Abstract
Objective
To examine the utility of using urea concentrations for determining synovial fluid (SF) joint volume in effused and non-effused joints.
Methods
Knee joint SF was aspirated from 159 human study participants with symptomatic OA of at least one knee either directly (165 knees) or by lavage (110 knees). Serum was obtained immediately prior to SF aspiration. Participants were asked to rate individual knee pain, aching or stiffness. SF and serum urea levels were determined using a specific enzymatic method run on an automated CMA600 analyzer. Cell counts were performed on direct SF aspirates when volume permitted. The formula for calculating SF joint volume was as follows: Vj=CD(VI)/(C−CD) with Vj=volume SF in entire joint, CD = concentration urea in diluted (lavage) SF, VI=volume saline injected into joint, and C = concentration urea in undiluted (neat) SF derived below where C=0.897(Cs), Cs = concentration urea in serum.
Results
There was an excellent correlation (r2=0.8588) between SF and serum urea in the direct aspirates with a ratio of 0.897 (SF/serum). Neither urea levels nor the SF/serum ratio showed any correlation with KL grade, or cell count. While urea levels increased with age there was no change in the ratio. Intraarticular SF volumes calculated for the lavaged knees ranged from 0.555ml to 71.71ml with a median volume of 3.048ml. There was no correlation of SF volume to KL grade but there was a positive correlation (p=0.001) between SF volume and self reported individual knee pain.
Conclusion
Our urea results for direct aspirates indicate an equilibrium state between serum and SF with regard to the water fraction. This equilibrium exists regardless of disease status (KL grade), inflammation (cell count), or age, making it possible to calculate intraarticular volume of lavaged joints based upon this urea method. Most of the joint volumes we calculated fell within the previously reported range for normal knees of 0.5–4.0mls. The positive correlation between SF volume and knee symptoms reinforces the clinical utility of this method for quantifying SF volume.
Keywords: urea, osteoarthritis, synovial fluid, pain
Introduction
Synovial Fluid (SF) derived markers are often used to monitor tissue degradation in joint diseases such as Rheumatoid Arthritis (RA) and Osteoarthritis (OA) in humans and animals. Monitoring of a single index joint is most directly done by analysis of joint fluid. At times it becomes desirable to estimate synovial fluid volume to determine total amount of a biomarker in the joint. This may be of special use in cases of effusion.
Estimations of synovial fluid volume have typically been performed with radioisotopic dilution techniques that include [113In]-indium chloride or sodium [99Tc]-pertechnetate.1 Nearly 2 decades ago, a non-radioactive method was reported for estimating intra-articular volume based upon albumin dilution upon intraarticular injection of saline.2 This method required aspirating an initial volume of synovial fluid directly. The retained or residual volume was estimated by injecting a known quantity of saline (10–50 mls) followed by 2 minutes of external joint massage to promote intraarticular mixing, followed by reaspiration of the diluted synovial fluid. The concentration of albumin in this latter sample, compared to the concentration of albumin in the undiluted sample aspirated first, allowed determination of the residual volume.
Another reported method of measuring synovial fluid volume relied upon injection of high molecular weight dextrans (one fluorescently tagged) to minimize potential efflux of marker from the joint after intraarticular injection.3 This study was limited by the need to inject a non-native molecule, and the use of a hypertonic solution to further counteract efflux of marker from the joint. In so doing however, the hypertonic solution may have promoted transsynovial fluid flux into the joint, leading to an artifactual increase in intraarticular volume. Interestingly, the intraarticular volumes determined by albumin dilution were comparable to estimations based upon proteoglycan dilution but not hyaluronan dilution. The authors posited that hyaluronan adhered to intraarticular surfaces, and thus underestimated intraarticular volumes, while albumin did not.
A more recent study estimated joint fluid volume in rabbits by dividing the total calcium concentration in the joint cavity lavage (μg/joint) by calcium concentration in the plasma (μg/ml).4 To confirm the relevance of the method, radioactivity in the plasma and joint cavity lavage was determined after intravenously injecting 3 H2O. Based upon the calcium ratio technique, joint fluid volumes ranged from a mean 401 μl/joint in normal rabbits to 680 μl/joint in arthritic rabbits. These correlated with results obtained from the radioisotope method (r = 0.985). An advantage of this study was that it relied upon a native molecule, however, calcium can be taken up by cells in the various joint tissues and therefore is subject to error dependent upon the metabolic activity of the cells.
We further explored a method of determining intraarticular synovial fluid volume, based upon measuring urea, that we previously showed was useful for quantifying dilution of synovial fluid after joint lavage.5 Urea is a small molecule (60 Da), it is neither synthesized nor metabolized by joint tissues, and it diffuses freely between the blood and intra-articular space. Maroudas has reported that urea also freely diffuses between an external solution and cartilage with a partition coefficient of 1.0 for 200–600μm slices stirred in solute for 30 minutes.6 Additionally, it has been shown that a large amount of albumin, another native molecule, resides in the periarticular tissue mass.7 For these reasons, we also examined the potential for this method to be confounded by a contribution of urea from cartilage during the course of a brief lavage procedure. We also examined the relationship between joint volume and self-reported knee pain.
Methods
Participants
A total of 159 participants were enrolled in the NIH funded POP study (Prediction of Osteoarthritis Progression). Participants met American College of Rheumatology criteria for symptomatic OA of at least one knee. In addition, all participants met radiographic criteria for OA with Kellgren Lawrence8 (KL) grade of ≥ 1 in at least one knee. Participants were excluded on the basis of bilateral knee replacement or bilateral knee OA of KL grade 4. Knee radiographs were read by two graders (VBK, GV). A total of 275 knees were included in the analysis.
Sample collection
Except for replaced knees (total of 10), synovial fluid aspiration was attempted for both knees of all participants. Synovial fluid was aspirated directly and without lavage (neat) from 165 knees. When direct aspiration failed to yield synovial fluid, the aspiration syringe was exchanged and 10 ml sterile saline (without preservative) was injected through the same needle, thus requiring only one needle stick per arthrocentesis for each knee assessed. The time between saline injection to aspiration never exceeded 2 minutes. Synovial fluid cell counts were performed when volumes permitted (greater than 0.1 ml available). Then synovial fluids were centrifuged at 3500 rpm for 10 mins and the supernatant was frozen at −80C. Immediately before the synovial fluid was aspirated, blood was obtained for serum urea assessments, and serum was frozen at −80C until analysis.
In-vitro cartilage experiment
Fresh cartilage surgical waste specimens were collected at the time of total knee replacement. Normal appearing full-thickness cartilage slices were harvested remote from any macroscopic lesions. Plugs (8mm2) were taken from this slice and embedded in a well of silicon, with the bottom and sides of the cartilage sealed and only the surface exposed. The plugs were equilibrated overnight with synovial fluid. The synovial fluid was then removed, the surface patted dry, and a X20 dilution of synovial fluid in saline was added to the surface of the cartilage for 2 minutes using only enough fluid (≈25μl) to cover the surface. This simulated the in vivo lavage methodology. The aqueous sample was then removed and analyzed for urea and compared with the urea concentration in the original X20 dilution of synovial fluid.
Urea measurements
Concentrations of urea were determined by a CMA600 microdialysis analyzer (CMA Microdialysis, Solna, Sweden) on 5μl samples of sera and joint fluid. Concentrations of urea were obtained by completely automated enzymatic reaction techniques. Quantification of urea in this assay depends on the rate of utilization of nicotinamide adenine dinucleotide (NAD) and was measured at 365 nm. A measurement cycle was completed in less than two minutes. All reagents were obtained from CMA microdialysis.
Pain measurements
Participants with OA were recruited through the community and a rheumatology clinic. Some participants had bilateral knee symptoms (n=117) and others unilateral knee symptoms (n=30) at the time of sample collection. Participants were asked to rate individual knee pain, aching or stiffness that occurred on most days of any one month in the last year as well as most days of the present month. Pain was rated as none, mild, moderate or severe.
Results
The sample consisted of 159 participants with symptomatic knee OA during the last year in at least one knee; 79 had bilateral moderate to severe radiographic knee OA (KL grades 2–4), and of the total 275 knees evaluated, 91 were KL grades 0–1. The participants (118 females, 41 males) ranged in age from 35 to 85 years old with a mean age of 64 years (12 years SD). We obtained synovial fluid by direct aspiration from 165 knees and by lavage from 110 knees for a total of 275 knees aspirated. Knees were only lavaged when it was not possible to obtain fluid directly. Neat knee aspirations yielded on average 2.3 mls (2.7 mls SD) synovial fluid and lavaged joints yielded 2.8mls (3.3 mls SD) synovial fluid. There was an excellent correlation (r2 = 0.8588) between synovial fluid and serum urea in the direct aspirates (Fig. 1) with a ratio of 0.897 (SF/serum). There was also an excellent correlation (r2 = 0.984) between synovial fluid urea concentrations between paired contralateral joints aspirated directly. As expected, neither urea concentrations nor the SF/serum ratio showed any correlation with KL grade or cell count. While urea concentrations increased with age there was no change in the ratio. In our cartilage explant diffusion study we found no evidence of cartilage contributing urea during a simulated two minute lavage procedure.
Figure 1.

Correlation of synovial fluid (SF) and serum urea concentrations (mmol/ml) in SF samples aspirated directly (without lavage).
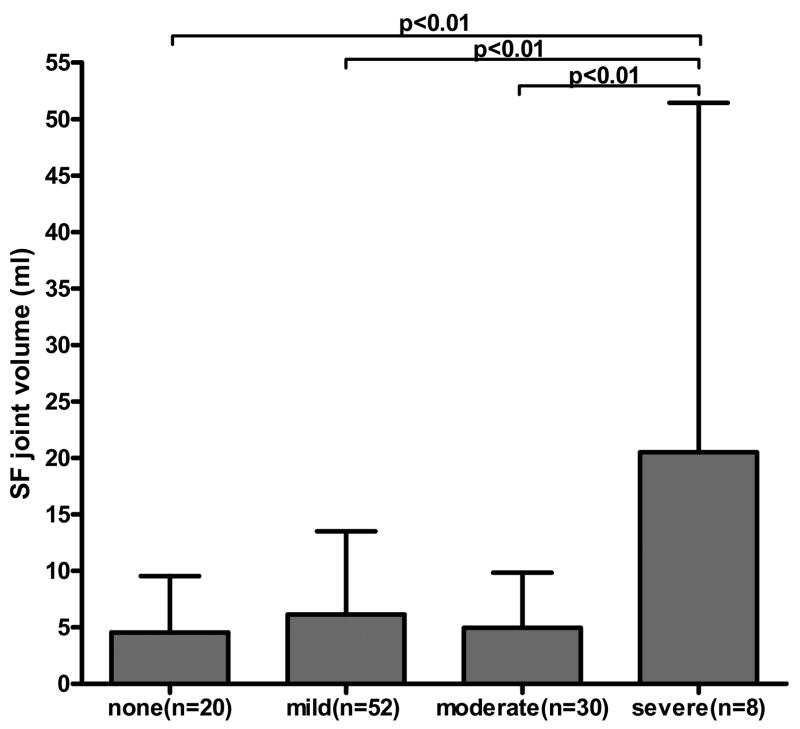
The formula for calculating synovial fluid joint volume in the lavaged joints, regardless of effusion, is a modification of the albumin dilution method2 which uses the measurement of a native molecule before and after dilution with a known amount of saline. The volume of saline can vary as long as it is sufficient to provide adequate mixing within the joint. The formula was as follows: Vj=CD(VI)/(C−CD) with Vj=volume SF in entire joint, CD = concentration urea in diluted (lavage) SF, VI=volume saline injected into joint, and C = concentration urea in undiluted (neat) SF. Since our method uses only one aspiration, the concentration of analyte before dilution (C) is derived using the serum urea (CS) as a proxy for the neat SF urea. Since the SF urea in neat samples is slightly lower but highly correlated to serum urea, C was derived from the ratio of SF/serum urea in neat samples as described above, multiplied by the concentration of urea in serum CS: C=0.897(CS). Intra-articular synovial fluid volumes calculated for the lavaged knees ranged from 0.56mls to 71.71mls with a median volume of 3.05mls. There was no correlation of SF volume to KL grade but there was a positive correlation by ANOVA (p=0.001) between SF volume and self reported individual knee pain on most days of the present month (Fig. 2) as well as most days of any month in the last year (p=0.001). The mean SF volume was 4.5mls for knees without pain (n=20). The mean SF volume was 7.0mls for knees with any pain (n=90). The eight knees with most severe pain were associated with the highest mean SF volume (20.5mls).
Fig. 2.
Knee joint synovial fluid (SF) volume by pain in knees aspirated by lavage (n=110). The mean (+SD) synovial fluid volume is shown relative to self-reported knee pain on most days of present month on a 4-point scale. Bonferroni's multiple comparison test is shown. Oneway ANOVA: p=0.001.
Discussion
We observed a SF/serum urea ratio of 0.897 in direct aspirates of OA knees. In another study, the SF/serum urea ratio in RA (N=10) and OA (N=11) patients was 1.14 (Ilya Tchetverikov and TeKoppele unpublished communication). Both these results are similar to the ratios of 1.06 in healthy canines5 and 0.95 in bovines9 with no signs of arthritis. This ratio indicates an equilibrium state between serum and synovial fluid with regard to the water fraction in RA/OA patients. This equilibrium exists regardless of disease severity (KL grade), inflammation (cell count), or age, making it possible to calculate intraarticular volume of lavaged joints based upon this urea method. This known relationship can be used to estimate intra-articular synovial fluid volume in normal joints and those with chronic arthritides.
Most of the joint volumes we calculated fell within the previously reported range for normal knees of 0.5 ml to 4.0 mls.10 The limitations of the method include the need for adequate mixing after saline injection as well as the probable need for a minimum of 10 mls of saline for lavage. Although a previous study recommended 15 mls or more of saline per injection,2 we chose to inject 10mls of saline as an amount that would give us good recovery while keeping participant discomfort to a minimum. This method may not be applicable in joints with acute effusions, for instance acute joint injury, as the serum to synovial fluid urea ratio may vary from what we have found for normal joints and chronically arthritic joints described here. As long as the time of lavage is kept to a minimum, urea washout from surrounding tissues should have no effect on the volume calculation. Since we did not test the efflux of urea from synovial tissue, we cannot exclude a contribution of urea from this source that would serve to result in an overestimation of intraarticular synovial fluid volume. However, we do not expect such confounding to be an issue for two reasons: first, the minimal duration of the lavage procedure; and second, our previous experience demonstrating an excellent correlation of four separate synovial fluid analytes measured in one knee from a neat sample and in the contralateral knee by the urea-corrected lavage method.5 It is nevertheless important to keep in mind that any confounding of intraarticular volume estimates by potential contributions of urea from adjacent joint tissues would be lessened by the presence of an effusion.
The positive correlation between SF volume and knee symptoms provides independent confirmation of the recently reported association of knee pain and stiffness with moderate and large joint effusions by magnetic resonance imaging (MRI).11 This correlation reinforces the clinical utility of this method for quantifying SF volume. It would be valuable to further validate this method with intraarticular volume estimation by MRI.
Acknowledgments
We wish to thank Norine Hall for database assistance.
Supported by the following funding sources: NIH/NIAMS grant RO1 AR4879, UO1 AR050898 and NIH/NIAMS P50 AR049056
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rekonen A, Oka M, Kuikka J. Measurement of synovial fluid volume by a radioisotope method. Scand J Rheumatol. 1973;2:33–35. [PubMed] [Google Scholar]
- 2.Geborek P, Saxne T, Heinegard D, Wollheim FA. Measurement of synovial fluid volume using albumin dilution upon intraarticular saline injection. J Rheumatol. 1988;15:91–94. [PubMed] [Google Scholar]
- 3.Delecrin J, Oka M. Measurement of synovial fluid volume: a new dilution method adapted to fluid permeation from the synovial cavity. J Rheumatol. 1992;34:1746–1752. [PubMed] [Google Scholar]
- 4.Matsuzaka S, Sato S, Miyauchi S. Estimation of joint fluid volume in the knee joint of rabbits by measuring the endogenous calcium concentration. Clin Exp Rheumatol. 2002;20:531–534. [PubMed] [Google Scholar]
- 5.Kraus VB, Huebner JL, Fink C, King JB, Brown S, Vail TP, et al. Urea as a passive transport marker for arthritis biomarker studies. Osteoarthritis Cartilage. 2002;46(2):420–427. doi: 10.1002/art.10124. [DOI] [PubMed] [Google Scholar]
- 6.Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys J. 1970;10:365–379. doi: 10.1016/S0006-3495(70)86307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman PJ, Scott D, Ray J, Mason RM, Levick JR. Hyaluronan secretion into the synovial cavity of rabbit knees and comparison with albumin turnover. J Physiol. 1997;503:645–656. doi: 10.1111/j.1469-7793.1997.645bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthritis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ropes MW, Bennett GA, Bauer W. The origin and nature of normal synovial fluid. J Clin Invest. 1939;18(3):351–372. doi: 10.1172/JCI101050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarty DJ. Synovial fluid. In: McCarty DJ, editor. Arthritis and Allied Conditions. Philadelphia: Lea and Febiger; 1979. pp. 51–69. [Google Scholar]
- 11.Kornaat PR, Bloem JL, Cuelemans RYT, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239(3):811–817. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]