Abstract
We identified the causal genetic variation for the difference in the thoracic trident pigmentation intensity between two wild-derived strains of Drosophila melanogaster. It was found to be the difference in expression level of ebony, which codes for an enzyme in the melanin-synthesis pathway and has pleiotropic effects on vision and behavior.
INTRASPECIFIC variation in the pattern and intensity of pigmentation exists widely in diverse insect taxa (reviewed in True 2003; Gray and Mckinnon 2006). In Drosophila melanogaster, variation in thoracic trident pigmentation intensity has been long understood (Morgan and Bridges 1919; Plough and Ives 1934, 1935; Jacobs 1960; David et al. 1985; Moreteau et al. 1995; Gibert et al. 2000, 2004; Munjal et al. 1997; Wittkopp et al. 2002). Some authors predicted that ebony is responsible for this phenotypic variation, mainly from the observation of mutants showing a dark trident pattern on their thorax segments (Jacobs 1960; Wittkopp et al. 2002). ebony mutants are also known to have defects in neuronal activities at photoreceptor terminals (Hotta and Benzer 1969; Heisenberg 1971; Borycz et al. 2002), arrhythmic locomotor activity (Newby and Jackson 1991), and unusual sexual behavior (Kyriacou et al. 1978; Kyriacou 1981). These pleiotropic effects are likely to stem from the EBONY expression in the nervous systems (Hovemann et al. 1998; Richardt et al. 2002, 2003; Wagner et al. 2007); thus, variation of this gene is likely to have some impact on individual fitness under natural conditions.
Despite the widely accepted prediction, no evidence directly links the ebony gene to the thoracic trident pigmentation variation in natural populations. We performed a genetic mapping using two wild-derived strains to identify the causal genetic differences underlying this naturally occurring phenotypic variation.
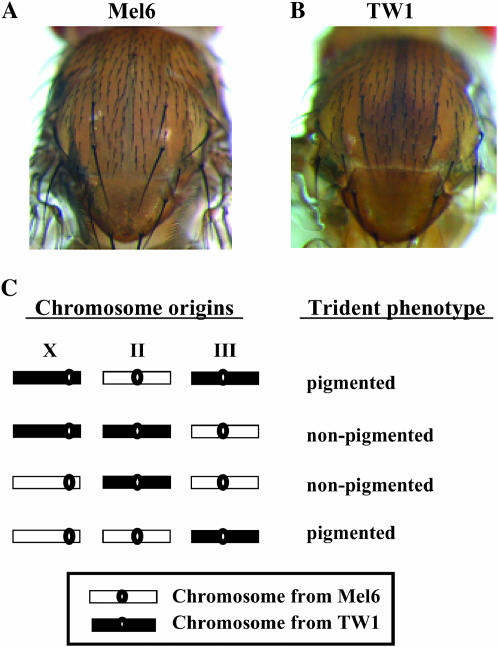
To conduct a systematic genetic mapping, we used two representative wild-derived inbred lines, Mel6 (G59) and TW1 (G23), which originated from Benin, West Africa, and Taiwan, respectively. These lines were subjected to 59 and 23 generations of sib mating, respectively, to construct nearly isogenic lines. Figure 1, A and B, shows the thoracic pigmentation pattern in these strains. The trident pattern shaped by dark pigmentation clearly appears in TW1 but not in Mel6. We checked the phenotype at three different temperatures—18°, 20°, and 25°—since temperature is known to affect the intensity of pigmentation (David et al. 1985; Moreteau et al. 1995; Gibert et al. 2000, 2004, 2007; Munjal et al. 1997). The pigmentation of the TW1 line darkens when reared in lower temperatures, but no obvious trident shape appeared in Mel6 in the temperature range we tested (data not shown). The phenotype of TW1 is completely recessive over Mel6 at least when reared at 25°.
Figure 1.—
Thoracic trident pigmentation pattern and its association with the third chromosome. (A) Thorax segment of Mel6 (G59) originated from Benin, West Africa. (B) Thorax segment of TW1 (G23) originated from Taiwan. Mel6 (G59) and TW1 (G23) were subjected to 23 and 59 generations of sib mating, respectively, to construct nearly isogenic lines. (C) Trident phenotypes of the whole-chromosome substitution lines between Mel6 and TW1. Chromosome origins and the trident phenotypes of the lines are indicated in the left and right columns, respectively. These lines were generated using balancer chromosomes by a similar scheme used in Hollocher et al. (1997). Pigmentation intensity was examined under the microscope.
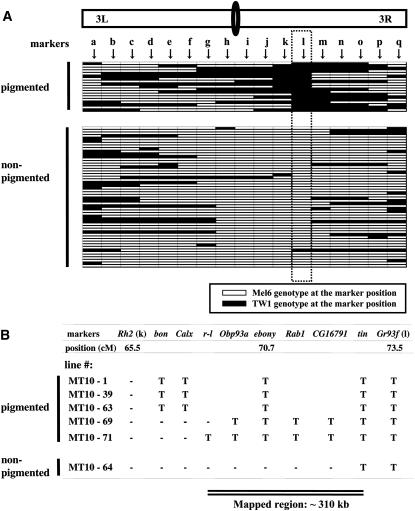
Chromosomal substitution lines were constructed (Figure 1C) to investigate which chromosomes have effects on the pigmentation pattern. Thoracic trident phenotypes of these lines indicate that only the third chromosome contributes to the phenotypic difference (Figure 1C). Next, we constructed 73 third chromosome recombinant lines in the Mel6 homozygous background. These lines were made homozygous using balancer chromosomes, and they were genotyped for 17 molecular markers. On the basis of the comparison of genotypes and phenotypes of these recombinants, there is likely to be only one major segregating factor between Rh2 and Gr93f for the trident pigmentation variation (Figure 2A).
Figure 2.—
Genetic mapping of the thoracic trident pigmentation phenotype using 73 homozygous recombinant lines constructed between TW1 and Mel6. (A) Genotypes of the pigmented (n = 19) and nonpigmented (n = 54) phenotype lines at 17 restriction fragment length polymorphism (RFLP) markers (a–q) on the third chromosome: a, Gr61d; b, gry; c, Gr64A; d, lama; e, Gr65c; f, Dhpr; g, Gr68d; h, Or74a; i, desat2; j, pnr; k, Rh2; l, Gr93f; m, RpS3; n, ssh; o, Pr; p, Gr98a; q, Ptx. Horizontal bars indicate genotypes of the recombinant lines. The dotted square indicates the genotypes at Gr93f marker that had almost complete association with the phenotype (r2 = 0.93). Note that one exceptional line that showed nonpigmented phenotype but had TW1 genotype at Gr93f is represented as MT10-64 in B. RFLP markers were developed by direct sequencing PCR fragments of ∼600–900 bp from Mel6 and TW1. Further information on RFLP markers can be provided upon request. Primers were designed by Primer3 software (Rozen and Skaletsky 2000) from the publicly available genome sequence (Adams et al. 2000). DNA was extracted from one male each from Mel6 and TW1 using the GeneElute Mammalian Genomic DNA kit (Sigma-Aldrich, St. Louis). PCR products were obtained from these genomic samples by the AmpliTaq Gold PCR kit (Applied Biosystems, Foster City, CA) and were purified using MultiScreen-FB filter plates (Millipore, Billerica, MA). These purified products were directly sequenced on both strands using the BigDye terminator cycle sequencing kit version 3 (Applied Biosystems). The third chromosome recombinant lines were constructed from F2 backcrossed individuals in the Mel6 homozygous background. The line was backcrossed twice (including the F2 backcross) to Mel6 and had two generations of recombination opportunity. The lines were made homozygous using balancers in the last step. Note that these third chromosome recombinants possess the X and the second chromosomes from Mel6. (B) Genotypes at eight additional RFLP markers in six recombinant lines from A that had their breakpoints between markers Rh2 (k) and Gr93f (l). T indicates TW1 genotype and “-” indicates Mel6 genotype. Two critical lines, MT10-69 and MT10-64, indicate that the ∼310-kb region between markers r-l and tin is responsible for the phenotype.
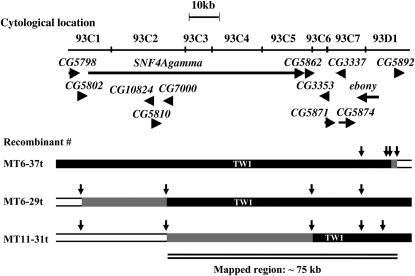
Finer breakpoint investigation on six recombinant lines that had their breakpoints between markers Rh2 and Gr93f showed that the critical region lies between markers r-l and tin (Figure 2B). Note that one line in Figure 2A that showed nonpigmented phenotype but had TW1 genotype at Gr93f is represented as MT10-64 in Figure 2B. This line actually had Mel6 genotype at most of the marker positions between Rh2 and Gr93f. To further map the gene, we used a laboratory population that was made from a cross between Mel6 and TW1 and experienced over 20 generations of free recombination. We chose 116 individuals showing the trident phenotype and screened for those that had recombination breakpoints between r-l and tin. Ten individuals met these criteria. Figure 3 shows the recombination breakpoints of the three most informative recombinants. From these results, we narrowed down the critical region for the responsible gene to ∼75 kb between CG7000 and CG5892.
Figure 3.—
Recombination breakpoints of the three most informative recombinants between Mel6 and TW1. The recombinants were screened from a laboratory population that experienced over 20 generations of recombination. These third chromosome recombinants had the X and the second chromosomes from Mel6. Horizontal arrows indicate annotated ORFs with their transcriptional directions. Vertical arrowheads indicate positions of the markers genotyped. Open and solid bars represent chromosome segments from Mel6 and TW1, respectively. Shaded bars indicate regions where recombinant breakpoints reside. The markers were genotyped by directly sequencing PCR fragments of ∼600–900 bp. The thoracic tridents of the three recombinants were all pigmented. Note that the critical interval of ∼75kb between CG7000 and CG5892 includes the candidate gene ebony.
The ∼75-kb region included seven annotated genes including ebony (CG3331), a strong candidate gene. We sequenced the coding regions of ebony gene from TW1 (AB301907) and Mel6 (AB301908). There were two amino acid differences between those sequences. Although we cannot completely exclude the possibility, it is less likely that these differences cause some effect on the phenotype, since those changes were not consistent with the phenotype in other inbred lines collected in Iriomote, Japan, or in other worldwide areas (data not shown).
Immunocytochemical and reporter gene assays have shown that EBONY is expressed in multiple tissues including the epidermal cells of thorax segment (Hovemann et al. 1998; Richardt et al. 2002, 2003; Wittkopp et al. 2002; Gibert et al. 2007; Wagner et al. 2007), in which the highest level of the EBONY expression is found in cells that produce the trident pigmentation in ebony mutants (Wittkopp et al. 2002). Also, e-lacZ transformants showed high expression of the ebony transcripts at the trident producing cells (Gibert et al. 2007). To see if the expression level of ebony is lower in the thorax of TW1 than that of Mel6, we performed quantitative reverse transcriptase PCR (qRT–PCR) as follows. Adult flies within 1 hr of eclosion were dissected under the microscope; this is when the pigmentation of the adult cuticle initiates. For each of Mel6 and TW1 strain, three replicate RNA were extracted from thoraces of 12 individuals using the QIAGEN RNeasy kit (QIAGEN, Valencia, CA) with subsequent DNase I digestion procedure. The first stranded cDNA was synthesized by using 2 μg of total RNA with SYBR ExScript RT–PCR kit (Perfect Real Time) from Takara-bio (Tokyo) in a 40-μl reaction volume. qRT–PCR was performed with SYBR ExScript RT–PCR kit (Perfect Real Time, Takara-bio) on a Thermal Cycler Dice TP800 from Takara-bio. For each cDNA, two qRT–PCR reactions were conducted.
The expression levels of ebony were measured three times with RNA samples extracted on different days. The expression level standardized by Actin57B (CG10067) was 1.69 ± 0.13 (SD) fold higher in the thorax of Mel6 when compared to that of TW1. This difference was statistically significant (t in pairwise Student's t-test = 16.16, d.f. = 2, P < 0.005). The same conclusion was obtained by using rp49 (CG7939) as the internal control (ratio of expression levels, Mel6/TW1 = 1.33 ± 0.06 (SD); t = 5.08, d.f. = 2, P < 0.05). This finding is consistent with the hypothesis that the thoracic trident pigmentation variation is caused by the difference in ebony expression level: low expression in the pigmented strain and high expression in the nonpigmented strain.
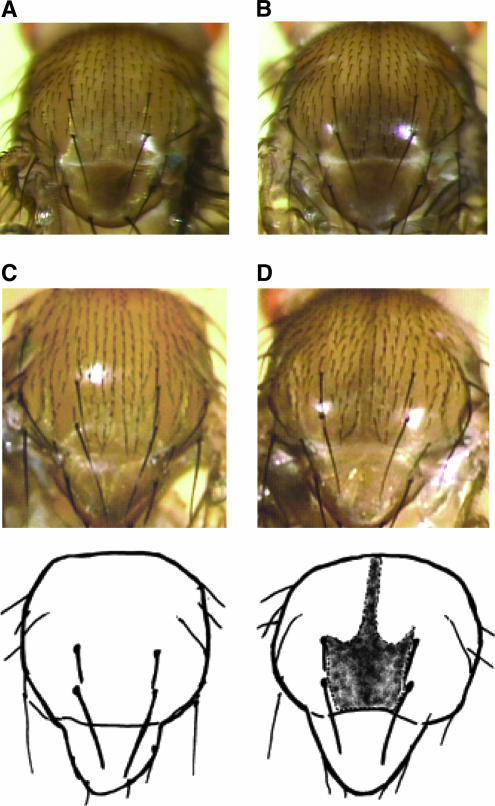
We also performed a complementation test using ebony mutants. TW1 showed pigmented phenotype under the heterozygous condition with the e1 mutant (Figure 4A), whereas Mel6 showed nonpigmented phenotype under the same condition (Figure 4B). The results were almost identical when we used eAFA, Df(3R)e-H4, and Df(3R)e-R1 as ebony mutants (data not shown). These results indicated that ebony is allelic to the locus we mapped.
Figure 4.—
Thoracic trident pigmentation phenotype obtained from complementation test with ebony and ebony RNAi knockdown experiment. (A) Dorsal view of the thorax segment of the Mel6/e1 heterozygote. (B) Dorsal view of the thorax segment of the TW1/e1 heterozygote. The flies in A and B were reared at 25°. The results were almost identical when eAFA, Df(3R)e-H4, and Df(3R)e-R1 were used as ebony mutants (data not shown). (C) Dorsal view of the thorax segment of the wild-type control, w1118; P{w+mc = Actin5C-GAL4}/+. (D) Dorsal view of the thorax segment of the RNAi knockdown individual, w1118; P{UAS-ebony-IR}/+; P{w+mc = Actin5C-GAL4}/+. The insert, P{UAS-ebony-IR} contains two fragments of 500 bp ebony cDNA as an inverted repeat (IR). These lines were crossed to w1118; P{w+mc = Act5C–GAL4}17bFO1/+ strain to induce IR RNA in the whole body. w1118; P{w+mc = Act5C–GAL4}17bFO1/+ was obtained by backcrossing y1 w*; P{w+mc = Act5C–GAL4}17bFO1/TM6B, Tb1 to w1118 strain for 10 generations. Adult flies carrying one copy of the UAS-IR construct and one copy of the GAL4 driver were investigated for the thorax pigmentation. The flies in C and D were reared at 20°.
Next, we designed another experiment to show that the ebony transcript is necessary to give rise to the nonpigmented-thorax phenotype. It is already known that loss-of-function mutations of ebony show pigmented phenotype. However, those mutant strains have various genetic backgrounds. To cleanly examine the effect of this single gene, we conducted an inducible RNAi experiment using GAL4-UAS system, which allowed us to knockdown this specific gene in a controlled genetic background (w1118). Figure 4, C and D, shows that the trident pigmentation appears when RNAi is induced by a ubiquitously expressed Actin5C GAL4 driver. This result showed that the reduced expression level of this single gene can produce the pigmented-trident phenotype in a controlled genetic background.
We have shown that the difference in expression level of ebony is responsible for the variation in thoracic trident pigmentation between the two representative lines. On the other hand, there are reports of mutations in other loci, which give rise to this trident-pigmented phenotype. In the early years of Drosophila genetics, Morgan and Bridges (1919) reported with (with trident) on 2-55 and Bridges discovered ptg (pentagon) around the same time (Lindsley and Grell 1968). Plough and Ives (1934, 1935) reported another locus, tri (trident). Nevertheless, none of these have been molecularly identified. These reports and phenotypes of other body color mutants such as black (Lindsley and Zimm 1992) suggest that ebony may not be the only segregating locus for the trident pigmentation variation in natural populations. Curiously, among the worldwide populations, latitudinal cline of the pigmentation intensity has been reported (David et al. 1985; Munjal et al. 1997). It is intriguing to investigate how much of this clinal variation can be explained by the expression level variation of the ebony gene.
Recently, Pool and Aquadro (2007) investigated female abdominal pigmentation in the lines from Africa. They compared the DNA sequences of the ebony gene region and found that darkest lines sequenced had nearly identical haplotypes spanning 14.5 kb upstream of the protein-coding exons of ebony. Neither of the two lines that we used in our study had dramatically dark abdominal pigmentation as seen in the darkest scored individuals photographed in their study. However, it is certainly of our interest to examine ebony's contributions to both abdominal and thoracic pigmentation and also whether the haplotype block found in the darkest African lines is present in other worldwide samples with various intensities of abdominal and thoracic pigmentation. The next step would be to investigate DNA sequences from worldwide strains and population samples to identify the specific site(s), region(s), or haplotype(s) responsible for the pigmentation variations in this species.
Many of the insect pigmentation patterns are controlled by the melanin-synthesis pathway, in which ebony has a catalytic role. This pathway involves dopamine, which is also utilized as a neurotransmitter. In Drosophila, the genes controlling this dopamine pathway, such as ebony and tan, are known to catalyze reactions of histamine at the photoreceptor terminals (Richardt et al. 2002, 2003; Borycz et al. 2002; True et al. 2005). Taking these factors into account, the natural variation that we identified in the expression level of ebony provides an excellent model system for studying the association between color morphology and visually affected behavior in insects.
Acknowledgments
We thank Yuriko Ishii for technical assistance. This work was supported in part by The Nissan Science Foundation and Grants-in-Aid for Young Scientists (B) and Grants-in-Aid for Scientific Research on Priority Areas (Comparative Genomics) from the Ministry of Education, Culture, Sports, Science and Technology of Japan to A.T. and Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan to T.T.- S.
References
- Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne et al., 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195. [DOI] [PubMed] [Google Scholar]
- Borycz, J., J. A. Borycz, M. Loubani and I. A. Meinertzhagen, 2002. tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J. Neurosci. 22: 10549–10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, J. R., P. Capy, V. Payant and S. Tsakas, 1985. Thoracic trident pigmentation in Drosophila melanogaster: differentiation of geographical populations. Genet. Sel. Evol. 17: 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert, P., B. Moreteau and J. R. David, 2000. Developmental constraints on an adaptive plasticity: reaction norms of pigmentation in adult segments of Drosophila melanogaster. Evol. Dev. 2: 249–260. [DOI] [PubMed] [Google Scholar]
- Gibert, P., P. Capy, A. Imasheva, B. Moreteau, J. P. Morin et al., 2004. Comparative analysis of morphological traits among Drosophila melanogaster and D. simulans: genetic variability, clines and phenotypic plasticity. Genetica 120: 165–179. [DOI] [PubMed] [Google Scholar]
- Gibert, J. M., F. Peronnet and C. Schlotterer, 2007. Phenotypic plasticity in Drosophila pigmentation caused by temperature sensitivity of a chromatin regulator network. PLoS Genet 3: 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, S. M., and J. S. McKinnon, 2006. Linking color polymorphism maintenance and speciation. Trends Ecol. Evol. 22: 71–79. [DOI] [PubMed] [Google Scholar]
- Heisenberg, M., 1971. Separation of receptor and lamina potentials in the electroretinogram of normal and mutant Drosophila. J. Exp. Biol. 55: 85–100. [DOI] [PubMed] [Google Scholar]
- Hollocher, H., C.-T. Ting, F. Pollack and C.-I Wu, 1997. Incipient speciation by sexual isolation in Drosophila melanogaster: variation in mating preference and correlation between sexes. Evolution 51: 1175–1181. [DOI] [PubMed] [Google Scholar]
- Hotta, Y., and S. Benzer, 1969. Abnormal electroretinograms in visual mutants of Drosophila. Nature 222: 354–356. [DOI] [PubMed] [Google Scholar]
- Hovemann, B. T., R. P. Ryseck, U. Walldorf, K. F. Stortkuhl, I. D. Dietzel et al., 1998. The Drosophila ebony gene is closely related to microbial peptide synthetases and shows specific cuticle and nervous system expression. Gene 221: 1–9. [DOI] [PubMed] [Google Scholar]
- Jacobs, M. E., 1960. Influence of light on mating of Drosophila melanogaster. Ecology 41: 182–188. [Google Scholar]
- Kyriacou, C. P., B. Burnet and K. Connolly, 1978. The behavioural basis of overdominance in competitive mating success at the ebony locus of Drosophila melanogaster. Anim. Behav. 26: 1195–1206. [Google Scholar]
- Kyriacou, C. P., 1981. The relationship between locomotor activity and sexual behaviour in ebony strains of Drosophila melanogaster. Anim. Behav. 29: 462–471. [Google Scholar]
- Lindsley, D. L., and E. H. Grell, 1968. Genetic Variations of Drosophila melanogaster. Publication. no. 627, Carnegie Institution, Washington, DC.
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego.
- Moreteau, B., P. Capy, A. Alonso-Moraga, A. Munoz-Serrano, J. Stockel et al., 1995. Genetic characterization of geographic populations using morphometrical traits in Drosophila melanogaster: isogroups versus isofemale lines. Genetica 96: 207–215. [DOI] [PubMed] [Google Scholar]
- Morgan, T. H., and C. B. Bridges, 1919. The inheritance of a fluctuating character. J. Gen. Physiol. 1: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjal, A. K., D. Karan, P. Gibert, B. Moreteau, R. Parkash et al., 1997. Thoracic trident pigmentation in Drosophila melanogaster: latitudinal and altitudinal clines in Indian populations. Genet. Sel. Evol. 29: 601–610. [Google Scholar]
- Newby, L. M., and F. R. Jackson, 1991. Drosophila ebony mutants have altered circadian activity rhythms but normal eclosion rhythms. J. Neurogenet. 7: 85–101. [DOI] [PubMed] [Google Scholar]
- Plough, H. H., and P. T. Ives, 1934. New mutants report. Drosoph. Inf. Serv. 1: 31–34. [Google Scholar]
- Plough, H. H., and P. T. Ives, 1935. Induction of mutations by high temperature in Drosophila. Genetics 20: 42–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool, J. E., and C. F. Aquadro, 2007. The genetic basis of adaptive pigmentation variation in Drosophila melanogaster. Mol. Ecol. 16: 2844–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardt, A., J. Rybak, K. F. Stortkuhl, I. A. Meinertzhagen and B. T. Hovemann, 2002. Ebony protein in the Drosophila nervous system: optic neuropile expression in glial cells. J. Comp. Neurol 452: 93–102. [DOI] [PubMed] [Google Scholar]
- Richardt, A., T. Kemme, S. Wagner, D. Schwarzer, M. A. Marahiel et al., 2003. Ebony, a novel nonribosomal peptide synthetase for β-Alanine conjugation with biogenic amines in Drosophila. J. Biol. Chem. 278: 41160–41166. [DOI] [PubMed] [Google Scholar]
- Rozen, S., and H. Skaletsky, 2000. Primer3 on the WWW for general users and for biologist programmers, pp. 365–386 in Bioinformatics Methods and Protocols: Methods in Molecular Biology, edited by S. Krawetz and S. Misener. Humana Press, Totowa, NJ. [DOI] [PubMed]
- True, J. R., 2003. Insect melanism: the molecules matter. Trends Ecol. Evol. 18: 640–647. [Google Scholar]
- True, J. R., S. D. Yeh, B. T. Hovemann, T. Kemme, I. A. Meinertzhagen et al., 2005. Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet. 1: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, S., C. Heseding, K. Szlachta, J. R. True, H. Prinz et al., 2007. Drosophila photoreceptors express cysteine peptidase tan. J. Comp. Neurol. 500: 601–611. [DOI] [PubMed] [Google Scholar]
- Wittkopp, P. J., J. R. True and S. B. Carroll, 2002. Reciprocal functions of the Drosophila Yellow and Ebony proteins in the development and evolution of pigment patterns. Development 129: 1849–1858. [DOI] [PubMed] [Google Scholar]