Abstract
At the proximal part of mouse chromosome 17 there are three well-defined genes affecting the axis of the embryo and consequently tail length: Brachyury, Brachyury the second, and the t-complex tail interaction (T1, T2, and tct). The existence of T1 and tct in fact defines the classical “t-complex” that occupies ∼40 cM of mouse chromosome 17. Their relationship to each other and various unlinked interacting genes has been enigmatic. The tint gene was the first of the latter to be identified. We report here its genetic mapping using a microsatellite scan together with outcrosses to Mus spretus and M. castaneous followed by a subsequent testcross to T, T1, and T2 mutants. Surprisingly, tint interacts with T2 but not with T1. The implications of our data suggest that T2 may be part of the T1 regulatory region through direct or indirect participation of tint.
A combination of classical genetics, gene cloning, and experimental embryology has revealed that neural tube defects in mice and, by implication, in humans are a developmentally heterogeneous group of malformations (Juriloff and Harris 2000; Copp et al. 2003). This heterogeneity and contributing environmental factors have been some of the reasons for the sporadic nature of these conditions. Furthermore, an expanding body of evidence indicates that neural tube development is a multigenic process that may involve several independently segregating genes (Estibeiro et al. 1993; Copp 1994; Helwig et al. 1995; Greco et al. 1996; Doudney and Stanier 2005). The combination of Brachyury (T) and tct is one of the oldest and most penetrant models for this developmental defect (Park et al. 1989) but has been incompletely understood.
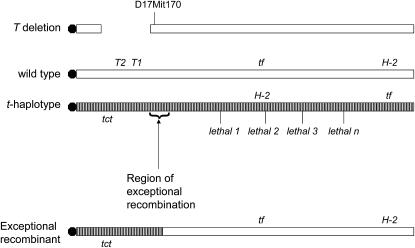
The t complex located on proximal third of mouse chromosome 17 is characterized by four inversions that prevent recombination between mutant and wild-type chromosomes (Figure 1, striped bars). Every t-bearing chromosome trapped in the wild in Europe and the Americas always contains at least one recessive embryo lethal (Silver 1981). In fact, in the days before mouse knockout technology, most of the known recessive embryo lethals in mice were mapped to the t-complex. None of them are cloned yet because of their complicated genetics. The net effect of the inversions is that t-haplotypes act like the balancer chromosomes of Drosophila locking up the entire region against recombination. However, exceptional recombination can occur at the rate of 1/1000 because of inverted repeats. Other remarkable features of t-haplotypes are male transmission ratio distortion making t's look like a virus in terms of population genetics. In fact, t-haplotypes are found in 10% of wild mice (Ardlie and Silver 1996).
Figure 1.—
Diagram of the proximal third of chromosome 17. The striped chromosome represents inverted regions in t-haplotypes and the solid circle, the centromere. Various markers are shown: H-2, the mouse major histocompability complex; tf, tufted, a visible hair growth pattern mutation; various lethals; and D17Mit170, a marker near the distal end of the T deletion. See text for explanation of T1, T2, and tct.
T is a deletion (see Table 1 for all genes mentioned) that includes a semidominant mutation that has been found in only non-t-carrying wild-type chromosomes. It causes a short tail in heterozygotes and is lethal in homozygotes. Affected embryos die shortly after gastrulation, presenting a complete loss of the posterior mesoderm due to primitive streak defects. Differentiation of anterior portions of the notochord is also disrupted followed by its complete absence in posterior portions of the embryo (Gruneberg 1958; Yanagisawa et al. 1981).
TABLE 1.
Summary of genes mentioned
| Gene symbol | Gene name | Allele symbol, name(s) | Map position (Chr: Mb) | Notes | Reference |
|---|---|---|---|---|---|
| T | Brachyury | Del(17)T, deletion on Chr 17, Brachyury | Chr 17: ∼8.04–8.54 | Deletion >200 kb | Herrmann (1990) |
| T1 | Brachyury the first | T1Wis, Brachyury Wisconsin | Chr 17: 8.27–8.28 | Transcription factor, hypermorph | Rennebeck (1998) |
| T2 | Brachyury the second | T2Bob, Brother of Brachyury, T2Tg(H2K)1Art | Chr 17: 8.23–8.26 | Transgene insertion | Rennebeck (1999) |
| tct | t complex tail interaction | Only existing allele | Chr 17: ∼4.02 cM | Not cloned, only in t-haplotypes | Silver (1981) |
| tint | t-interacting factor | Only existing allele | Chr 6: 81.09–88.09 | Not cloned | This article |
| Noto | Notochord homolog (Xenopus laevis) | Nototc, truncate | Chr 6: 85.38–85.39 | Point mutation in the homeobox | Abdelkhalek(2004) |
| tf | tufted | None | Chr 17: 13.3 cM | Abnormal hair growth, not cloned | Lyon (1956) |
| Sp5 | trans-acting transcription factor 5 | Sp5tm1Rbe, targeted mutation 1, Rosa Beddington | Chr 2: 70.28–70.27 | Transcription factor | Harrison (2000) |
| Aft | abnormal feet and tail | Only existing allele | Chr 9: 32 cM | Not cloned | Ruvinsky (2002) |
| brm1 | brachyury modifier 1 | QTL | Chr 9: 57.695–57.696 | QTL | Agulnik (1998) |
| brm2 | brachyury modifier 2 | QTL | Chr 15: 82.468–82.469 | QTL | Agulnik (1999) |
| D17Mit170 | DNA segment, Chr 17, MIT 170 | Not relevant | Chr 17: 8.54 | Marker for distal end of Del(17)T | This article |
In the mouse, studies have shown that, although the Brachyury phenotype is dependent on the presence of a mutation mapping to the T/t locus, its severity can be profoundly affected by changes in genetic background (Wittman and Hamburgh 1968; Mikova and Ivanyi 1974). This suggests that the Brachyury gene product might interact with multiple factors during embryo development that can affect the variability at which the “T pathway” functions.
Finally, and most important for this study, every wild trapped t-haplotype by definition carries a mutation proximally known as tct (t-complex interaction factor) (see Figure 1). tct as studied in exceptional recombinants without a linked lethal has no phenotype on its own even in homozygotes. The classical identification of a t-haplotype is that in compound heterozygotes (T/tct), it produces a tailless rather than a short-tailed mouse. Thus, tct interacts with the classical T deletion.
Great effort has gone into the analysis of the relationship between T and tct (Justice and Bode 1988). The interaction between these two mutations suggests that the lesions are in the same gene. However, genetic analysis of this phenomenon has been immensely complicated by the fact that tct is carried exclusively by all t-haplotypes that contain multiple inversions (Howard et al. 1990) that have historically made recombinational analysis impossible. Over the years, the assessment remained that either T and tct were two alleles at a single locus or mutations at two closely linked loci (Rennebeck et al. 1995, 1998). tct is still not cloned.
The cloning of the T gene (Herrmann et al. 1990) did not resolve the one vs. two gene question because the t-haplotype copy of T has not been shown to have a mutation.
The Brachyury gene, hereafter designated as T1 (Table 1), is the founding member of the T-box gene family of transcriptional factors (Papaioannou 1997; Showell et al. 2004). T1 is a classical transcriptional activator that is localized to the nucleus, binds DNA, and functions cell autonomously (Kispert and Herrmann 1993; Wilson et al. 1993; Conlon et al. 1996). The N-terminal region of the protein contains a rather large DNA-binding core followed by an activation domain at the C terminus. The finding of repressor domains together with activation domains has generated some debate since the former has not been conserved among vertebrate species (Kispert et al. 1995). Brachyury homologs have been identified in many different organisms: Xenopus laevis (Xbra), zebrafish (ntl), Halocynthia (As-T), and Ciona (CiBra and CsBra) (Smith et al. 1991; Schulte-Merker et al. 1994; Yasuo et al. 1996; Corbo et al. 1997). Comparison of their function has shown that Brachyury's role in morphogenesis and mesodermal cell fate is evolutionarily conserved. Curiously, subtle phenotypic differences among Brachyury mutations in different species suggest that its regulation, despite being considered conserved, requires different molecular players (Halpern et al. 1993).
A second Brachyury gene, named “Brachyury the second” (T2), was identified and cloned (Rennebeck et al. 1995). T2Bob is the only mutation of this gene identified so far. It was found by the analysis of a transgenic insertion that is known to map 12 kb upstream of the first T1 gene on the same (+) DNA strand. The original T mutation has been shown to be a deletion of about 200 kb with the T1 gene located approximately in the middle (Herrmann et al. 1990) and is now known to delete both the T1 and T2 genes. T1 and T2 do not compliment each other or tct. The phenotype of T2/T2 is similar to T1 but is less affected in all measurable ways. The embryological defect in T2Bob starts more caudally than in T1 embryos and is mostly limited to the notochord.
The genome sequencing in mice revealed that there are no genes directly telomeric to T1; however, in addition to T2, there are several coding units centromeric that could also be included in the original T deletion. These genes include chemokine receptor 6 (Ccr6); brain protein 44-like (Brp44l); SFT2 domain containing 1 (Sft2d1), a SNARE-like protein believed to be required for traffic through the Golgi complex (Banfield et al. 1995); and two genes of unknown function, 4930506C21Rik and 9630019k15Rik. Although the T1 and T2 genes map physically close to one another, they are unrelated in sequence.
For decades, simple maintenance of exceptional proximal tct stocks constituted an unheralded enhancer screen. Homozygous tct/tct, without the presence of a lethal, produces exclusively normal tailed progeny; however, the appearance of a single short-tailed offspring led to the identification of a new mutation, tint (Artzt et al. 1987). tint is unlinked to T as measured by independent segregation of the visible marker tufted (tf). It was the first non T/t complex, non-chromosome 17 tail interacting factor that by itself has no phenotype in homozygotes but interacts with great specificity with both the original T deletion and tct.
We report here the genetic mapping of tint. A microsatellite scan was used together with outcrosses to Mus spretus and M. castaneous followed by a subsequent testcross to T, T1, and T2 mutants. Surprisingly, tint interacts with T2Bob but not with T1Wis. The implications of such interaction suggest that T2 may be part of T1 regulation through indirect or direct participation of tint.
MATERIALS AND METHODS
Mice:
The inbred strain homozygous for tint is designated tint/Art (Artzt et al. 1987) and has been brother sister mated since 1986 (more than F96 generations). However, this strain had many genetic inputs including random bred and wild mice and thus their microsatellite genotype was unknown. tint/tint have normal tails because the gene is only evident in compound with either T or tct from the t complex on chromosome 17. To produce a heterozygous F1, tint/tint mice were crossed to a “non inbred” subline of M. spretus (Spr/Jls-RP), which took origin in Spain and was maintained by the late Verne Chapman at Roswell Park until 1990 when it was transferred to the JLS colony at the University of California at Davis. The more commonly used SPRET/Ei strain was obtained from the Jackson Laboratory and was used in later experiments as a control for microsatellite mapping. Cast/Ei also came from Jackson. BTBRTF/Art (hereafter referred to as BTBR) was originated by L. C. Dunn and transferred to the colony of the late D. Bennett and K. Artzt. In preliminary microsatellite typing BTBR was found to be very similar, but not identical, to tint/Art. truncate (tc) homozygotes were kindly provided by Achim Gossler (Medizinische Hochschule, Hannover, Germany). The Brachyury mutant (Twis) was a gift from Virginia Pappaionnou (Columbia University, New York).
Genotyping:
DNA samples were isolated from tail or ear clippings using a protocol adapted from Sambrook et al. (1989) and normalized to concentrations of 50 or 100 ng/μl. Primer sets were synthesized by IDT (Coralville, IA) and checked for polymorphisms. Briefly, primers were 5′ end-labeled with 32P using T4 polynucleotide kinase (Invitrogen, Frederick, MD) as follows: primer mix, 5 pmol final concentration; 5× forward reaction buffer, 1 μl; T4 polynucleotide kinase (10 units/μl), 0.5 μl; 32P dATP (10 μCi/μl), 1 μl; water to 10 μl. The reaction was incubated at 37° for 30 min and then used for PCR analysis. PCR reactions were prepared according to a protocol adapted from HotMaster Taq DNA polymerase (Eppendorf AG, Hamburg, Germany). Specifically, a reaction mix cocktail was prepared containing the following final concentrations per reaction: 1× HotMaster Taq buffer with Mg2+, 2.5 mm Mg2+; dNTP mix (Roche Diagnostics, Mannheim, Germany), 0.2 mm; 32P dATP-labeled primer mix, 0.08 μm; DNA template, 50 or 100 ng; HotMaster Taq DNA polymerase, 2.5 units; water to 25 μl. PCR conditions were as follows: 94°, 2 min; (94°, 20 sec; 56°, 20 sec; 65°, 45 sec) 35 cycles; 65°, 5 min. Optimal annealing temperatures for individual primer sets varied from 54.5° to 58.0°. A 20-μl aliquot of the PCR product was loaded onto nondenaturing polyacrylamide gels containing 1× TBE and ranging from 6 to 10% polyacrylamide to resolve the products. Gels were run at 40 V overnight or 150 V for 3 hr, transferred to filter paper, dried, and transferred to a Kodak (Rochester, NY) BioMax MS intensifying phosphor screen for 1–2 hr. The gels were scanned using a Bio-Rad (Hercules, CA) molecular imager FX.
Our initial strategy in the genome-wide microsatellite scan was to assay with markers that were spaced ∼30 cM apart. Later we used the MB positions defined by the mouse genome mapping project (Ensembl v.45, June 2007). All of the markers used are listed in supplemental Table S1 at http://www.genetics.org/supplemental/. When linkage was found, more closely spaced markers were used to refine the tint map position. All recombination was tested in females.
RESULTS
Design of crosses:
The design of the mapping crosses for tint is shown in Table 2. We first outcrossed tint/Art to spretus or castaneous and then test crossed the F1 to the original Brachyury deletion for the presence of tint. Of the testcross progeny, all normal tails were discarded at birth since they segregate genotypes that are phenotypically indistinguishable and therefore are not informative.
TABLE 2.
Mapping crosses and tail phenotypes at birth
|
spretus cross
|
||||||
|---|---|---|---|---|---|---|
| Chr 17 | Chr 6 | Chr 17 | Chr 6 | |||
| P | +Ttint/+Ttint | int/int | × | +Tsp +Tsp/+Tsp +Tsp | sp/sp | |
| F1 | +Ttint/+Tsp +Tsp | int/sp | × | T/+Tsp +Tsp | BT/BT | |
| Progeny | Phenotype | Expected (%) | Observed no. | Observed (%) | ||
| Test cross | +Ttint/+TBT | sp/BT | Normal tail | |||
| +TBT/+Tsp +Tsp | sp/BT | Normal tail | ||||
| +Ttint/+TBT | int/BT | Normal tail | 62.5 | 478 | 67 | |
| +TBT/+Tsp +Tsp | int/BT | Normal tail | ||||
| T/+Tsp +Tsp | sp/BT | Normal tail | ||||
| T/+Tsp +Tsp | int/BT | Short tail | ||||
| 25 | 148 | 21 | ||||
| T/+Ttint | sp/BT | Short tail | ||||
| T/+Ttint | int/BT | Tailless | 12.5 | 87 | 12 | |
| Total | 100 | 713 | 100 | |||
|
castaneus cross
|
||||||
| Chr 17 | Chr 6 | Chr 17 | Chr 6 | |||
| P | +Ttint/+Ttint | int/int | × | +Tcas/+Tcas | cas/cas | |
| F1 | +Ttint/+Tcas | int/cas | × | T/+TBT | BT/BT | |
| Progeny | Phenotype | Expected (%) | Observed no. | Observed (%) | ||
| Test cross | +Ttint/+TBT | cas/BT | Normal tail | |||
| +TBT/+Tcas | cas/BT | Normal tail | 50 | 269 | 51.7 | |
| +Ttint/+TBT | int/BT | Normal tail | ||||
| +TBT/+Tcas | int/BT | Normal tail | ||||
| T/+Tcas | cas/BT | Short tail | ||||
| 25 | 136 | 26.2 | ||||
| T/+Ttint | cas/BT | Short tail | ||||
| T/+Tcas | int/BT | Tailless | ||||
| 25 | 115 | 22.1 | ||||
| T/+Ttint | int/BT | Tailless | ||||
| Total | 100 | 520 | 100 | |||
Gene symbols: +Ttint, wild type of T from the tint strain; +Tsp, wild type of T from spretus (for clarity, we assume it is duplicated); +TBT, wild type of T from BTBRTF; +Tcas, wild type of T from castaneous; T, the original Bracyhury deletion. The chromosome 6 column refers to the tint locus, mutant “int,” wild type from spretus “spret,” from castaneous “cast,” or from BTBRTF “BT.”
The remaining mice that receive T in the testcross are of two genotypes with respect to tint and these are expected in equal numbers. They segregate for +tint vs. M. spretus (S) on chromosome 17. For the markers close to where tint resides, all the short-tail mice are S and all the tailless mice are expected to be tint (I) type (except for recombinants). For all other chromosomes, the short-tail mice and tailless mice should consist of an equal number of S and I types. A similar situation applies to the M. castaneous cross where markers were designated as (C) types.
There are two noteworthy considerations in the design of this cross. Since the tint parental strain and the T/+ parent used for the test crosses are on similar genetic backgrounds, we expect them to be nonpolymorphic with most microsatellite markers. Thus, for most markers, an S- or C-specific PCR product was necessary. In the case where I and BTBRTF/Art were polymorphic, the I product could be used to type regardless of the other band. Furthermore, it is important to distinguish S and I type in most cases. The litter sizes of these backcross mice averages 11–12 pups so that normal tail mice can be culled at birth and there are still reasonable numbers of test cross progeny to genotype.
Phenotypic overlap problems:
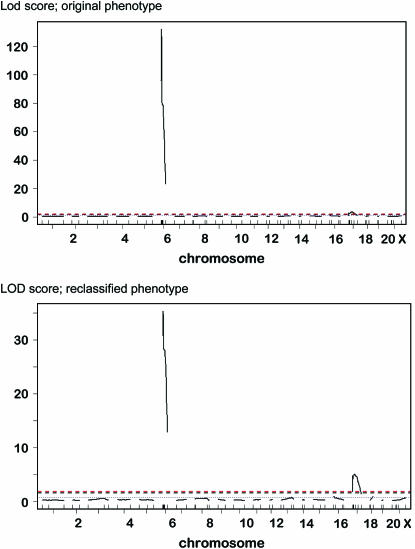
In the spretus testcross, an excess over the predicted number of normal tailed were initially recorded: 63 vs. the 50% expected (Table 2). Thus, it was apparent early on that either M. spretus had a duplication of Brachyury (T1) and the closely linked Brachyury the second (T2) makes twice as much product or M. spretus contains dominant suppressors of short tail. We tested this hypothesis by crossing T heterozygotes to M. spretus and found that only normal-tailed offspring were produced (n = 48), thus confirming one of the possibilities above. M. castaneous crossed to T/+ produced short- and normal-tailed offspring in the expected proportions (50:50). Thus, we decided to map tint in both M. spretus and M. castaneous crosses. In both test crosses to T/+, there was a dearth of tailless progeny. For this, it was necessary to type each individual mouse to be certain it was carrying the T deletion. We ascertained a microsatellite maker (D17mit170) near the distal deletion breakpoint that was polymorphic for BTBRTF mice segregating for T or +T. This marker became a useful tool for genotyping T. However, after eliminating all the noncarriers of T, there were still less than the original expected numbers of tailless mice born. It soon became clear that tint is on chromosome 6 (see below). In the castaneous cross where there was no genetic complication of a possible duplication, we retrospectively classified all mice on the basis of having the T deletion and an I (tint) or C (M. castaneous) genotype for the relevant region of chromosome 6. We tested the feasibility of this hypothesis in the M. castaneous cross by blindly typing an additional 28 mice from the testcross whose tails had been measured. They were typed using several markers for carrying T and being I or C for the relevant region of chromosome 6. In every case but two, which turned out to be recombinants, the measured tail length correlated with the genotype (Table 3). However, it was evident that there is phenotypic overlap in the tail length range of 42% of normal. These mice, including the two recombinants, were then included with the castaneous cross typing results (Figure 2). When we ran pair wise recombination fractions and LOD scores on both models using R/qtl (Li et al. 2006), it appeared that the retroactive classification based on the presence of T and chromosome 6 genotype seemed robust (Figure 3).
TABLE 3.
Results of “blind” typing in castaneous test cross
| Probe
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual phenotype | Tail length (mm)a | D6mit386 | D6mit22 | D6mit323 | D6mit40 | D6mit263 | D6mit131 | D6mit365 | D6mit104 | D17mit170 | |||
| Mouse | % normal tail | Mb position: | 81.94 | 85.09 | 86.87 | 88.08 | 88.95 | 90.32 | 94.07 | 110.93 | 8.54 | ||
| 1 | Brachy | 48 | 84 | C | C | C | C | C | C | C | ND | T | |
| 2 | Brachy | 46 | 80 | C | C | C | C | C | C | C | ND | T | |
| 3 | Long Brachy | 39 | 68 | C | C | C | C | C | C | C | ND | T | |
| 4 | Brachy | 37 | 65 | C | C | C | C | C | C | C | ND | T | |
| 5 | Brachy | 24 | 42 | C | C | C | C | C | C | C | ND | T | |
| 6 | Brachy | 24 | 42 | I | I | I | I | I | I | I | ND | T | |
| 7 | Short Brachy | 14 | 24 | I | I | I | I | I | I | I | ND | T | |
| 8 | Short Brachy | 10 | 17 | I | I | I | I | I | I | I | ND | T | |
| 9 | Curved Brachy | 6–9 | 13 | I | I | I | I | I | I | I | ND | T | |
| 10 | Short Brachy | 4 | 7 | I | I | I | I | I | I | I | ND | T | |
| 11 | Short Brachy | 2 | 4 | I | I | I | I | I | I | I | ND | T | |
| 12 | Short Brachy | 2 | 4 | I | I | I | I | I | I | I | ND | T | |
| 13 | Short Brachy | 2 | 4 | I | I | I | I | I | I | I | ND | T | |
| 14 | Short Brachy | 2 | 4 | I | I | I | I | I | I | I | ND | T | |
| 15 | Short Brachy | 2 | 4 | NDb | I | I | I | I | I | I | ND | T | |
| 16 | Tailless stump | 1 | 2 | I | I | I | I | I | I | I | ND | T | |
| 17 | Tailless stump | 1 | 2 | I | I | I | C | C | C | C | C | T | |
| 18 | Tailless stump | 1 | 2 | I | I | I | I | I | I | I | ND | T | |
| 19 | Tailless stump | 1 | 2 | I | I | I | I | I | I | I | ND | T | |
| 20 | Tailless stump | 1 | 2 | I | I | I | I | I | I | I | ND | T | |
| 21 | Tailless | 0 | 0 | I | ND | I | I | I | I | I | ND | T | |
| 22 | Tailless | 0 | 0 | I | I | I | I | I | I | I | ND | T | |
| 23 | Tailless | 0 | 0 | I | I | I | I | I | I | I | ND | T | |
| 24 | Tailless | 0 | 0 | ND | I | I | I | I | I | I | ND | T | |
| 25 | Tailless | 0 | 0 | I | I | I | I | I | I | I | ND | T | |
| 26 | Tailless | 0 | 0 | I | I | I | I | I | I | I | ND | T | |
| 27 | Tailless | 0 | 0 | I | I | ND | I | I | I | I | ND | T | |
| 28 | Tailless | 0 | 0 | C | I | I | I | I | ND | I | I | T | |
The two recombinants are 17 and 28; marker megabase positions Ensembl Dec 2006. I, tint; C, castaneous type.
At the age measured (2 weeks), normal tail length was 57 mm.
ND, not done.
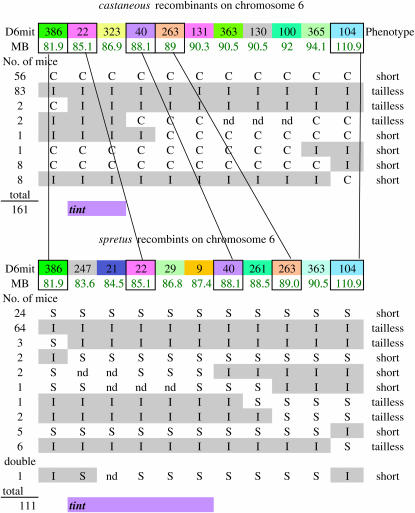
Figure 2.—
All recombinants for chromosome 6.
Figure 3.—
LOD scores for both models. Top, original phenotype; bottom, reclassified phenotype based on typing for T and the tint region of chromosome 6.
Tint maps to chromosome 6:
The major component of the tint and T interaction maps to chromosome 6 in both crosses. With respect to markers on chromosome 6, we examined 161 mice from M. castaneous and 111 mice from the M. spretus cross. Recombinants in the critical regions are shown in Figure 2. Only some of the probes could be used in both crosses because not all of them were dually polymorphic. Except for recombinants, all the tailless mice were I and all short-tailed mice were C or S for the relevant region. Both crosses are in agreement and the smallest possible region is defined by recombinants in the M. castaneous cross marked by D6mit386 at 81.09 Mb and D6mit40 at 88.1 Mb. This is a relatively large region and we were unable to divide it further using microsatellites. Regardless of this area being gene dense with >30 genes, we have not made an attempt to further define the interval but rather opted for the candidate gene approach.
tint is not Noto:
The genetic map in this region contained at least one probable candidate. The truncated (tc) mutation has now been cloned and renamed Noto (notochord homolog, Xenopus laevis) and resides on chromosome 6 at 85.39 Mb (Pavlova et al. 1998; Abdelkhalek et al. 2004). The phenotype of tc is recessive and not completely penetrant. Nototc causes an interruption of the notochord at E9.5 leading to a short or absent tail. We tested Nototc for interaction with both tint and the T deletion and found no more than the expected number of short tails, suggesting no interaction between the 2 genes (n = 49 tint; n = 86 for T; data not shown). In addition, the sequence of the three coding exons of Noto in tint/tint showed no significant differences in BTBRTF/Art, M. spretus, or M. castaneous.
tint interacts with T2 and not with T1:
Since T2 was not identified at the time tint was discovered to interact with the original T deletion, it seemed reasonable to retest separately the interaction of tint with T1 and T2. This was accomplished by crossing tint/tint to T1Wis and T2Bob independently. T1Wis is a hypermorph allele of T1 that causes the carrier to have no tail at all (Herrmann 1991). Any interaction with tint would therefore be expected to have less than no tail or sacroccygeal spina bifida, usually detected by a blood blister on the rump (Park et al. 1989). Since some of these might not be viable, all pregnant females were inspected for newborns twice daily. In cases of severe spina bifida, there might even be a loss of T1Wis embryos. T2Bob carriers consistently have a 50% tail length on the BTNTTF/Art background; thus, any reduction in tail length was easily recognized. Much to our surprise, it was clear that tint interacted with T2 and not with T1 (Table 4). Whereas T1Wis maintains a relatively constant ratio of normal tails to tailless offspring in all three crosses, the T2Bob mutation shows a dramatic shortening of the tail in successive crosses to tint. The proportion of tailless mice rose from 0 in the control to 17% in the F1 cross and to 33% in the backcross. Thus, remarkably, the real interaction of tint is with T2 and not T1.
TABLE 4.
tint interaction with T1Wis and T2Bob
| Control: T1wis, +/+ × +/+, +/+
|
Control: T2Bob, +/+ × +/+, +/+
|
|||
|---|---|---|---|---|
| Phenotype | No. | % | No. | % |
| Normal tail | 69 | 51.1 | 91 | 52.0 |
| Short tail 50% | 0 | 0 | 84 | 48.0 |
| Short tail 25% | 5 | 3.7 | 0 | 0 |
| Stump <10% | 6 | 4.4 | 0 | 0 |
| Tailless | 55 | 40.7 | 0 | 0 |
| Total | 135 | 100 | 175 | 100 |
| F1: T1wis, +/+ × tint/tint
|
F1: T2Bob, +/+ × tint/tint
|
|||
| Phenotype | No. | % | No. | % |
| Normal tail | 150 | 52.4 | 168 | 47.7 |
| Short tail 50% | 0 | 0 | 1 | 0 |
| Short tail 25% | 0 | 0 | 98 | 27.8 |
| Stump <10% | 0 | 0 | 23 | 6.5 |
| Tailless | 136 | 47.6 | 62 | 17.6 |
| Total | 286 | 100 | 352 | 100 |
| BC1: T1wis, +/tint × tint/tint
|
BC1: T2Bob, +/tint × tint/tint
|
|||
| Phenotype | No. | % | No. | % |
| Normal tail | 304 | 54.2 | 150 | 47.6 |
| Short tail 50% | 0 | 0 | 0 | 0 |
| Short tail 25% | 0 | 0 | 44 | 14 |
| Stump <10% | 0 | 0 | 17 | 5.4 |
| Tailless | 255 | 45.5 | 104 | 33 |
| Total | 560 | 100 | 315 | 100 |
DISCUSSION
tint maps to chromosome 6 and interacts with T2:
tint maps between D6mit386 at 81.1 Mb and D6mit40 at 88.1 Mb on chromosome 6. This region contains >30 genes and among these Noto is the only particularly striking candidate. Initially named truncate, Nototc shows decreased tail length, truncated notochord, abnormal somite, and sclerotome formation (Theiler 1959; Abdelkhalek et al. 2004). While Nototc shows a defect in axial development, we saw no interaction when crossed to the T deletion. Furthermore, sequence of noto's three exons in tint, M. castaneous, and M. spretus mice did not show any significant changes. Thus, Noto is excluded as a candidate for tint.
We were surprised to discover tint's interaction is with T2 rather than with T1. The transgenic insertion in T2Bob was thought not to affect the T1 gene, its defined regulatory region, its expression in the primitive streak, or in the few notochord cells present (Rennebeck et al. 1995). At E9.5, T2Bob embryos show quite a distinct phenotype from T1Wis and T embryos, being less affected in all measurable ways. The embryological defect in T2Bob starts more caudally than in T1 embryos and is mostly limited to the notochord. Furthermore, the presence of floor plate in T2Bob indicates that notochord cells were present, at least in the initial process of the notochord formation, but they were not maintained in later stages. Therefore, the interaction of tint with T2 might modulate the development of axial or paraxial mesoderm or both.
Two other genes and two QTL have been reported to interact with T, but in all cases their interaction has been tested against the original T deletion that deletes both T1 and T2. In 2000, the laboratory of Rosa Beddington identified the trans-acting transcription factor (Sp5) mapping to chromosome 2 at 70.2 Mb. It is similar to tint in that it has no homozygous phenotype and genetically interacts with the T deletion (Harrison et al. 2000). Ruvinsky et al. (2002) showed that the dominant mutation Abnormal feet and tail (Aft) located on chromosome 9 at 32 cM also interacts with the T deletion. In this case, the compound heterozygote mice display kinky tails and syndactyly in the hindlimbs. The two T interacting QTL (brm1 and brm2) described by Agulnik et al. (1998) are on chromosomes 9 and 15, respectively. Thus, in all four cases cited above, it is not clear whether these genes are interacting with T1 or T2. Perhaps an important lesson learned from the results presented here is that establishing genetic interactions using a deletion, however small, is not an appropriate strategy.
Is the notochord enhancer of Brachyury embedded in T2?
Numerous studies have revealed that Activin A (TGFβ family) and basic FGF are the main inducers of T1 expression (Smith et al. 1991; Amaya et al. 1993; Labonne and Whitman 1994). Transgenic studies in mice and manipulation experiments in frogs have identified a minimal region (∼400 bp upstream of the initiation site) of the T1 gene that is responsible for the mesoderm and notochord expression in frogs. However, in mice, a larger region is probably required since there was no rescue of the Brachyury homozygous phenotype (Stott et al. 1993). This minimal region contains an E-box and two canonical Lef1/Tcf binding sites that, in mice, are responsible for partially rescuing the Brachyury phenotype (Stott et al. 1993; Clements et al. 1996). Differences among vertebrate species vouch for the complexity of the regulation of Brachyury. Other binding sites such as goosecoid, Mix1 (now named Mixl1), and Otx2 that function as repressors of Xbra expression have being identified in frogs (Latinkic et al. 1997). Interestingly, these regulatory regions are not present in the mouse promoter, suggesting that the regulatory elements may vary between vertebrate species (Yamaguchi et al. 1999). Although most of these studies show the regulation of Brachyury in the mesoderm, regulatory elements controlling its expression in the notochord are not completely understood in mice.
Although T2 appears to be a bona fide gene in all respects, it is possible that it is important solely in rodents, since no conservation has been identified in any other vertebrate. In contrast, genome comparisons have shown that there are highly conserved sequences between mice and humans that map to the distal end of T2 (Figure 4). As mentioned above, the original mutation T2Bob is a transgenic mutation that has inserted ∼200 kb between the last two exons of T2 followed by a deletion of 3 kb of the intronic sequence (Rennebeck et al. 1998).
Figure 4.—
Conserved genomic sequences in mouse T2 are circled in red.
We cannot exclude the possibility that T2 is an important gene in mice and rats, or that it is probably allelic with tct since the tct copy of T2 has a significant amino acid change at the carboxyl terminus (Rennebeck et al. 1998). However, taken together, our results lead us to the following alternate hypothesis from our original interpretation of T2. It is possible that the prevailing defect in the T2Bob mutants is that its transgenic insertion at the 3′ end interferes with a previously unidentified T1 notochord enhancer. After the insertion that putative enhancer would be separated from T1 by ∼200 kb instead of 12 kb or may have even been deleted with the 3-kb intronic sequence.
While a full understanding of the genes involved in the vertebrate neural tube formation is still evolving, the impact of tint interaction with T2Bob can be further appreciated by the identification of Brachyury's notochord regulatory sequences.
Acknowledgments
This work was supported by National Institutes of Health Grant HD10668 (to K.A.).
We dedicate this article to the memory of our dear colleague Lori Flaherty (1946-2006).
References
- Abdelkhalek, H. B., A. Beckers, K. Schuster-Gossler, M. N. Pavlova, H. Burkhardt et al., 2004. The mouse homeobox gene Not is required for caudal notochord development and affected by the truncate mutation. Genes Dev. 18: 1725–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulnik, I. I., S. I. Agulnik, B. D. Saatkamp and L. M. Silver, 1998. Sex-specific modifiers of tail development in mice heterozygous for the brachyury (T) mutation. Mamm. Genome 9: 107–110. [DOI] [PubMed] [Google Scholar]
- Amaya, E., P. A. Stein, T. J. Musci and M. W. Kirschner, 1993. FGF signalling in the early specification of mesoderm in Xenopus. Development 118: 477–487. [DOI] [PubMed] [Google Scholar]
- Ardlie, K. G., and L. M. Silver, 1996. Low frequency of mouse t haplotypes in wild populations is not explained by modifiers of meiotic drive. Genetics 144: 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artzt, K., J. Cookingham and D. Bennett, 1987. A new mutation (t-int) interacts with the mutations of the mouse T/t complex that affect the tail. Genetics 116: 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield, D. K., M. J. Lewis and H. R. Pelham, 1995. A SNARE-like protein required for traffic through the Golgi complex. Nature 375: 806–809. [DOI] [PubMed] [Google Scholar]
- Clements, D., H. C. Taylor, B. G. Herrmann and D. Stott, 1996. Distinct regulatory control of the Brachyury gene in axial and non-axial mesoderm suggests separation of mesoderm lineages early in mouse gastrulation. Mech. Dev. 56: 139–149. [DOI] [PubMed] [Google Scholar]
- Conlon, F. L., S. G. Sedgwick, K. M. Weston and J. C. Smith, 1996. Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development 122: 2427–2435. [DOI] [PubMed] [Google Scholar]
- Copp, A. J., 1994. Genetic models of mammalian neural tube defects. Ciba Found. Symp. 181: 118–134, discussion 134–143. [DOI] [PubMed] [Google Scholar]
- Copp, A. J., N. D. Greene and J. N. Murdoch, 2003. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 4: 784–793. [DOI] [PubMed] [Google Scholar]
- Corbo, J. C., M. Levine and R. W. Zeller, 1997. Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development 124: 589–602. [DOI] [PubMed] [Google Scholar]
- Doudney, K., and P. Stanier, 2005. Epithelial cell polarity genes are required for neural tube closure. Am. J. Med. Genet. C Semin. Med. Genet. 135: 42–47. [DOI] [PubMed] [Google Scholar]
- Estibeiro, J. P., F. A. Brook and A. J. Copp, 1993. Interaction between splotch (Sp) and curly tail (ct) mouse mutants in the embryonic development of neural tube defects. Development 119: 113–121. [DOI] [PubMed] [Google Scholar]
- Greco, T. L., S. Takada, M. M. Newhouse, J. A. McMahon, A. P. McMahon et al., 1996. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 10: 313–324. [DOI] [PubMed] [Google Scholar]
- Gruneberg, H., 1958. Genetical studies on the skeleton of the mouse. XXIII. The development of brachyury and anury. J. Embryol. Exp. Morphol. 6: 424–443. [PubMed] [Google Scholar]
- Halpern, M. E., R. K. Ho, C. Walker and C. B. Kimmel, 1993. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 75: 99–111. [PubMed] [Google Scholar]
- Harrison, S. M., D. Houzelstein, S. L. Dunwoodie and R. S. Beddington, 2000. Sp5, a new member of the Sp1 family, is dynamically expressed during development and genetically interacts with Brachyury. Dev. Biol. 227: 358–372. [DOI] [PubMed] [Google Scholar]
- Helwig, U., K. Imai, W. Schmahl, B. E. Thomas, D. S. Varnum et al., 1995. Interaction between undulated and Patch leads to an extreme form of spina bifida in double-mutant mice. Nat. Genet. 11: 60–63. [DOI] [PubMed] [Google Scholar]
- Herrmann, B. G., 1991. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development 113: 913–917. [DOI] [PubMed] [Google Scholar]
- Herrmann, B. G., S. Labeit, A. Poustka, T. R. King and H. Lehrach, 1990. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 343: 617–622. [DOI] [PubMed] [Google Scholar]
- Howard, C. A., G. R. Gummere, M. F. Lyon, D. Bennett and K. Artzt, 1990. Genetic and molecular analysis of the proximal region of the mouse t-complex using new molecular probes and partial t-haplotypes. Genetics 126: 1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriloff, D. M., and M. J. Harris, 2000. Mouse models for neural tube closure defects. Hum. Mol. Genet. 9: 993–1000. [DOI] [PubMed] [Google Scholar]
- Justice, M. J., and V. C. Bode, 1988. New evidence supporting the allelism of T and tct. Mouse News Lett. 80: 168–170. [Google Scholar]
- Kispert, A., and B. G. Herrmann, 1993. The Brachyury gene encodes a novel DNA binding protein. EMBO J. 12: 3211–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert, A., B. Koschorz and B. G. Herrmann, 1995. The T protein encoded by Brachyury is a tissue-specific transcription factor. EMBO J. 14: 4763–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne, C., and M. Whitman, 1994. Mesoderm induction by activin requires FGF-mediated intracellular signals. Development 120: 463–472. [DOI] [PubMed] [Google Scholar]
- Latinkic, B. V., M. Umbhauer, K. A. Neal, W. Lerchner, J. C. Smith et al., 1997. The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev. 11: 3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R., S. W. Tsaih, K. Shockley, I. M. Stylianou, J. Wergedal et al., 2006. Structural model analysis of multiple quantitative traits. PLoS Genet. 2: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon, M. F., 1956. Hereditary hair loss in the tufted mutant of the house mouse. J. Hered. 47: 101–103. [Google Scholar]
- Mickova, M., and P. Ivanyi, 1974. Sex-dependent and H-2-linked influence on expressivity of the Brachyury gene in mice. J. Hered. 65(6): 369–372. [DOI] [PubMed] [Google Scholar]
- Papaioannou, V. E., 1997. T-box family reunion. Trends Genet. 13: 212–213. [DOI] [PubMed] [Google Scholar]
- Park, C. H., J. H. Pruitt and D. Bennett, 1989. A mouse model for neural tube defects: the curtailed (Tc) mutation produces spina bifida occulta in Tc/+ animals and spina bifida with meningomyelocele in Tc/t. Teratology 39: 303–312. [DOI] [PubMed] [Google Scholar]
- Pavlova, M. N., A. M. Clark and A. Gossler, 1998. High-resolution mapping of the truncate (tc) locus on mouse chromosome 6. Mamm. Genome 9: 843–845. [DOI] [PubMed] [Google Scholar]
- Rennebeck, G., E. Lader, A. Fujimoto, E. P. Lei and K. Artzt, 1998. Mouse Brachyury the Second (T2) is a gene next to classical T and a candidate gene for tct. Genetics 150: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennebeck, G. M., E. Lader, Q. Chen, R. A. Bohm, Z. S. Cai et al., 1995. Is there a Brachyury the Second? Analysis of a transgenic mutation involved in notochord maintenance in mice. Dev. Biol. 172: 206–217. [DOI] [PubMed] [Google Scholar]
- Ruvinsky, I., O. Chertkov, X. V. Borue, S. I. Agulnik, J. J. Gibson-Brown et al., 2002. Genetics analysis of mouse mutations. Abnormal feet and tail and rough coat, which cause developmental abnormalities and alopecia. Mamm. Genome. 13: 675–679. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E. F. Fritsch and T. Maniatis, 1989. Molecular Cloning: A Laboratory Manual, Ed. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schulte-Merker, S., M. Hammerschmidt, D. Beuchle, K. W. Cho, E. M. De Robertis et al., 1994. Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development 120: 843–852. [DOI] [PubMed] [Google Scholar]
- Showell, C., O. Binder and F. L. Conlon, 2004. T-box genes in early embryogenesis. Dev. Dyn. 229: 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, L. M., 1981. Genetic organization of the mouse t complex. Cell. 27: 239–240. [DOI] [PubMed] [Google Scholar]
- Smith, J. C., B. M. Price, J. B. Green, D. Weigel and B. G. Herrmann, 1991. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67: 79–87. [DOI] [PubMed] [Google Scholar]
- Stott, D., A. Kispert and B. G. Herrmann, 1993. Rescue of the tail defect of Brachyury mice. Genes Dev. 7: 197–203. [DOI] [PubMed] [Google Scholar]
- Theiler, K., 1959. Anatomy and development of the “truncate” (boneless) mutation in the mouse. Am. J. Anat. 104: 319–343. [DOI] [PubMed] [Google Scholar]
- Wilson, V., P. Rashbass and R. S. Beddington, 1993. Chimeric analysis of T (Brachyury) gene function. Development 117: 1321–1331. [DOI] [PubMed] [Google Scholar]
- Wittman, K. S., and M. Hamburgh, 1968. The development and effect of genetic background on expressivity and penetrance of the Brachyury mutation in the mouse: a study in developmental genetics. J. Exp. Zool. 168: 137–145. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, H., T. Niimi, Y. Kitagawa and K. Miki, 1999. Brachyury (T) expression in embryonal carcinoma P19 cells resembles its expression in primitive streak and tail-bud but not that in notochord. Dev. Growth Differ. 41: 253–264. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, K. O., H. Fujimoto and H. Urushihara, 1981. Effects of the brachyury (T) mutation on morphogenetic movement in the mouse embryo. Dev. Biol. 87: 242–248. [DOI] [PubMed] [Google Scholar]
- Yasuo, H., M. Kobayashi, Y. Shimauchi and N. Satoh, 1996. The ascidian genome contains another T-domain gene that is expressed in differentiating muscle and the tip of the tail of the embryo. Dev. Biol. 180: 773–779. [DOI] [PubMed] [Google Scholar]