Abstract
Myasthenia gravis (MG) is a T cell-regulated, antibody-mediated autoimmune disease. Two peptides representing sequences of the human acetylcholine receptor α-subunit, p195-212 and p259-271, were previously shown to stimulate peripheral blood lymphocytes of patients with MG and were found to be immunodominant T cell epitopes in SJL and BALB/c mice, respectively. Single amino acid substituted analogs of p195-212 (analog Ala-207) and p259-271 (analog Lys-262) were synthesized. We showed that analogs Ala-207 and Lys-262 inhibited, in vitro and in vivo, the proliferative responses of T cell lines specific to the relevant peptide and lymph node cells of mice immunized to p195-212 and p259-271, respectively. To inhibit T cell responses to both peptides (p195-212 and p259-271), we synthesized dual analogs composed of the tandemly arranged two single (Ala-207 and Lys-262) analogs (dual analog) either sequentially (Ala-207–Lys-262) or reciprocally (Lys-262–Ala-207). In the present study, we report that both dual analogs could bind to major histocompatibility complex class II molecules on antigen-presenting cells of SJL and BALB/c mice. Analog Lys-262–Ala-207, which bound more efficiently to major histocompatibility complex class II molecules, was found to inhibit the proliferative responses of both p195-212- and p259-271-specific T cell lines. Furthermore, the analog inhibited the in vivo priming of lymph node cells of both SJL and BALB/c mice when administered i.v., i.p., or per os. The dual analog Lys-262–Ala-207 could also immunomodulate myasthenogenic manifestations in mice with experimental autoimmune MG induced by inoculation of a pathogenic T cell line. Thus, a single peptide that is composed of analogs to two epitope specificities can be used to regulate T cell responses and disease associated with each epitope.
Keywords: T cell activation, binding to major histocompatibility complex class II, altered peptide ligand, experimental autoimmune myasthenia gravis, immunotherapy
Myasthenia gravis (MG) is an autoimmune disease caused by antibody-mediated autoimmune responses to the nicotinic acetylcholine receptor (1, 2). Although autoantibodies play a pivotal role in the development of MG, T cells were shown to be important in the pathogenesis of this disease (3–6). Two peptides representing sequences of the human acetylcholine receptor α-subunit, namely p195-212 and p259-271, were previously shown by our laboratory to significantly stimulate peripheral blood lymphocytes of patients with MG and to serve as immunodominant T cell epitopes of SJL and BALB/c mice, respectively (7, 8). T cell lines specific to peptides p195-212 and p259-271 (TCSJL195-212 and TCBALB/c259-271) were established in our laboratory from lymph node (LN) cells of immunized SJL and BALB/c mice, respectively, and were found to induce experimental autoimmune MG (EAMG) following inoculation into naive syngeneic mice (9).
The current treatment of MG is nonspecific (reviewed in ref. 1). It has been proposed that T cell responses may be inhibited by peptides that bind to major histocompatibility complex (MHC) class II restriction elements but that do not activate specific T cells. Such inhibitory peptides may be used to specifically treat autoimmune diseases by inhibiting the pathogenic T cell responses (10–12). It has been also demonstrated that peptide analogs related in sequence to the native peptides have a greater capacity to inhibit specific T cell responses in comparison to peptides that are unrelated to the native peptides (13–15). In an attempt to inhibit myasthenogenic autoreactive T cells in a specific manner, we synthesized single amino acid substituted analogs of peptides p195-212 and p259-271: analog Ala-207 (previously designated no. 455) of p195-212 and analog Lys-262 (previously designated no. 306) of p259-271 (16). We showed that analogs Ala-207 and Lys-262 inhibited in vitro the proliferative response of cells of the TCSJL195-212 and TCBALB/c259-271 lines, respectively. Moreover, when the analogs were administered either i.v. or i.p. to mice immunized with the appropriate myasthenogenic peptide, the proliferative responses of their LN cells to the immunogens were inhibited (16).
One of our goals is to design an analog that will be effective in inhibiting T cell responses to both peptides p195-212 and p259-271. To this end, we synthesized two analogs composed of the tandemly arranged two single (Ala-207 and Lys-262) analogs (dual analog) either sequentially (Ala-207–Lys-262) or reciprocally (Lys-262–Ala-207). In the present study, we report that both dual analogs could bind to MHC class II molecules on antigen-presenting cells (APC) of SJL and BALB/c mice. However, analog Lys-262–Ala-207 was more efficient in its binding to MHC class II of both strains. The latter analog inhibited the proliferative responses of both p195-212- and p259-271-specific T cell lines. Furthermore, it inhibited the in vivo priming of LN cells of both SJL and BALB/c mice when administered i.v., i.p., or per os, concomitant with immunization with the myasthenogenic peptides. We also show the efficient inhibitory capacity of the dual analog when it is administered 7 days postimmunization with the myasthenogenic peptides. Furthermore, the dual analog (Lys-262–Ala-207) could immunomodulate myasthenogenic manifestations in mice with EAMG induced by inoculation of a pathogenic T cell line.
MATERIALS AND METHODS
Mice.
Mice of the inbred strains SJL (The Jackson Laboratory) and BALB/c (Olac, Bichester, U.K.) were used at the age of 8–12 weeks.
Synthetic Peptides and Peptide Analogs.
Peptides p195-212 (DTPYLDITYHFVMQRLPL) and p259-271 (VIVELIPSTSSAV), peptide analogs (Ala-207, DTPYLDITYHFVAQRLPL, and Lys-262, VIVKLIPSTSSAV), the dual analogs (Ala-207–Lys-262, DTPYLDITYHFVAQRLPLVIVKLIPSTSSAV, and Lys-262–Ala-207, VIVKLIPSTSSAVDTPYLDITYHFVAQRLPL), and a reversed peptide of the latter dual analog (LPLRQAVFHYTIDLYPTDVASSTSPILKVIV) that was used as control were prepared with an automated synthesizer (model AMS 422; Abimed Analyses-Technik, Langenfeld, Germany), while the side chain groups were protected following the company’s protocol for 9-fluorenylmethoxycarbonyl (N-Fmoc) strategy. (Boldface type indicates modified residues.) Cleavage of peptides from the polymeric carrier was achieved by reacting with a mixture of trifluoroacetic acid (TFA)/H2O/triethylsilane (90:5:5, vol/vol), for 2 hr at room temperature. Crude peptides were purified to homogeneity by semipreparative HPLC on a Lichrosorb RP-8 column (7 μM, 250 × 10 mM; Merck) by using a linear gradient established between 0.1% trifluoroacetic acid in double-distilled water (DDW) and 75% acetonitrile in DDW containing 0.1% TFA. Purity of the peptides and peptide analogs was confirmed by analytical HPLC (RP-18, 125 × 4 mM; Merck) using the above gradient and by amino acid analysis, following exhaustive acid (6 M HCl) hydrolysis.
Immunizations.
For priming of LN cells, mice were injected intradermally into the hindfoot pads with 10–20 μg of peptide in complete Freund’s adjuvant (CFA; Difco; 100 μl total volume) and LN cells were harvested 10–12 days later. For inhibition of in vivo priming, the analog was administered either i.p., i.v., or orally in 0.2–0.5 ml of PBS (pH 7.2), either concomitant with priming of the LN cells or 7 days later.
Culture of Antigen-Specific T Cell Lines.
T cell lines specific to p195-212 and to p259-271 were established as previously described (16) and maintained in culture in enriched RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; GIBCO/BRL) and 10% supernatant of concanavalin A (Con-A) stimulated splenocytes at 2–5 × 106 cells per flask (17). Cells were exposed to the stimulating peptide (50–100 μg/ml) presented on irradiated (3,000 rad) syngeneic spleen cells every 14 days (17).
Proliferative Responses of T Cell Lines.
The capacity of the analog to inhibit specific proliferative responses of the T cell lines was tested 7 or 10 days after antigenic stimulation. Cells (104 per well) were cultured with 0.5 × 106 irradiated (3,000 rad) syngeneic spleen cells in the presence of different concentrations of the peptides, with or without the analog. Cultures were established in 200 μl of enriched RPMI 1640 medium (17) containing 10% FCS in flat-bottomed microtiter plates. At the end of a 48-hr incubation period, 0.5 μCi (1 Ci = 37 GBq) of [3H]thymidine (5 Ci/mmol; Nuclear Research Center, Negev, Israel) was added. Cells were harvested and radioactivity counted 16 hr later.
Proliferative Responses of LN Cells.
Popliteal LN cells (0.5 × 106) obtained from immunized mice were cultured in enriched RPMI 1640 medium (17) supplemented with 1% normal mouse serum, in the presence of various concentrations of antigen, for 96 hr. Then 0.5 μCi of [3H]thymidine was added, and 16 hr later plates were harvested onto filter paper and radioactivity was counted.
Biotinylation of Peptides.
N-terminal biotinylation of peptide p195-212 and the analogs Ala-207 and Ala-207–Lys-262 was performed with excess biotin-N-hydroxysuccinimide (Sigma; ref. 18). N-terminal biotinylation of p259-271, Lys-262, and Lys-262–Ala-207 was performed with excess biotinamidocaproate N-hydroxysuccinimide ester (Sigma) as described (19).
Binding of Peptides to Splenic Adherent Cells.
Splenic cells (1 × 108) suspended in RPMI 1640 medium and supplemented with 5% FCS were incubated in Petri dishes at 37°C for 1 hr. Thereafter, nonadherent cells were removed and the plates were washed and placed on ice. Adherent cells were collected and were incubated (1 × 106 cells per sample) in the presence of different concentrations of the biotinylated peptides, in PBS containing 0.1% BSA, in a 37°C incubator containing 5% CO2. Following 20 hr of incubation, the cells were washed and then incubated with 25 μl of phycoerythrin-conjugated streptavidin diluted 1:70 in PBS (Jackson ImmunoResearch). After washing, the cells were further incubated with biotinylated goat anti-streptavidin (Vector Laboratories). The cells were washed and were incubated again with 25 μl of phycoerythrin-conjugated streptavidin. After washing, the cells were resuspended in PBS and then analyzed. The fluorescence profile of the stained cells was determined using a fluorescence-activated cell sorter and a cellquest software (FACSort; Becton Dickinson; ref. 19).
Induction of EAMG.
For the induction of EAMG, BALB/c mice were injected i.v. with 5 × 106 cells of the TCBALB/c259-271 T cell line. The cells were exposed to their specific peptide for 3–4 days and then transferred to antigen-free medium containing 10% Con-A supernatant for 7 days. At this time, only T cells could be detected in the culture. Before injection, cells were washed thoroughly and resuspended in PBS (0.5 ml total volume).
Electrophysiological Evaluations.
Mice were anesthetized with sodium pentothal (1.2 mg per mouse) and were restrained on a dissecting board. Stimulation was performed with paired needle electrodes inserted percutaneously at the sciatic notch. Stimulation and recording were done with conventional electromyography equipment (Medelec, Old Woking Surrey, U.K.). The decrement in the compound muscle action potentials (CMAPs) was calculated as the difference between the first and fifth CMAP (peak to peak) and expressed as percentage of the first CMAP. Recordings were obtained from at least four locations within the gastrocnemius muscle and were considered as positive for myasthenic decrement only if a decremental response of 10% or more was obtained in at least two different recording sites within the muscle (20). Electromyographies were performed with the examiner blinded to whether mice belonged to control or experimental groups.
Statistical Analyses.
The Fisher’s exact test was performed to analyze the in vivo experiments (21).
RESULTS
Binding of the Myasthenogenic Peptides and of the Dual Analogs to Murine APC.
It was of interest to compare the binding capacity of the myasthenogenic peptides to APC of SJL and BALB/c mice to that of the two dual analogs. To this end, direct binding assays were performed with the different peptides. Peptides p195-212 and p259-271 and the dual analogs Ala-207–Lys-262 and Lys-262–Ala-207 were covalently bound to a biotin reagent via their N termini. The biotinylated peptides were added to adherent cells of SJL and BALB/c splenocytes at different concentrations in the range of 0.1–100 μM, and binding was monitored by FACS analysis. The efficiency of binding was calculated for the biotinylated peptides out of the binding assays, and the results are summarized in Table 1. The binding efficiencies of the two analogs (based on their KD-like values) to MHC determinants on APCs of each mouse strain were similar to that determined for the respective myasthenogenic peptide. Nevertheless, the binding efficiency of analog Lys-262–Ala-207 appeared to be greater than that of analog Ala-207–Lys-262 for APCs of both mouse strains and similar to that of the relevant myasthenogenic peptide. Thus, further experiments were performed with the former analog.
Table 1.
Binding of peptides to MHC class II on APCs
| Biotinylated peptide
|
||||
|---|---|---|---|---|
| Mouse strain | p195–212 | p259–271 | Ala-207–Lys-262 | Lys-262–Ala-202 |
| SJL | 0.3 × 10−6 M | No binding | 5 × 10−6 M | 0.5 × 10−6 M |
| BALB/c | Not done | 3.4 × 10−6 M | 8 × 10−6 M | 3.9 × 10−6 M |
APCs (106 cells per sample) of SJL and BALB/c mice were incubated for 20 hr in the presence and absence of each of the biotinylated peptides: p195-212, p259-271, 207-Ala–Lys-262, and Lys-262–Ala-207. Phycoerythrin–streptavidin staining was followed as described. Thereafter, cells were subjected to flow cytometric analysis. Double-reciprocal conversion of the net mean fluorescence intensity (MFI) values (after subtraction of background fluorescence) was used to calculate the Kd-like constants.
Inhibition of the Proliferative Responses of the Lines by Analog Lys-262–Ala-207.
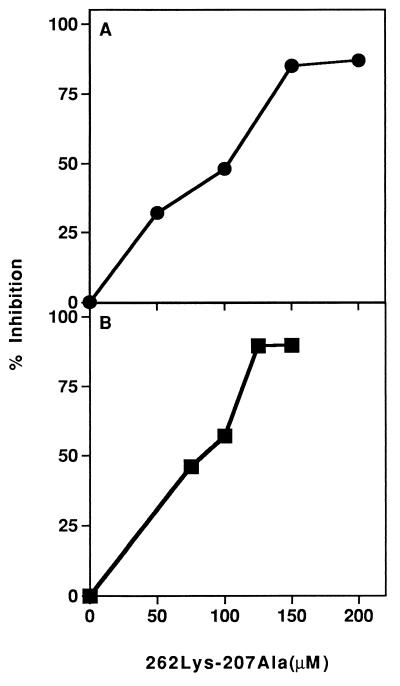
The dual analog Lys-262–Ala-207 was tested for its ability to inhibit the proliferative responses of both TCSJL195-212 and TCBALB/c259-271 T cell lines when cocultured with their stimulating peptides p195-212 and p259-271, respectively. Fig. 1 demonstrates representative experiments of inhibition of the proliferative responses of both T cell lines. As can be seen, analog Lys-262–Ala-207 inhibited the proliferative responses of both lines in a dose-dependent manner, with a maximum inhibition of 87% and 90% for lines TCSJL195-212 and TCBALB/c259-271, respectively. In addition, the dual analog did not inhibit nonspecifically the proliferative responses of the line to Con-A supernatant (data not shown), suggesting that the inhibitory activity of the dual analog Lys-262–Ala-207 is specific.
Figure 1.
Inhibition of proliferative responses of T cell lines specific to myasthenogenic peptides. Different concentrations of analog Lys-262–Ala-207 were added to (A) cultures of cells of the TCSJL195-212 line and 5 μM p195-212 or to (B) cells of the TCBALB/c259-271 line in the presence of 20 μM p259-271. After 48 hr of incubation, [3H]thymidine was added. Sixteen hours later, plates were harvested onto filter paper. Results are expressed as percent inhibition of the specific proliferative response of peptides p195-212 • (stimulation index = 43) or p259-271 ▪ (stimulation index = 79).
In Vivo Inhibition of Priming of LN Cells by the Dual Analog.
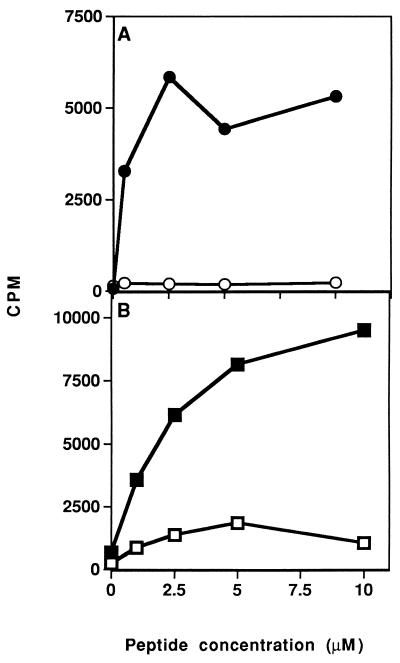
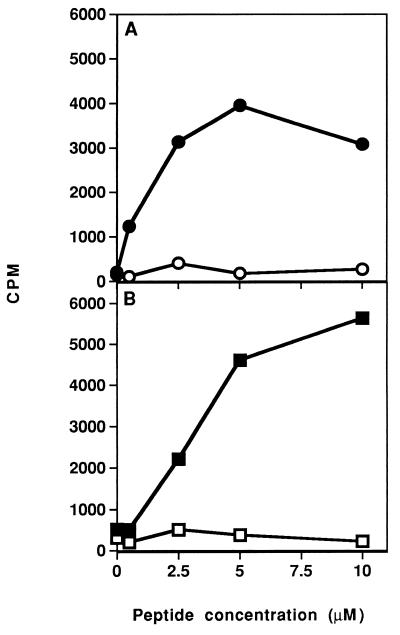
After determining that the dual analog could inhibit in vitro proliferative responses of both lines, its ability to inhibit the in vivo priming of LN cells was tested. To this end, SJL and BALB/c mice were immunized intradermally with their appropriate myasthenogenic peptides (either p195-212 or p259-271, respectively) in CFA, concomitant with administration in aqueous solution of the dual analog either i.p. (for SJL) or i.v. (for BALB/c). Thereafter, the proliferative capacity of the LN cells in response to the priming peptide was tested in vitro. Fig. 2 represents typical results of such experiments. As can be seen, inoculation of both strains of mice with Lys-262–Ala-207 inhibited the ability of their LN cells to proliferate (in SJL up to 99% inhibition and in BALB/c up to 90% inhibition) in response to the relevant myasthenogenic peptide. No significant inhibition was observed in the group inoculated with the dual analog when the response to the mitogen Con-A was tested (in SJL mice, a mean of 9,614 cpm in the control group as compared with 9,876 cpm in the dual analog-inoculated group, and in BALB/c mice, a mean of 6,900 cpm in the control group as compared with 11,000 cpm in the dual analog-inoculated group). It is noteworthy that a peptide that was synthesized in the reversed sequence of the dual analog Lys-262–Ala-207 and that served as a control peptide either did not inhibit at all or inhibited to a significantly lesser extent the proliferative response of primed LN cells (data not shown). It has been of further interest to find out whether the inhibitory effect of the dual analog could be detected when it is administered at the stage that the LN cells are already activated. To this end, 200 μg of analog Lys-262–Ala-207 was administered either i.p. or i.v. 7 days after immunization of SJL or BALB/c mice with their myasthenogenic peptide (either p195-212 or p259-271, respectively). Thereafter, the proliferative capacity of the LN cells in response to the priming peptide was tested in vitro. As demonstrated in Fig. 3, a delayed inoculation with analog Lys-262–Ala-207 resulted in an efficient inhibition (in SJL up to 98% inhibition and in BALB/c up to 99% inhibition) of the capacity of both p195-212 and p259-271 primed LN cells to proliferate to their immunizing peptide. In this case as well, no significant inhibition was observed in the group inoculated with the dual analog when the response to the mitogen Con-A was determined (in SJL mice, a mean of 13,006 cpm in the control group as compared with 12,123 cpm in the dual analog-inoculated group, and, in BALB/c mice, a mean of 4,600 cpm in the control group as compared with 4,450 cpm in the dual analog-inoculated group). These results that were reproduced in a series of experiments indicate that the dual analog can inhibit cell activation to both myasthenogenic peptides, even when administered to the mouse when its immunocytes are already activated.
Figure 2.
Inhibition of in vivo priming of LN cells to the myasthenogenic peptides. (A) SJL mice were injected intradermally to the hindfoot pads with 10 μg of p195-212 in CFA, with ○ or without • concomitant i.p. inoculation of analog Lys-262–Ala-207 (200 μg in 500 μl of PBS). (B) BALB/c mice were injected intradermally to the hindfoot pads with 20 μg of p259-271 in CFA (100 μl), with □ or without ▪ concomitant i.v. inoculation of analog Lys-262–Ala-207 (200 μg in 500 μl PBS). LN cells taken from the mice 10 days later were incubated in the presence of various concentrations of the relevant peptide for 96 hr. Thereafter, [3H]thymidine was added and 16 hr later cells were harvested and radioactivity was counted. Results are expressed as mean cpm of triplicate cultures. SD values did not exceed 10%.
Figure 3.
Inhibition of the in vivo priming of LN cells to the myasthenogenic peptides by the dual analog given 7 days following immunization. (A) SJL and (B) BALB/c mice were injected intradermally in the hindfoot pads with 10 μg of p195-212 and 20 μg of p259-271, respectively, in CFA. Analog Lys-262–Ala-207 (200 μg in 500 μl of PBS) was injected i.p. (SJL mice, ○) and i.v. (BALB/c mice, □) 7 days after the priming. LN cells obtained from the mice were incubated in the presence of various concentrations of p195-212 or p259-271 for 96 hr. Thereafter, [3H]thymidine was added and 16 hr later cells were harvested and radioactivity was counted. Results are expressed as mean cpm of triplicate cultures. SD values did not exceed 10%.
Inhibition of Priming of LN Cells, with the Dual Analog Administered per Os.
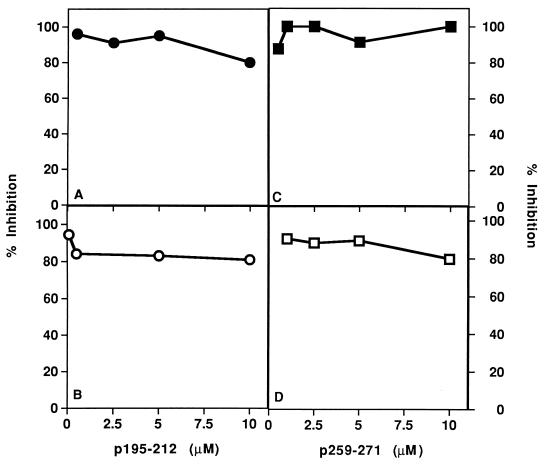
The ability of analog Lys-262–Ala-207 to inhibit priming of LN cells with the myasthenogenic peptides was then tested following the administration of the analog per os (orally). To this end SJL and BALB/c mice were immunized intradermally with either p195-212 or p259-271, respectively, in CFA. The dual analog was given to the mice per os along with or 7 days postpriming. Dose–response experiments showed that for efficient inhibition 500 μg of the dual analog were usually needed when given per os as compared with 200 μg used for the i.p. or i.v. administration. The results of representative experiments shown in Fig. 4 indicate that the dual analog that was given per os inhibited efficiently the priming of LN cells of both SJL mice by p195–212 and of BALB/c mice by p259–271. It is noteworthy that the LN cell proliferation in response to Con-A was not affected significantly by the oral administration of Lys-262–Ala-207 (between 0% inhibition in most experiments up to 20% in a few cases).
Figure 4.
Inhibition of the in vivo priming by oral administration of a dual analog. SJL mice (A and B) and BALB/c mice (C and D) were injected intradermally in the hindfoot pads with 10 μg of p195-212 and 20 μg p259-271, respectively, in CFA. Analog Lys-262–Ala-207 (500 μg in 300 μl of PBS) was administered per os either concomitant with (A, •, and C, ▪) or a week after (B, ○, and D, □) the priming. LN cells obtained from mice 10 days following immunization were incubated in the presence of various concentrations of p195-212 or p259-271 for 96 hr. Thereafter, [3H]thymidine was added and 16 hr later cells were harvested and radioactivity was counted. Results are expressed as percent of inhibition of the specific proliferative responses to the myasthenogenic peptide, as measured in LN cells of mice that were primed but not inhibited (for SJL, 71 cpm background, 5,826 cpm at optimal peptide concentration; for BALB/c, 246 cpm background, 6,904 cpm at optimal peptide concentration).
Inhibition of EAMG Manifestations in Mice by the Dual Analog.
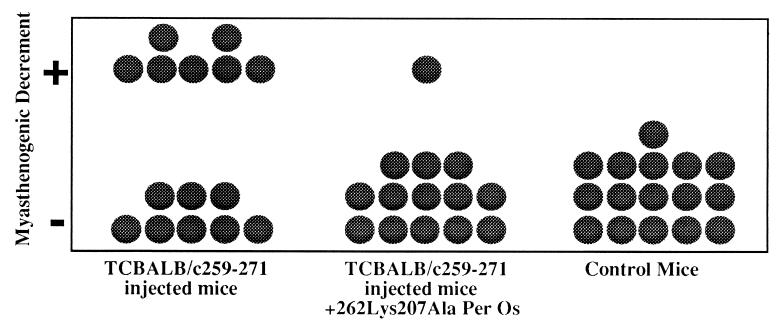
The dual analog Lys-262–Ala-207 was further tested for its ability to reverse EAMG manifestations in BALB/c mice, which were inoculated with cells of the TCBALB/c259-271 line. To this end, BALB/c mice were injected i.v. with cells of the line (5 × 106 cells). Fourteen days later (a period in which half of the mice developed impairment of neuromuscular transmission), the mice were divided into two groups. One group was treated with the dual analog administered per os (500 μg, twice a week for 4 weeks), and the other group of mice was not treated. At the end of this treatment protocol, mice were subjected to electromyography evaluation. Muscle weakness was diagnosed in the mice if their electrophysiological evaluation revealed a myasthenogenic decrement. The results demonstrated in Fig. 5 indicate that a myasthenogenic decrement was detected in 7 of 15 mice that were inoculated with cells of the TCBALB/c259-271 line, but in only 1 of 14 mice that were inoculated with cells of the line and were afterwards treated with analog Lys-262–Ala-207. Thus, the dual analog Lys-262–Ala-207 appears to be capable of reversing EAMG manifestations.
Figure 5.
Immunomodulation of EAMG manifestations by oral administration of the dual analog. Stimulation of mice was performed with paired needle electrodes inserted percutaneously at the sciatic notch. CMAPs were recorded during 3 Hz repetitive stimulation (eight stimuli) administrated at supramaximal stimulus intensity. Decrement was calculated as the difference between the first and the fifth CMAP (peak to peak) and was expressed as percent of the first CMAP. Recordings were obtained from at least four locations within the muscle and were considered as positive for myasthenogenic decrement, only if a decremental response of 10% or more was found in at least two different recording sites within the muscle. The Fisher’s exact test was performed to test the null hypothesis of no association between treatment with Lys-262–Ala-207 and response. The probability value was 0.022, which allowed us to reject the null hypothesis.
DISCUSSION
The main findings of the present study are that a dual analog of two myasthenogenic T cell epitopes can inhibit antigen-specific lymphocyte responses in SJL and BALB/c mice both in vitro and in vivo. Moreover, this dual analog could reverse established EAMG in BALB/c mice. The results of these studies suggest that the in vitro activity of the dual analog corresponds with an ability to modulate immune responses in vivo.
Mechanisms by which peptidic analogs of native T cell epitopes inhibit T cell responses have been studied by a number of groups. In vitro studies have revealed that peptidic analogs may modulate T cell responses in one or more of three ways: (i) by blockade of MHC presentation using molar excess of competitor peptides restricted to the same class II molecule as the stimulatory peptide (13, 15, 22); (ii) by TCR antagonism or inhibition of T cell activation by engagement of the TCR in the absence of signal delivery (13, 14); and (iii) by partial TCR agonism that leads to incomplete or altered signal delivery (23).
In addition to the mechanisms of T cell modulation revealed by in vitro studies, in vivo studies have shown that altered peptide ligands can control CD4+ T cell differentiation (24), by shifting the response toward secretion of Th2-type suppressive cytokines. This was demonstrated in the experimental autoimmune encephalomyelitis system for analogs of two autoantigens involved in disease induction: a peptide analog of proteolipid protein (25) and copolymer 1, a synthetic polypeptide analog of myelin basic protein, which was found effective in suppressing both experimental autoimmune encephalomyelitis and relapsing–remitting multiple sclerosis (26).
MHC blockade has been well documented both in our laboratory (22) as well as in other laboratories (13, 15). The ability of the dual analog to inhibit in vitro proliferative responses of the peptide specific T cell lines may be due in part or all to MHC blockade. However, in vivo inhibitory capacity of the dual analog when delivered at the same time as the stimulatory peptide is not likely to be due solely to MHC blockade, since a single administration of a 5- to 10-fold excess of the analog led to successful inhibition. Moreover, MHC blockade cannot explain the ability of the dual analog to inhibit previously induced immune responses. Thus the ability of the dual analog to inhibit in vivo priming of LN cells 1 week after the primary immunization, as well as its ability to reverse established EAMG, suggest that it operates through an antigen-specific mechanism, by being either a T cell antagonist or a partial agonist.
One goal of this study was to determine whether the dual analog could inhibit T cell responses in vivo when administered orally. Larger amounts of Lys-262–Ala-207 (2.5 fold) were needed to obtain the same effect when the peptide was delivered per os than when it was delivered i.v. or i.p. This is not surprising, considering the acidic conditions and the presence of proteolytic enzymes in the stomach (27, 28). Oral administration of Lys-262–Ala-207 7 days postimmunization with the myasthenogenic peptides was as effective as both oral administration on day 0 and i.p. or i.v. inoculaton on day 0 and day 7. This suggests that the dual analog Lys-262–Ala-207 may be therapeutically useful when administered orally. Weiner et al. (29) have suggested two mechanisms by which peptides may leave the gut. (i) Direct passage to the circulatory system, leading to systemic antigen presentation, usually occurs when high doses are administered. (ii) Passage through the Peyer’s patches, leading to local presentation by gut associated APC and to the generation of regulatory T cells, usually occurs when low doses, as in the present study, are administered.
For the dual peptide to be considered of therapeutic potential, it must be able to treat an established disease. Indeed, per os administration of Lys-262–Ala-207 to EAMG afflicted BALB/c mice reversed the myasthenogenic symptoms of those mice. Reversal of an established autoimmune disease such experimental autoimmune encephalomyelitis was previously demonstrated by copolymer 1 (26) and recently also by a peptide analog of myelin basic protein (30). Thus, there is increasing experimental evidence that peptidic analogs of pathogenic T cell epitopes are viable candidates for specific immunomodulatory therapy.
We have shown here that it has been possible to design and synthesize a single peptide that is composed of analogs to two T cell epitopes and, as demonstrated in the present study, is capable of immunomodulating autoimmune T cell responses and disease manifestations associated with each epitope. We have recently demonstrated that p195-212- and p259-271-specific in vitro proliferative responses of peripheral blood lymphocytes from MG patients could be inhibited significantly by the appropriate single amino acid analog(s) and by the dual analog (19). Collectively, the results of our murine systems described here and the previously described in vitro inhibition of peripheral blood lymphocyte response of human MG patients to the myasthenogenic epitopes suggest that the dual analog Lys-262–Ala-207 is a good candidate for inhibition of T cell responses of MG patients and might have a therapeutic potential.
Acknowledgments
We gratefully acknowledge Prof. Irun R. Cohen for helpful discussions and critical review of the manuscript. This research was supported by Teva Pharmaceutical Industries Ltd., Petach-Tikva, Israel (M.S. and E.M.).
ABBREVIATIONS
- MG
myasthenia gravis
- LN
lymph node
- EAMG
experimental autoimmune MG
- MHC
major histocompatibility complex
- APC
antigen-presenting cell
- CFA
complete Freund’s adjuvant
- Con-A
concanavalin A
- CMAP
compound muscle action potential
References
- 1.Drachman D B. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom J, Shelton D, Fuji Y. Adv Immunol. 1988;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- 3.Hohlfeld R, Toyka K V, Heininger K, Grosse-Wilde H, Kalies I. Nature (London) 1984;310:244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- 4.Melms A, Chrestel S, Schalke B C G, Wekerle H, Mauron A, Ballivet M, Barkas T. J Clin Invest. 1989;83:785–790. doi: 10.1172/JCI113958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Link H, Xu Z Y, Melms A, Kalbacher H, Sun J B, Wang Z Y, Fredrikson S, Olsson T. Scand J Immunol. 1992;36:405–14. doi: 10.1111/j.1365-3083.1992.tb02954.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z Y, Link H, Huang W X. Scand J Immunol. 1993;37:615–622. doi: 10.1111/j.1365-3083.1993.tb02580.x. [DOI] [PubMed] [Google Scholar]
- 7.Brocke S, Brautbar C, Steinman L, Abramsky O, Rothbard J, Neumann D, Fuchs S, Mozes E. J Clin Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brocke S, Dayan M, Rothbard J, Fuchs S, Mozes E. Immunology. 1990;69:495–500. [PMC free article] [PubMed] [Google Scholar]
- 9.Kirshner S L, Katz-Levy Y, Wirguin Y, Argov Z, Mozes E. Cell Immunol. 1994;157:11–28. doi: 10.1006/cimm.1994.1201. [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum D, Aharoni R, Arnon R, Sela M. Proc Natl Acad Sci USA. 1988;85:9724–9728. doi: 10.1073/pnas.85.24.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai K, Zamvil S S, Mitchell D J, Hodgkinson S, Rothbard J B, Steinman L. Proc Natl Acad Sci USA. 1989;86:9470–9474. doi: 10.1073/pnas.86.23.9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gautman A M, Glynn P. J Immunol. 1990;144:1177–1180. [PubMed] [Google Scholar]
- 13.Sette A, Alexander J, Ruppert J, Snoke K, Franco A, Ishioka G, Grey H M. Annu Rev Immunol. 1994;12:413–431. doi: 10.1146/annurev.iy.12.040194.002213. [DOI] [PubMed] [Google Scholar]
- 14.de Magistris M T, Alexander J, Coggeshall M, Altman A, Gaeta F C, Grey H M, Sette A. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 15.Wauben M H M, Boog C J P, van der Zee R, Joosten I, Schlief A, van Eden W. J Exp Med. 1992;176:667–677. doi: 10.1084/jem.176.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz-Levy Y, Kirshner S L, Sela M, Mozes E. Proc Natl Acad Sci USA. 1993;90:7000–7004. doi: 10.1073/pnas.90.15.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axelrod O, Mozes E. Immunobiology. 1986;172:99–109. doi: 10.1016/S0171-2985(86)80056-0. [DOI] [PubMed] [Google Scholar]
- 18.Mozes E, Dayan M, Zisman E, Brocke S, Licht A, Pecht I. EMBO J. 1989;8:4049–4052. doi: 10.1002/j.1460-2075.1989.tb08588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zisman E, Katz-Levy Y, Dayan M, Kirshner S L, Paas-Rozner M, Karni A, Abramsky O, Brautbar H, Fridkin M, Sela M, Mozes E. Proc Natl Acad Sci USA. 1996;93:4492–4497. doi: 10.1073/pnas.93.9.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berman P W, Patric J. J Exp Med. 1980;151:204–210. doi: 10.1084/jem.151.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodges J L J, Lehmann E L. Basic Concepts of Probability and Statistics. 2nd Ed. San Francisco: Holden–Day; 1970. [Google Scholar]
- 22.Brocke S, Dayan M, Steinman L, Rothbard J, Mozes E. Int Immunol. 1990;2:735–742. doi: 10.1093/intimm/2.8.735. [DOI] [PubMed] [Google Scholar]
- 23.Sloan-Lancaster J, Allen P M. Curr Opin Immunol. 1995;7:103–109. doi: 10.1016/0952-7915(95)80035-2. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson L B, Greer J M, Sobel R A, Lees M B, Kuchroo V K. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 26.Teitelbaum D, Arnon R, Sela M. Cell Mol Life Sci. 1997;53:24–28. doi: 10.1007/PL00000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanson D G, Roy M J, Miller S D, Seidman E G, Thomas M J, Sanderson I R, Udall J N, Ely I, Green G M. Reg Immunol. 1993;5:85–93. [PubMed] [Google Scholar]
- 28.Roberts P R, Zaloga G P. New Horiz. 1994;2:237–243. [PubMed] [Google Scholar]
- 29.Weiner H L, Friedman A, Miller A, Khoury S J, Al-Sabbaj A, Santos L, Sayegh M, Nussenblatt R B, Trentham D E, Hafler D A. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 30.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Steinman L. Nature (London) 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]