 161 | 137 | 1.17 (0.93-1.47) | 0.17 |
| Ischemic | 123 | 148 | 0.83 (0.66-1.06) | 0.13 | 121 | 150 | 0.79 (0.62-1.01) | 0.06 | 143 | 128 | 1.12 (0.88-1.42) | 0.37 |
| Hemorrhagic | 13 | 12 | 1.09 (0.50-2.39) | 0.83 | 15 | 10 | 1.47 (0.66-3.27) | 0.35 | 17 | 8 | 2.13 (0.92-4.93) | 0.08 |
| Fatal | 15 | 18 | 0.84 (0.42-1.67) | 0.63 | 18 | 15 | 1.15 (0.58-2.28) | 0.69 | 22 | 11 | 1.98 (0.96-4.07) | 0.07 |
| Nonfatal | 123 | 142 | 0.87 (0.68-1.10) | 0.25 | 119 | 146 | 0.80 (0.63-1.02) | 0.08 | 139 | 126 | 1.10 (0.87-1.40) | 0.42 |
| TIA | 203 | 218 | 0.93 (0.77-1.13) | 0.49 | 205 | 216 | 0.94 (0.78-1.14) | 0.54 | 201 | 220 | 0.91 (0.75-1.10) | 0.35 |
| CVD death‡ | 206 | 189 | 1.10 (0.90-1.33) | 0.37 | 193 | 202 | 0.94 (0.77-1.15) | 0.56 | 211 | 184 | 1.14 (0.94-1.39) | 0.18 |
| Total mortality | 504 | 491 | 1.03 (0.91-1.17) | 0.62 | 502 | 493 | 1.00 (0.89-1.14) | 0.95 | 505 | 490 | 1.03 (0.91-1.17) | 0.65 |
Abbreviations: CVD = cardiovascular disease; MI = myocardial infarction; CHD = coronary heart disease (includes MI, revascularization, CHD death); TIA = transient ischemic attack; RR = relative risk; CI = confidence interval; Plac = Placebo.
Primary outcome, includes MI, stroke, revascularization procedure or death due to cardiovascular cause.
Secondary outcome
Figure 2.
Cumulative incidence of major vascular disease (myocardial infarction, stroke, coronary revascularization, or cardiovascular death), by randomized antioxidant intervention in the Women’s Antioxidant Cardiovascular Study (WACS). P-value is from log-rank test.
Table 3.
Relative risk of major cardiovascular outcomes by randomized intervention within subgroups in the Women’s Antioxidant Cardiovascular Study.*
| Vitamin C |
Vitamin E |
Beta-carotene |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Factor | Active | Placebo | RR (95% CI) | p | Active | Placebo | RR (95% CI) | p | Active | Placebo | RR (95% CI) | p |
| Age (years) | ||||||||||||
| 40-54 | 111 | 123 | 0.90 (0.70-1.17) | 0.44 | 117 | 117 | 1.01 (0.78-1.30) | 0.95 | 115 | 119 | 0.97 (0.75-1.25) | 0.82 |
| 55-64 | 269 | 244 | 1.12 (0.94-1.33) | 0.20 | 246 | 267 | 0.91 (0.77-1.09) | 0.30 | 252 | 261 | 0.96 (0.81-1.15) | 0.68 |
| 65+ | 351 | 352 | 0.99 (0.85-1.15) | 0.91 | 345 | 358 | 0.94 (0.81-1.09) | 0.39 | 364 | 339 | 1.08 (0.93-1.25) | 0.31 |
| Health history | ||||||||||||
| Prior CVD | 592 | 567 | 1.05 (0.93-1.18) | 0.43 | 558 | 601 | 0.89 (0.79-1.00) | 0.04 | 580 | 579 | 1.02 (0.91-1.14) | 0.77 |
| 3+ risk factors | 139 | 152 | 0.93 (0.74-1.16) | 0.51 | 150 | 141 | 1.13 (0.89-1.42) | 0.32 | 151 | 140 | 1.06 (0.84-1.34) | 0.60 |
| Smoking status | ||||||||||||
| Current | 139 | 158 | 0.92 (0.73-1.16) | 0.48 | 151 | 146 | 1.05 (0.84-1.32) | 0.68 | 152 | 145 | 1.12 (0.89-1.40) | 0.34 |
| Past or never | 592 | 561 | 1.05 (0.94-1.18) | 0.38 | 557 | 596 | 0.91 (0.81-1.02) | 0.12 | 579 | 574 | 1.00 (0.89-1.12) | 0.98 |
| Alcohol use in past year | ||||||||||||
| Never | 443 | 442 | 0.97 (0.85-1.11) | 0.68 | 427 | 458 | 0.95 (0.83-1.08) | 0.43 | 461 | 424 | 1.08 (0.95-1.23) | 0.24 |
| Ever | 288 | 277 | 1.08 (0.92-1.28) | 0.35 | 281 | 284 | 0.94 (0.79-1.11) | 0.44 | 270 | 295 | 0.92 (0.78-1.09) | 0.35 |
| Body mass index | ||||||||||||
| <25 kg/m2 | 192 | 170 | 1.17 (0.95-1.44) | 0.14 | 167 | 195 | 0.88 (0.71-1.08) | 0.22 | 197 | 165 | 1.09 (0.88-1.34) | 0.43 |
| 25-<30 kg/m2 | 229 | 225 | 0.98 (0.81-1.18) | 0.82 | 233 | 221 | 0.98 (0.81-1.18) | 0.81 | 210 | 244 | 0.89 (0.74-1.07) | 0.21 |
| 30+ kg/m2 | 310 | 323 | 0.97 (0.83-1.14) | 0.73 | 307 | 326 | 0.94 (0.80-1.10) | 0.43 | 323 | 310 | 1.08 (0.93-1.27) | 0.32 |
| History of hypertension | ||||||||||||
| Yes | 583 | 591 | 0.98 (0.87-1.09) | 0.68 | 570 | 604 | 0.95 (0.84-1.06) | 0.35 | 595 | 579 | 1.01 (0.90-1.13) | 0.87 |
| No | 148 | 128 | 1.21 (0.95-1.53) | 0.12 | 138 | 138 | 0.93 (0.73-1.18) | 0.54 | 136 | 140 | 1.03 (0.82-1.31) | 0.78 |
| Diabetes | ||||||||||||
| Yes | 232 | 235 | 0.96 (0.80-1.15) | 0.63 | 222 | 245 | 0.92 (0.77-1.11) | 0.38 | 244 | 223 | 1.09 (0.91-1.31) | 0.34 |
| No | 499 | 484 | 1.04 (0.92-1.18) | 0.52 | 486 | 497 | 0.95 (0.84-1.08) | 0.45 | 487 | 496 | 0.99 (0.87-1.12) | 0.82 |
| Menopause and HT use | ||||||||||||
| Premenopausal | 26 | 30 | 0.92 (0.54-1.55) | 0.74 | 31 | 25 | 1.26 (0.74-2.13 | 0.40† | 24 | 32 | 0.82 (0.48-1.40) | 0.47 |
| Uncertain | 77 | 65 | 1.16 (0.83-1.61) | 0.39 | 60 | 82 | 0.70 (0.50-0.98) | 0.04 | 74 | 68 | 1.06 (0.77-1.48) | 0.71 |
| Post, current HT | 277 | 252 | 1.07 (0.90-1.27) | 0.45 | 279 | 250 | 1.10 (0.93-1.31) | 0.27 | 280 | 249 | 1.10 (0.92-1.30) | 0.29 |
| Post, no HT | 337 | 359 | 0.96 (0.83-1.11) | 0.59 | 328 | 368 | 0.88 (0.76-1.02) | 0.08‡ | 338 | 358 | 0.96 (0.83-1.11) | 0.60 |
| Current multivitamin use | ||||||||||||
| Yes | 173 | 194 | 0.89 (0.72-1.09) | 0.26 | 179 | 188 | 0.92 (0.75-1.13) | 0.42 | 183 | 184 | 0.99 (0.81-1.22) | 0.94 |
| No | 551 | 518 | 1.07 (0.95-1.21) | 0.28 | 525 | 544 | 0.96 (0.85-1.08) | 0.46 | 540 | 529 | 1.02 (0.90-1.15) | 0.76 |
Abbreviations: CVD = cardiovascular disease; Vit = vitamin; Plac = placebo; RR = relative risk; CI = confidence interval; HT = hormone therapy; Post = postmenopausal. Baseline factors are defined as in Table 1.
P for interaction < 0.05.
Among post-menopausal women, p < 0.05 for interaction comparing HT users vs. non-users.
Vitamin E
No differences were seen in the primary end point by randomized vitamin E assignment (RR=0.94, 95% CI = 0.85-1.04, p=0.23) (Table 2 and Figure 2), with no significant variation in the relative risk over time. We found a non-significant 16% reduction in total stroke, comprised of a 21% reduction in ischemic stroke (p=0.06) and an increase in hemorrhagic stroke based on small numbers. There was an overall 10% reduction in the combination of MI, stroke and CVD death, with a suggestion of a decrease (p=0.08) in benefit over time. No difference in total mortality by vitamin E group was found.
Censoring participants upon noncompliance led to a significant 13% reduction in the primary endpoint (RR=0.87, 95% CI = 0.76-0.99, p=0.04). Reductions in secondary study endpoints were also stronger, with a 22% reduction in MI (RR=0.78, 95% CI = 0.58-1.06, p=0.11), a 27% reduction in stroke (RR=0.73, 95% CI = 0.54-0.98, p=0.04), and a 9% reduction in CVD mortality (RR=0.91, 95% CI = 0.66-1.25, p=0.55). There was a 23% reduction in the combination of MI, stroke or CVD death (RR=0.77, 95% CI = 0.64-0.92, p=0.005). Among those with prior CVD the active vitamin E group experienced fewer major CVD events (RR=0.89, 95% CI = 0.79-1.00, p=0.04, p for interaction = 0.07) (Table 3).
Beta-Carotene
For beta-carotene, there was no difference in the primary end point (RR=1.02, 95% CI = 0.92-1.13, p=0.71) (Table 2 and Figure 2), with no variation over time. There were no significant treatment effects on individual secondary endpoints. There was a non-significant 14% increase in CVD mortality in the active group, with a significant decline over time in the effect on CVD deaths (p=0.04), but no difference in total mortality. When participants were censored upon noncompliance, the effect on the primary endpoint remained non-significant (RR for major vascular disease = 1.09, 95% CI = 0.96-1.24, p=0.18), but an increase in CVD mortality appeared to emerge (RR=1.48, 95% CI = 1.08-2.02, p=0.02).
No statistically significant subgroup effects were seen for the primary endpoint. In particular, in the pre-specified subgroup of smokers, we did not find any elevation in the risk of the primary endpoint (Table 3) or any of the individual components (data not shown).
Combinations of antioxidants
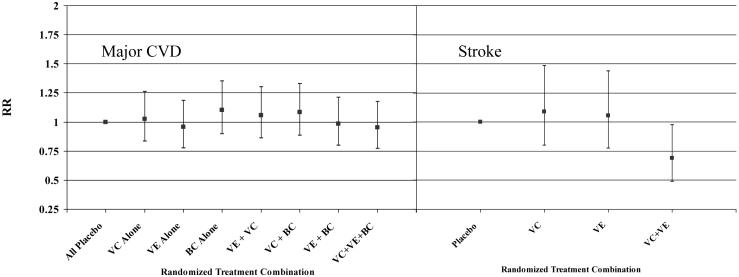
There were no significant two-way or three-way interactions among the agents for the primary endpoint. The effects for each of the combinations of active agents compared to the group with all three placebos is shown in Figure 3 (right). There were also no interactions for the secondary endpoints of MI or cardiovascular death. For stroke, we found a significant two-way interaction between vitamins C and E (p=0.03). Those in the active groups for both agents experienced fewer strokes compared to those in the placebo group for both agents (RR=0.69, 95% CI = 0.49-0.98, p=0.04) (Figure 3, left).
Figure 3.
Relative risk (RR) of major cardiovascular disease by eight combinations of all three active antioxidant assignments relative to the all placebo group (right); or of stroke by combinations of active vitamin C and vitamin E assignments relative to the groups with placebo vitamins C and E (left). VC = vitamin C, VE = vitamin E, BC = beta-carotene, CVD = cardiovascular disease.
Side Effects
We examined reports of bleeding (including gastrointestinal bleeds, hematuria, easy bruising, epistaxis), of gastrointestinal symptoms (including peptic ulcer, gastric upset, nausea, constipation, diarrhea), or of fatigue or drowsiness. There were no statistically significant differences by any of the randomized antioxidant groups except for a small increase in reports of symptoms suggestive of gastric upset among those in the active beta-carotene group (2785 vs. 2717 reports, RR=1.06, 95% CI = 1.00-1.11, p=0.05).
DISCUSSION
In this large-scale randomized trial among high-risk women, we found no overall effects of vitamins E, C or beta-carotene on the primary endpoint of major vascular disease over a long-term follow-up of more than nine years. These null results are consistent with the majority of trials of these antioxidants in both primary and secondary prevention. When combinations of agents were examined, there were no significant interactions, except for a possible reduction in stroke among those taking both active vitamin C and active vitamin E. In contrast to a recent meta-analysis of antioxidant supplements,15 we found no detrimental effects of any of these agents on total or CVD mortality.
No previous trial has considered the effect of vitamin C alone on CVD prevention. A few trials, however, have considered vitamin C as one component of an antioxidant cocktail, but have found no benefit for cardiovascular disease either in primary16, 17 or in secondary18, 19 prevention. Studies of atherosclerotic progression among patients with coronary disease20 or hypercholesterolemia21 have shown inconsistent results for combinations of vitamins C and E. The null results for the primary endpoint seen in WACS further limit enthusiasm for the role of vitamin C in cardiovascular risk protection.
For vitamin E, the lack of effect in primary prevention is consistent with results from three previous intervention trials of vitamin E as a single agent.22-24 As in the Women’s Health Study,24 there was some suggestion of an early effect of vitamin E in WACS (Figure 2), but this diminished over time and was null overall. We also found no evidence of an increase in total mortality at the 600 IU every other day dose tested, as suggested for higher doses in a prior meta-analysis.25 We did find a significant reduction in the primary endpoint in the subgroup of women with prior CVD. Previous trials of vitamin E in secondary prevention have been inconsistent, with significant decreases in CVD found in two trials of shorter 1.4 years duration.26, 27 Later and longer trials have generally not upheld26 this effect. Some found a reduction in secondary outcomes only,28, 29 and another reported no effect at all in a combined high risk primary and secondary prevention population.30, 31 Meta-analyses of patients with prior CVD enrolled in these trials may provide additional insights as to whether there may still be a role for vitamin E in the secondary prevention of cardiovascular disease.
For beta-carotene, we found no overall benefit or harm, a lack of effect that is consistent with studies using a similar 50 mg alternate day regimen.32, 33 The ATBC22 and CARET34 trials found some increase in total and cardiovascular deaths among smokers or those exposed to asbestos, but we found little difference in these endpoints. Compliance-adjusted analyses found some increase in CVD mortality, but these were not adjusted for time-varying risk factors associated with compliance, and are more prone to bias than intent-to-treat analyses. Previous studies in secondary prevention had been small and inconsistent,29, 35 and we found no evidence for benefit or harm in this pre-specified sub-group.
For vitamin E, there have been suggestions that γ-tocopherol is a more powerful antioxidant. Supplementation with α-tocopherol depletes γ-tocopherol, which may explain the lack of effect seen in vitamin E trials.36 Single antioxidants may not reflect the complex vitamins and nutrients found in foods, which may explain the discrepancies between most intervention trials and studies of fruits and vegetables.37 Trials using an antioxidant combination, however, also have not shown a clear benefit for CVD.16-19 Questions concerning the complex nature of dietary effects on lipid peroxidation, however, are deserving of further study.
Limitations of the trial include the lack of complete follow-up and compliance. Mortality information follow-up was virtually complete through 2003, then 93% complete for the remaining two years. Overall, however, mortality follow-up as a percentage of person-time was over 99% complete. Although suboptimal, compliance in WACS over time is relatively comparable to several other trials of vitamin supplementation with at least four years duration in secondary,19, 29 as well as primary17, 38, 39 prevention.
In summary, results from WACS and other antioxidant trials have not found consistent preventive effects on CVD. Overall, we found no benefit on the primary combined endpoint for any of the antioxidant agents tested, alone or in combination. We also found no evidence for harm. While additional research into combinations of agents, particularly for stroke, may be of interest, widespread use of these individual agents for cardiovascular protection does not appear warranted.
Acknowledgements
We acknowledge the invaluable contributions of the WACS staff, including Marilyn Chown, Shamikhah Curry, Margarette Haubourg, Felicia Zangi, Tony Laurinaitis, Geneva McNair, Philomena Quinn, Harriet Samuelson, Ara Sarkissian, and Martin Van Denburgh. We also thank the endpoints reviewers, including Michelle Albert, Gavin Blake, Claudia Chae, Wendy Chen, Bill Christen, Carlos Kase, Tobias Kurth, I-Min Lee, Aruna Pradhan, Paul Ridker, Jackie Suk, and James Taylor. Finally, we are indebted to the 8,171 dedicated WACS participants.
Footnotes
Funding/Support: This study was supported by investigator-initiated grant HL47959 from the National Heart, Lung, and Blood Institute. Vitamin E and its placebo were supplied by Cognis Corporation (LaGrange, IL). All other agents and their placebos were supplied by BASF Corporation (Mount Olive, NJ). Pill packaging was provided by Cognis and BASF.
Role of the Sponsors: Neither Cognis nor BASF provided any input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Other Financial Disclosures: None.
REFERENCES
- 1.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 2.Diaz MN, Frei B, Vita JA, Keaney JFJ. Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 4.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006;136:2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 5.Stampfer MJ, Hennekens CH, Manson JE, et al. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 6.Rimm EB, Stampfer MJ, Asherio A, et al. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328:1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 7.Kushi LH, Folsum AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med. 1996;334:1156–1162. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 8.Losonczy KG, Harris TB, Havlik RJ. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: The Iowa Women′s Health Study. Am J Clin Nutr. 1996;64:190–196. doi: 10.1093/ajcn/64.2.190. [DOI] [PubMed] [Google Scholar]
- 9.Hak AE, Ma J, Powell CB, et al. Prospective study of plasma carotenoids and tocopherols in relation to risk of ischemic stroke. Stroke. 2004;35:1584–1588. doi: 10.1161/01.STR.0000132197.67350.bd. [DOI] [PubMed] [Google Scholar]
- 10.Buijsse B, Feskens JM, Schlettwein-Gsell D, et al. Plasma carotene and α-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA) Am J Clin Nutr. 2005;82:879–886. doi: 10.1093/ajcn/82.4.879. [DOI] [PubMed] [Google Scholar]
- 11.Hak AE, Stampfer MJ, Campos H, et al. Plasma carotenoids and tocopherols and risk of myocardial infarction in a low-risk population of US male physicians. Circ. 2003;108:802–807. doi: 10.1161/01.CIR.0000084546.82738.89. [DOI] [PubMed] [Google Scholar]
- 12.Evans RW, Shaten BJ, Day BW, et al. Prospective association between lipid soluble antioxidants and risk of myocardial infarction in the elderly: The Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1998;147:180–186. doi: 10.1093/oxfordjournals.aje.a009432. [DOI] [PubMed] [Google Scholar]
- 13.Bassuk SS, Manson JE, Gaziano JM. Antioxidant vitamins. In: Manson JE, Buring JE, Ridker PM, Gaziano JM, editors. Clinical Trials in Heart Disease. Elsevier; Philadelphia, PA: 2004. [Google Scholar]
- 14.Bassuk SS, Albert CM, Cook NR, et al. The Women′s Antioxidant Cardiovascular Study: Design and baseline characteristics of participants. J Women′s Health. 2004;13:99–117. doi: 10.1089/154099904322836519. [DOI] [PubMed] [Google Scholar]
- 15.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 16.Blot WJ, Li JY, Taylor PR, et al. Nutritional intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:483–492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 17.Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 18.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 19.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20 536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 20.Waters DD, Alderman EL, Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 21.Salonen RM, Nyyssönen K, Kaikkonen J, et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: The Antioxidant Supplementation in Atherosclerosis Prevention Study (ASAP) Circ. 2003;107:947–953. doi: 10.1161/01.cir.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- 22.Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 23.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: A randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 24.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer. The Women′s Health Study: A randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 25.Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 26.Stephens NG, Parsons A, Schofield PM, et al. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 27.Boaz M, Smetana S, Weinstein T, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomized placebo-controlled trial. Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 28.GISSI-Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell′Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 29.Rapola JM, Virtamo J, Ripatti JK, et al. Randomized trial of α-tocopherol and β-carotene supplements on incidence of major coronary events in men with previous myocardial infarction. Lancet. 1997;349:1715–1720. doi: 10.1016/S0140-6736(97)01234-8. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Dagenais G, Pogue J, et al. Vitamin E supplementation and cardiovascular events in high-risk patients: The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 31.The HOPE and HOPE-TOO Trial Investigators Effects of long-term vitamin E supplementation on cardiovascular events and cancer. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 32.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 33.Lee IM, Cook NR, Manson JE, et al. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: The Women′s Health Study. J Natl Cancer Inst. 1999;91:2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 34.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 35.Gaziano JM, Manson JE, Ridker PM, Buring JE, Hennekens CH. Beta carotene therapy for chronic stable angina [abstract] Circ. 1990;82:III-202. [Google Scholar]
- 36.Devaraj S, Jialel I. Failure of vitamin E in clinical trials: Is gamma-tocopherol the answer? Nutr Rev. 2005;63:290–293. doi: 10.1111/j.1753-4887.2005.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 37.Blomhoff R. Dietary antioxidants and cardiovascular disease. Curr Opin Lipidol. 2005;16:47–54. doi: 10.1097/00041433-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg ER, Baron JA, Karagas MR, et al. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA. 1996;275:699–703. doi: 10.1001/jama.1996.03530330043027. [DOI] [PubMed] [Google Scholar]