Abstract
An upper-level genetics research course was developed to expose undergraduates to investigative science. Students are immersed in a research project with the ultimate goal of identifying proteins important for chromosome transmission in mitosis. After mutagenizing yeast Saccharomyces cerevisiae cells, students implement a genetic screen that allows for visual detection of mutants with an increased loss of an ADE2-marked yeast artificial chromosome (YAC). Students then genetically characterize the mutants and begin efforts to identify the defective genes in these mutants. While engaged in this research project, students practice a variety of technical skills in both classical and molecular genetics. Furthermore, students learn to collaborate and gain experience in sharing scientific findings with others in the form of written papers, poster presentations, and oral presentations. Previous students indicated that, relative to a traditional laboratory course, this research course improved their understanding of scientific concepts and technical skills and helped them make connections between concepts. Moreover, this course allowed students to experience scientific inquiry and was influential for students as they considered future endeavors.
LABORATORIES play a critical role in illustrating and extending concepts learned in the classroom. Organizations committed to improving undergraduate education in the sciences recommend that traditional “cookbook” laboratories be replaced by inquiry, project-based laboratories (e.g., National Research Council Committee on Undergraduate Biology Education to Prepare Research Scientists for the 21st Century 2003; Wood 2003). Investigative laboratories offer many advantages for student learning and development. They provide opportunities for students to ask scientific questions, design experiments, collect data, interpret data, and contribute to an interdisciplinary body of scientific knowledge. When a project-based laboratory is organized with student groups collecting subsets of an overall data set, students learn to collaborate and visualize how their data fit into a bigger picture (Bell 2001). In addition to acquiring technical skills, inquiry-based laboratories can help students develop skills in presenting scientific information in written and oral formats. Educators are responding to recommendations to use inquiry-based learning by incorporating investigative laboratories into the science curriculum (e.g., Odom and Grossel 2002; Griffin et al. 2003; Dibartolomeis and Moné 2003; Gammie and Erdeniz 2004; Howard and Miskowski 2005; Frantz et al. 2006).
In an effort to expose students to a “real” research experience, an upper-level undergraduate genetics research course was developed. This course involves an interdisciplinary research project aimed at identifying proteins important for chromosome transmission during mitosis in the excellent genetic model organism Saccharomyces cerevisiae (common baker's yeast). Previous screens in yeast designed for this purpose have been successful (e.g., McGrew et al. 1989; Hoyt et al. 1990; Spencer et al. 1990; Kouprina et al. 1993; Runge and Zakian 1993; Ouspenski et al. 1999; Baetz et al. 2004; Measday et al. 2005).
As illustrated in the project overview (Figure 1), students mutagenize yeast cells and implement a genetic screen to isolate mutants that display increased loss of a yeast artificial chromosome (YAC) (Burke et al. 1987). YACs contain cis-acting DNA elements known to be required for chromosome replication, segregation, and stability (i.e., centromere, origin of replication, and telomeres). Importantly, since YACs are not essential for the viability of a yeast cell, they are useful for the analysis of chromosome segregation as their loss and/or rearrangement can be monitored without detrimental effects to the cell. Students also genetically characterize the mutants and begin efforts to identify the defective genes in these mutants. This article provides a description of the research project, examples of students' data, and assessment of the impact of this course on student learning and future decisions.
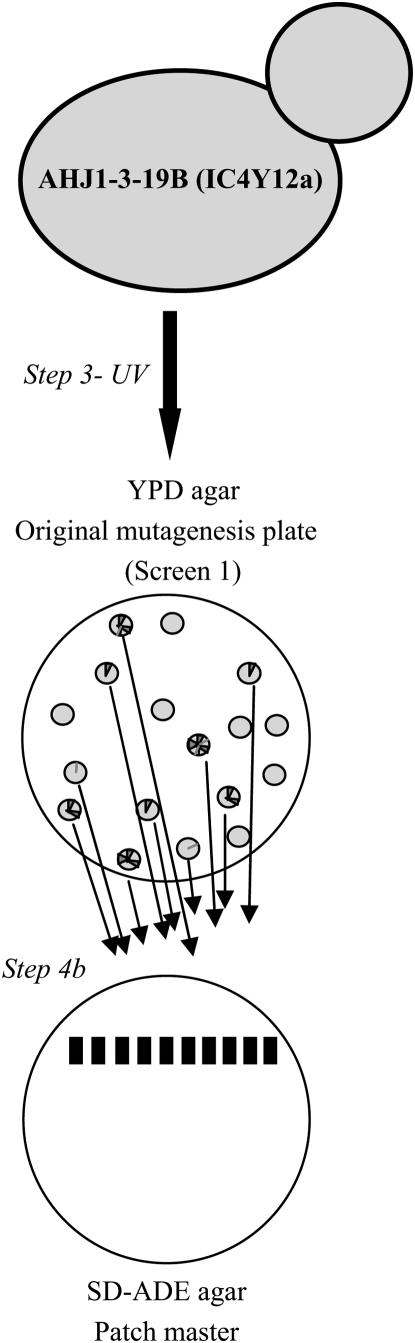
Figure 1.—
Overview of course research project to isolate and characterize yeast mutants with defects in mitotic chromosome segregation. The research project was conducted in seven steps. (1) Yeast strains were tested for correct genotype/phenotype. (2) The YAC loss rate in the wild-type unmutagenized yeast strain was determined. (3) The YAC-containing yeast strain was mutagenized with UV light. (4) Mutants with increased YAC loss were identified by a series of three screens: (a) screen 1, in which mutagenized colonies were visually screened for increased sectoring phenotype; (b) screen 2, in which an SD–ADE patch master was made using the white portion of a sectored colony from screen 1, the resulting SD–ADE patches were single-colony purified on YPD, and the sectoring phenotype was observed; and (c) screen 3, in which a fresh SD–ADE patch master was made using the white portion of a sectored colony from the YPD plate in screen 2, the resulting SD–ADE patches were single-colony purified on YPD, and the sectoring phenotype was observed. (5) Mutants were analyzed genetically for mode of inheritance (i.e., dominant, recessive), complementation analysis, and temperature sensitivity. (6) YAC loss rate was determined in the mutants. (7) Independent experiments were designed and conducted.
ORGANIZATION OF THE RESEARCH COURSE
Drake University's BIO106: “Research in Genetics” course is an upper-level undergraduate inquiry-based laboratory course designed to expose students to using scientific methods to solve a biological problem involving yeast as a genetics model organism. An introductory genetics course is a prerequisite for BIO106. This three-credit course includes two 3  -hr laboratory sessions and one 50-min discussion session per week. Students also commit a minimal amount of time outside of class to maintaining experiments. Enrollment has ranged from 8 to 15 students (15 is the maximum), and students collaborate in groups of 3 in the laboratory. A total of 48 students (42% male, 58% female; 23% sophomores, 31% juniors, 46% seniors) participated in BIO106 over the four semesters that it was offered. These students represented three science majors: biology (65%), biochemistry (33%), and chemistry (2%). On average, students entering the course have higher cumulative grade point averages (GPAs) than biology majors (BIO106 students' average GPA = 3.32 ± 0.44 (n = 48); biology majors' average GPA = 3.09 ± 0.62 (n = 303; fall 2003, spring 2005, and spring 2007); t-test, P = 0.02).
-hr laboratory sessions and one 50-min discussion session per week. Students also commit a minimal amount of time outside of class to maintaining experiments. Enrollment has ranged from 8 to 15 students (15 is the maximum), and students collaborate in groups of 3 in the laboratory. A total of 48 students (42% male, 58% female; 23% sophomores, 31% juniors, 46% seniors) participated in BIO106 over the four semesters that it was offered. These students represented three science majors: biology (65%), biochemistry (33%), and chemistry (2%). On average, students entering the course have higher cumulative grade point averages (GPAs) than biology majors (BIO106 students' average GPA = 3.32 ± 0.44 (n = 48); biology majors' average GPA = 3.09 ± 0.62 (n = 303; fall 2003, spring 2005, and spring 2007); t-test, P = 0.02).
Students have the opportunity to “do science” the way a scientist would. They design experiments, collect data, interpret data, and formally present findings. While engaged in this research project, students learn many genetics and molecular biology concepts and acquire technical skills in both classical and molecular genetics. Furthermore, they gain experience in the critical reading of scientific literature and navigation of scientific databases. They learn to work cooperatively as members of a research team. Importantly, they also practice scientific writing and oral presentation. An abbreviated list of course objectives and activities for assessing these objectives is provided in Table 1. Activities are modified each time the course is offered, but in general, repeated attempts are made to improve student understanding of how and why each step of the project is done and to make connections between individual experiments within the framework of an overall “big picture” of the project.
TABLE 1.
Activities to assess course objectives
| Major course objective | Assignment/activity to assess objective |
|---|---|
| To expose students to scientific inquiry | Participation in course research project, laboratory notebook, journal article discussions |
| To facilitate student learning of fundamental genetics and cell biology concepts related to the research project | Written quizzes and problem sets related to peer-reviewed journal articles |
| To provide opportunities for students to acquire technical skills related to genetics | Participation in course research project, practical quiz of techniques |
| To allow students to collaborate in small groups and contribute to a larger project | Group project, course poster, self and peer evaluations |
| To provide opportunities for students to practice presenting scientific data in written and oral formats | Individual lab reports, group research paper, mini-research proposal, group research poster, group PowerPoint presentation |
Students are exposed to scientific inquiry and concepts related to the course project through hands-on research and discussion of journal articles (supplemental Table 1 at http://www.genetics.org/supplemental/). Students either complete short answer questions related to each paper or lead a discussion of an assigned article. To encourage student preparation and participation, each student is required to submit a question related to the paper to the presenters via Blackboard (online course management system) prior to the in-class discussion of a journal article. In addition, students independently complete activities related to solution preparation, use of the metric system, calculation of cell concentrations, serial dilutions, and navigation of scientific databases (supplemental Table 1). To assess student learning of course-related concepts, students complete two written quizzes with questions about the research project, in-class discussions, and assigned journal articles. A practical quiz is administered by the instructor to assess the technical skills of each individual student (e.g., replica plating, cell plating, single-colony purification, sterile technique).
Three types of activities allow students to practice writing skills: lab reports, final research paper, and mini-research proposal. Each student prepares two brief lab reports with each focusing on a single experiment (e.g., isolation of ysm mutants, determination of YAC loss rate, independent experiment). Students within the same group prepare reports on different experiments such that each experiment is presented by at least one student in the group. For guidance, students are given a good-quality sample lab report, and in-class discussions focus on data interpretation, data presentation, and lab report format. The instructor grades each report and provides suggestions for improvement. After considering instructor feedback, students within a group compile the contents of their individual lab reports and add cohesive introductions and discussions to create a final research paper in scientific format (Abstract, Introduction, Materials and Methods, Results, Discussion, Acknowledgments, References). For additional guidance on preparing the final paper, students are referred to “instructions for authors” from a peer-reviewed genetics journal and the rubric that is used to evaluate the final paper (supplemental Figure 1 at http://www.genetics.org/supplemental/). The third type of written assignment is a mini-research proposal in which students (as a group) propose experiments designed to extend the research completed in the course.
Two types of assignments focus on oral presentation skills: a PowerPoint presentation and a poster presentation. Each group prepares a 10- to 15-min PowerPoint presentation with emphasis on the group's independent experiments. The presentations are evaluated by the instructor and peers using the rubric in supplemental Figure 2 at http://www.genetics.org/supplemental/. In addition, all groups collaborate to prepare a single large “class” poster (Bjordahl et al. 2005; Bjorge et al. 2007). Each student takes part in presenting the poster to interested students, faculty, and staff at the annual Drake University Conference on Undergraduate Research in the Sciences (Sleister et al. 2004).
EXPERIMENTAL METHODS
Strains and media:
S. cerevisiae strains used in this study are listed in Table 2. IC4Y12a is a 195-kb YAC containing human chromosome 4 DNA (Sleister et al. 1992). This YAC has TRP1 and URA3 telomeric markers and ADE2 integrated within the human DNA of the YAC. Bacterial strain DH5α is also used in this study: F−, ϕ80dlacZΔM15, Δ(lacZYA-argF)U169, deoR, recA1, endA1, hsdR17(rk−, mk+), phoA, supE44, λ−, thi-1, gyrA96, relA1.
TABLE 2.
Yeast strains
| Strain | Genotype/description | Source |
|---|---|---|
| BY4736 | MATa, ade2DhisG, trp1D63, ura3D, his3D200, met15D0 | ATCC 200898 |
| AHJ1-3-19B | MATα, ade2-1, trp1, ura3-1, lys2-1, leu2-3,112, his5, tyr1-1, can1R | Sleister et al. (1992) |
| AHJ1-3-19B [IC4Y12a] | AHJ1-3-19B containing 195 kb YAC [IC4Y12a] | Constructed in Robert Malone's laboratory, University of Iowa |
| HS100-4A | MATa, ade2, trp1, ura3, lys2-1, leu2-3,112, his3, tyr1-1, CAN1S | Meiotic segregant from diploid AHJ1-3-19B X BY4736 |
| HS100-4A [IC4Y12a] | HS100-4A containing 195 kb YAC [IC4Y12a] | Constructed by H. M. Sleister |
| ysm3 | YAC stability in mitosis mutant isolate 3 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm5 | YAC stability in mitosis mutant isolate 5 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm12 | YAC stability in mitosis mutant isolate 12 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm21 | YAC stability in mitosis mutant isolate 21 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm26 | YAC stability in mitosis mutant isolate 26 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm45 | YAC stability in mitosis mutant isolate 45 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm52 | YAC stability in mitosis mutant isolate 52 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm56 | YAC stability in mitosis mutant isolate 56 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm76 | YAC stability in mitosis mutant isolate 76 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm77 | YAC stability in mitosis mutant isolate 77 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm83 | YAC stability in mitosis mutant isolate 83 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm84 | YAC stability in mitosis mutant isolate 84 derived from AHJ1-3-19B [IC4Y12a] | This study |
| ysm102 | YAC stability in mitosis mutant isolate 102 derived from HS100-4A [IC4Y12a] | This study |
| ysm106 | YAC stability in mitosis mutant isolate 106 derived from HS100-4A [IC4Y12a] | This study |
| ysm107 | YAC stability in mitosis mutant isolate 107 derived from HS100-4A [IC4Y12a] | This study |
| ysm109 | YAC stability in mitosis mutant isolate 109 derived from HS100-4A [IC4Y12a] | This study |
| ysm110 | YAC stability in mitosis mutant isolate 110 derived from HS100-4A [IC4Y12a] | This study |
| ysm116 | YAC stability in mitosis mutant isolate 116 derived from HS100-4A [IC4Y12a] | This study |
| ysm123 | YAC stability in mitosis mutant isolate 123 derived from HS100-4A [IC4Y12a] | This study |
| ysm130 | YAC stability in mitosis mutant isolate 130 derived from HS100-4A [IC4Y12a] | This study |
Yeast cells were grown at 28° in YPD (1% yeast extract, 2% peptone, 2% glucose) or synthetic defined (SD) media lacking histidine (SD–HIS) or adenine (SD–ADE) (QBiogene, Irvine, CA). Diploids were sporulated in SPO media containing 1% potassium acetate and 0.25% yeast extract. Bacterial cells were grown at 37° in yeast extract tryptone media (1.6% tryptone, 1% yeast extract, 0.5% NaCl).
UV mutagenesis and genetic visual screen for increased YAC loss:
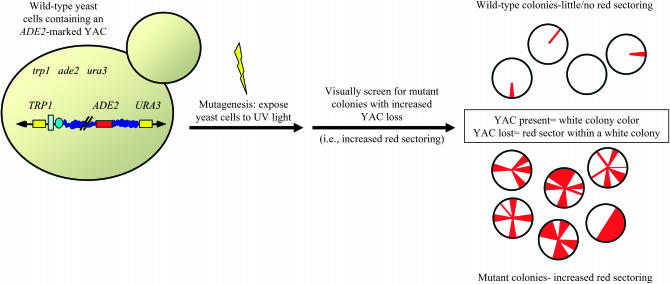
An initial goal of this research project is to isolate yeast mutants that are defective in mitotic chromosome transmission. A simple color visual screen of yeast colonies allows for detection of cells with increased YAC loss. AHJ1-3-19B [IC4Y12a] and HS100-4A [IC4Y12a] cells have a defective nuclear ade2 gene, but contain an ADE2-marked YAC. Mutant ade2 cells form red colonies, whereas ade2 cells containing an ADE2-marked YAC form white colonies (Jones and Fink 1982). Cells that lose the YAC during mitotic divisions give rise to red sectors within a white colony (Figure 2). The low loss rate of the YAC in wild-type cells (3.2 × 10−5 cell/generation; Sleister et al. 1992) is manifested as white colonies with no or very few tiny red sectors.
Figure 2.—
Genetic screen for yeast mutants with defects in chromosome segregation. To monitor chromosome loss, a yeast strain containing a YAC was used. The strain is mutant for trp1, ura3, and ade2 genes and contains a YAC with selectable markers TRP1, URA3, and ADE2. The YAC also contains a centromere, origin of replication, and telomeres. Cells are spread on agar plates, exposed to UV light, and allowed to form colonies. A visual screen for red-sectored colonies is used to isolate mutants with increased YAC loss.
Previous studies suggest that an appropriate level of mutagenesis by ultraviolet (UV) light for isolation of mutants will result in ∼10–50% cell survival (Lawrence 2002). To determine the length of UV exposure required for our yeast strains and UV setup (http://www.phys.ksu.edu/gene/RAD.html), students plate known numbers of yeast cells onto YPD agar and expose them to UV light for various times: 0, 90, 120, 180, and 240 sec. The plates are wrapped in foil and stored in the dark for 24 hr at room temperature. The foil is removed from the plates and colonies are allowed to grow for 5 days at 28°.
To isolate mutants with increased loss of the YAC, ∼60,000 AHJ1-3-19B [IC4Y12a] and HS100-4A [IC4Y12a] cells were plated onto YPD agar plates at a density of 750 cells/plate and exposed to UV light for 3 min. Following incubation in the dark at 25° for 24 hr, plates were placed at 28° for 5 days. Mutant colonies with more red sectors than the unmutagenized wild-type control strain were retested for the red sectoring phenotype by single-colony purification. Mutants that redisplayed the increased red sectoring phenotype were named YAC stability in mitosis (ysm) mutants.
Genetic analysis of ysm mutants:
To determine the mode of inheritance of each ysm mutant's red sectoring phenotype (i.e., chromosome loss phenotype), each mutant derived from AHJ1-3-19B [IC4Y12a] was mated with BY4736, and each mutant derived from HS100-4A [IC4Y12a] was mated with AHJ1-3-19B. Assuming that the YAC loss phenotype is due to a single mutation, the resulting diploids were heterozygous for the ysm mutation (YSM+/ysm−) and had one copy of the YAC. Heterozygous (YSM+/ysm−) diploids were selected on SD–HIS agar. After replica plating diploids on SD–ADE agar to select for the presence of the YAC, diploids were single colony purified on YPD agar. The colony red-sectoring phenotypes of the heterozygous diploids were compared to those of the parent haploid strains and diploid controls to determine the mode of inheritance.
To begin to estimate the number of genes represented in the ysm mutant collection, partial complementation analysis was performed with recessive mutants displaying a red-sectoring phenotype markedly greater than that of the wild-type strain. MATa, YAC-containing mutants derived from HS100-4A [IC4Y12a] (ysm's 102, 106, 107, 109, 110, 116, 123) were crossed with MATα, YAC-lacking mutants derived from AHJ1-3-19B [IC4Y12a] (ysm's 12, 21, 45, 52, 76, 77, 83, 84). Following diploid selection on SD–HIS and replica plating to SD–ADE for selection of the YAC, diploids were single colony purified on YPD. Complementation analysis was completed by comparing the red-sectoring phenotypes of the resulting diploids to those of the relevant haploid ysm parents and wild-type strain.
To determine whether any of the ysm mutants were temperature sensitive (ts), all ysm mutants were replica plated to YPD and complete media and incubated at 28° and 37° for 2 days.
Determination of YAC loss rate:
To calculate the loss rate of the YAC in the wild-type strains AHJ1-3-19B [IC4Y12a] and HS100-4A [IC4Y12a] and in a subset of the ysm mutant strains, 2-ml YPD cultures were inoculated with 100 YAC-containing cells and allowed to grow to saturation (48 hr) at 150 rpm at 28°. Approximately 300 cells were spread onto each of eight YPD agar plates. The frequency of YAC loss was calculated as the percentage of red Trp− Ura− Ade− colonies. The rate of YAC loss was calculated by the following formula: (0.4343) (loss frequency)/(log Nt − log No), where No is the initial concentration of the culture, and Nt is the concentration of the culture at the time of plating (Drake 1970). YAC loss rates were calculated from at least five cultures for each ysm strain tested. YAC loss rates in ysm strains relative to wild-type strains were statistically analyzed by t-test.
EXAMPLES OF STUDENTS' EXPERIMENTAL RESULTS
Length of UV exposure for mutagenesis:
Before implementing a genetic screen to isolate mutants with a defect in YAC segregation, students test the effect of a range of UV exposure times (0, 90, 120, 180, and 240 sec) on the viability of the wild-type AHJ1-3-19B [IC4Y12a] yeast strain. Students concluded that 180 sec is an adequate length of UV exposure to achieve an appropriate level of mutagenesis (38.6% cell survival at 180 sec; supplemental Table 2 at http://www.genetics.org/supplemental/; Lawrence 2002).
Genetic screen for YAC loss:
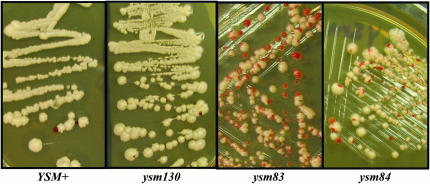
A visual screen was implemented to isolate yeast mutants that are defective in segregating a YAC (Figure 2). Wild-type YAC-containing yeast produce white colonies, and mutants that lose an ADE2-marked YAC at an elevated rate produce white colonies with red sectors. Compilation of student data revealed that UV mutagenesis of ∼42,000 yeast cells resulted in the survival of 16,204 yeast colonies (12,211 from strain AHJ1-3-19B [IC4Y12a] and 3993 from strain HS100-4A [IC4Y12a]). Red-sectored colonies from the original UV mutagenesis plates were rescreened at least two times for the mutant red-sectoring phenotype (Figure 1). A total of 132 mutants displayed a red-sectoring phenotype in at least two of three screens, and these mutant strains were named ysm mutants. Mutant strains ysm1–ysm84 were derived from strain AHJ1-3-19B [IC4Y12a], and mutant strains ysm100–ysm147 were derived from HS100-4A [IC4Y12a]. As illustrated in Figure 3, the level of red sectoring ranges from minimal sectoring (e.g., ysm130) to severe sectoring (e.g., ysm83 and ysm84).
Figure 3.—
Colony red-sectoring phenotype for a wild-type yeast strain (YSM+) and three ysm mutants. The level of red sectoring reflects the loss rate of an ADE2-marked YAC. Mutants ysm83 and ysm84 display much greater red sectoring (i.e., YAC loss) than the wild-type strain, whereas ysm130 displays slightly more red sectoring than the wild-type strain.
Genetic analysis of ysm mutants:
Analysis of the mode of inheritance of the red-sectoring phenotype of the 132 isolated ysm mutants revealed that 34 (25.8%) are recessive and 60 (45.5%) are dominant or incompletely dominant. The mode of inheritance of the remaining 38 ysm mutants was inconclusive because the red-sectoring phenotype of the haploid mutant overlapped that of the wild-type strain. Interpretation of the results and examples of student data are shown in supplemental Table 3 and supplemental Figure 3 at http://www.genetics.org/supplemental/.
Complementation analysis was performed to determine the minimum number of genes in the ysm mutant collection. Students crossed haploid recessive ysm mutants of opposite mating types and observed the red-sectoring phenotype of the resulting diploids in comparison to the relevant haploid ysm− parents and YSM+ wild-type strain. Student interpretation of complementation analysis data is presented in supplemental Table 4 and supplemental Figure 4 at http://www.genetics.org/supplemental/.
Only three ysm mutants were ts. Although the ts phenotype would be useful in functional complementation efforts, all three of these ts mutants had red-sectoring phenotypes very similar to those of the wild-type strain. Nonetheless, students discuss how they would experimentally determine if the YAC loss and ts phenotypes are caused by the same mutation. This leads to further discussion and a search for other phenotypes (e.g., sensitivity to a microtubule-destabilizing drug) that would facilitate efforts to identify the defective genes within the ysm mutants.
Determination of YAC loss rate:
The ysm mutants were isolated from a qualitative visual screen for increased red sectoring (i.e., increased YAC loss) in comparison to the isogenic wild-type strain. Each student group experimentally quantitates the YAC loss rate for two of their group's ysm mutants and the isogenic wild-type control strain. As an example, one group calculated that the YAC is lost at a 12.4-fold higher rate in the ysm3 mutant strain than in the wild-type strain (t-test, P = 0.045; supplemental Table 5 at http://www.genetics.org/supplemental/).
Independent experiments:
In addition to completing the experiments described above, each team of students proposes and completes two independent experiments to further characterize their mutants. A main objective of the independent experiments is to provide students an opportunity to apply their knowledge of the project and related literature to advance the project. Students are encouraged to ask questions about their mutant(s) and propose rational experiments that can be completed in a relatively short time frame to answer these questions. Ideally, the independent experiments will lead to the identification of phenotypes that may facilitate cloning the wild-type YSM genes that are defective in the ysm mutant strains. Examples of the types of questions that students ask and relevant experimental approaches are provided in Table 3.
TABLE 3.
Examples of independent experiments
| Question | Experimental approach |
|---|---|
| Is my favorite ysm mutant defective in meiosis (i.e., sporulation)? | A ysm homozygous diploid is constructed, and the sporulation frequency of this diploid is compared to that of a congenic wild-type diploid. |
| Is the genetic lesion causing the sectoring phenotype (i.e., YAC loss) located within the YAC or a yeast nuclear gene? | YSM+/ysm− heterozygous diploids are constructed, sporulated, and tetrads are dissected. The red-sectoring phenotype is analyzed in YAC-containing spores. If a particular ysm mutation is cis (within the YAC), then 100% of the YAC-containing spores derived from that mutant are expected to display the mutant red-sectoring phenotype. In contrast, if the ysm mutation is trans (within a yeast nuclear gene), then the mutation is expected to segregate 2:2 in meiosis. As a result, only 50% of the YAC-containing spores from this particular mutant are expected to display the mutant red-sectoring phenotype. |
| Are microtubules defective in my favorite ysm mutant? | A ysm mutant haploid and isogenic wild-type strain are compared for growth sensitivity to the microtubule-destabilizing drug benomyl. |
| Does my favorite ysm mutant display a defect in DNA replication? | Growth is compared between a ysm mutant haploid and isogenic wild-type strain on agar plates containing the DNA replication inhibitor hydroxyurea. |
| Is my favorite ysm mutant able to repair damaged DNA? | Cell viability is compared between a ysm mutant haploid and isogenic wild-type strain on agar plates upon exposure to mutagens (e.g., ultraviolet light, methyl methanesulfonate). |
| Does my favorite ysm mutant have an elevated mutation frequency? | The frequency of forward mutation at the CAN1 gene (i.e., production of canavanine-resistant cells) is compared in ysm mutant haploid and isogenic wild-type haploid cells. |
| Is the cell cycle progression of my favorite ysm mutant similar to that of the isogenic wild-type strain? | Wild-type and mutant ysm cells are synchronized with α-factor. α-factor is removed, and cell cycle progression [as assessed by yeast cell bud size (Alberts et al. 2001)] is examined by microscopic observation over time. |
| Does my favorite ysm mutant have an abnormal cell morphology? | The cell morphology and size of the ysm mutant and isogenic wild-type cells are compared by microscopy. |
DISCUSSION
Assessment of course objectives:
To assess whether the research course is effective for student learning of concepts, in two of the four semesters in which the course was taught students were given a short pretest on the first day of class (supplemental Table 6 at http://www.genetics.org/supplemental/). These same questions were included in one of the two in-class quizzes later in the semester. The mean number of correct answers on the post-test (17.8 ± 1.5) was significantly greater than the mean number of correct answers on the pretest (11.5 ± 5); (t-test, P = 0.012, n = 19; supplemental Table 6). Nearly all of the students correctly answered question 1 (basic understanding of the metric system) prior to the laboratory course. Questions 2, 3, and 4 are related to specific practical skills used repeatedly during the course, and questions 5 and 6 are related to concepts important for understanding the research project. Furthermore, students indicated in a course evaluation that participation in the research course improved their understanding of technical/scientific concepts (Table 4). To informally monitor student learning, students are invited to complete a “minute paper” containing the following questions: “What were the most important concepts/skills you learned today? Are there any concepts/skills that you find confusing/difficult that you would like the class to review? Other comments?” This provides immediate feedback for the instructor that is valuable for student-centered learning. These papers are submitted anonymously; therefore, a student who is struggling with a concept or technique can ask for the topic to be reviewed without revealing a deficiency to classmates.
TABLE 4.
Course assessment
| Average ± standard deviationa | As compared to before participating in BIO106, after taking BIO106 I noted an improvement in: |
|---|---|
| 4.34 ± 0.61 | My understanding of technical/scientific concepts (e.g., PCR, molecular cloning). |
| 4.69 ± 0.47 | My technical skills in frequently performed methods (e.g., pipeting, sterile technique, streaking cells on agar plates, etc.). |
| 4.38 ± 0.62 | My understanding of how science is done (i.e., the range of activities from asking a biological question to conducting an experiment and interpreting data). |
| 3.83 ± 0.76 | My technical writing skills (i.e., writing using the format Introduction, Methods, Results, Discussion). |
| 3.69 ± 0.71 | My oral presentation skills (for scientific/technical information). |
| 4.45 ± 0.74 | My comfort or efficiency in collaborating with a small research team of two to three students. |
| 4.45 ± 0.63 | My ability to make connections between individual experiments (e.g., by repeatedly discussing the “big picture” of the project). |
| 4.03 ± 0.68 | My ability to interpret and/or graphically present experimental data. |
| 4.00 ± 0.72 | My ability to search databases to find relevant scientific information (e.g., PubMed, NCBI Blast, Yeast Genome Database). |
| 4.45 ± 0.69 | My ability to carry out multiple tasks simultaneously (e.g., multiple experiments). |
| 4.21 ± 0.74 | My ability to think critically. |
| 3.79 ± 0.56 | My ability to critically read journal articles. |
| 4.19 ± 0.37 | Average |
| Average ± standard deviationa | Relative to other more traditional science laboratory courses, I believe: |
| 4.50 ± 0.58 | The concepts and technical skills I gained while participating in “Research in Genetics” helped me/will help me in my further studies at Drake and/or beyond. |
| 4.29 ± 0.85 | The concepts and skills I gained while participating in “Research in Genetics” will help me in my future career. |
| 4.48 ± 0.63 | The inquiry-based approach used in BIO106 helped me better understand genetics concepts and methods (e.g., chromosome segregation, gel electrophoresis). |
| 4.59 ± 0.63 | The inquiry-based approach helped me to make connections between different concepts/experiments. |
| 4.38 ± 0.62 | The inquiry-based approach helped me realize that research is interdisciplinary (e.g., a mutation at the level of DNA affects YAC loss at the cellular/biochemical level; methods are required from more than one subject area—e.g., genetics and chemistry). |
| 4.45 ± 0.49 | Average |
This survey was completed by 29 students; 14 completed the survey 1–2 years after completing the course (most had already graduated), and 15 completed the survey at the end of the enrolled semester. While the students who responded to the survey were enrolled in the course, 10 were sophomores, 5 were juniors, and 14 were seniors. Students reported the following postcourse plans: 12, health profession (medical school, dental school, veterinary school); 9, graduate school; 1, education; 4, job related to major (e.g., research); 3, unknown. Of the 29 survey responders, 11/29 had participated in a research project prior to the course, and 22/29 participated in a research project following enrollment in the course.
Using the scale 1–5, students were asked to indicate the level to which they agreed with the following statements: 5, strongly agree; 4, agree; 3, neutral; 2, disagree; 1, strongly disagree.
Most students entering the “Research in Genetics” course have limited exposure to genetics and/or molecular biology-related methods. Through frequent application, students gain competency in common genetics techniques (e.g., single-colony purification, agarose gel electrophoresis, replica plating, cell plating, PCR). A practical exam revealed that at the end of the semester nearly all students were proficient (mean = 4.9 of 5 points) in the technical skills assessed (e.g., pipeting, replica plating, cell plating on agar, single-colony purification). When surveyed after completion of the course, students noted improvement in frequently performed laboratory methods (Table 4). Importantly, both high-level (A) and average-level (C) students successfully learned concepts and technical skills related to the research project.
Smith et al. (2005) reported that students' learning is enhanced by collaboration with other students and faculty. An important aspect of this research course is that students collaborate in small groups to produce subsets of data for the overall course project. An end-of-semester evaluation indicates that students' comfort or efficiency in collaborating in a small research team improves as a result of taking the course (Table 4). Importantly, since much of a student's grade is affected by the other members of his/her research group, each student evaluates his/her efforts during the semester as well as the efforts of other members of the team. Typically, peer evaluations are very positive. Students compliment the dedication of teammates and the role that each person plays in the group.
Students have the opportunity to practice technical writing skills through a mini-research proposal, laboratory reports, and a final research paper. The intent of the lab report is to encourage students to interpret and present their data prior to writing the final research paper. Lab reports also inform the instructor of weaknesses in student understanding and writing. The most common mistakes observed in the lab reports include inadequate labels on figures, misinterpretation of data (e.g., over-interpretation), and inefficient data presentation (e.g., extensive descriptive text used in place of a graph). The exercise of completing lab reports prior to the final paper results in considerably greater qualities of final papers. When asked about the helpfulness of lab reports in a postcourse questionnaire, 100% of the students who responded (n = 29) indicated that the feedback received on the lab reports was very helpful for preparation of the final paper. While many of the final papers are excellent, some student groups could benefit by rewriting the final paper. Morgan and Fraga (2007) presented an effective “all-or-nothing” strategy in which students have multiple opportunities (if needed) to rewrite laboratory reports to create a high-quality report in the format of a scientific paper. It might also be beneficial for students to mimic the peer-review process used by scientists through critiquing one another's papers (Guilford 2001).
The ability to present data orally is also very important and sometimes lacking in the undergraduate science curriculum. In this course, oral presentation skills are assessed primarily in group PowerPoint presentations that are scored by both students and the instructor. These presentations are valuable as they demonstrate the extent of knowledge that the students gain in the course. Furthermore, student presenters must understand the project well enough to quickly answer viewers' questions. The quality of the presentations is impressive. Students are professional, well prepared, and collegial. The two most recent “Research in Genetics” cohorts completed and presented a “class” poster at Drake's annual undergraduate research conference (Sleister et al. 2004; Bjordahl et al. 2005; Bjorge et al. 2007). Of nearly 40 posters at the 2005 conference, the “Research in Genetics” Spring 2005 students won top poster award and were recognized by the President and Provost of the University. In addition to encouraging students to talk about their work to a broad audience, this experience is important as students realize that the university community values and is impressed by their work.
Challenges of the research course:
While accomplishing course objectives, students experience an important reality of science, namely that experiments do not always work and that there are consequences to making errors. For example, when transforming yeast cells with a yeast genomic library to screen for suppressors of the YAC loss phenotype, students typically had to repeat the transformation to achieve an adequate number of transformants for screening. A method that students found particularly difficult was recovering plasmids from yeast cells for amplification in Escherichia coli. After repeated attempts and modifications to the protocol, all student groups were able to isolate some, but not all, of their plasmids. Some lessons were learned the hard way. For example, if a group's experiment failed because of an error in an important experimental detail (such as an incubation temperature), the group had to repeat the procedure.
The research course also poses challenges for the instructor. The time required for preparation of research and curricular materials is significant. In addition, meeting the needs of each student and research team can be very demanding, particularly when multiple groups need assistance at the same time. Although there is not a laboratory assistant assigned to Drake's BIO106 course, it is expected that an assistant would minimize some of the instructor's challenges.
Past and future of the genetics research course and chromosome transmission project:
The first two semesters in which this course was taught, students isolated mutants defective in segregating a YAC (as described in this article). In a later semester, students implemented a genetic screen for genes that disrupt chromosome transmission in yeast when overproduced (i.e., when present on a high-copy plasmid). An advantage of this approach is that students spend a significant amount of time learning molecular genetics techniques (e.g., plasmid isolation, transformation of yeast and E. coli, restriction digestion, gel electrophoresis, PCR, and sequence analysis). Students most recently enrolled in the course extended the work of previous BIO106 students by isolating yeast genomic plasmid suppressors of their favorite ysm mutants.
In future offerings of the genetics research course, students will focus on identification of the defective genes in the previously isolated ysm mutant strains. Initially, this will involve complementation testing with the well-characterized set of chromosome transmission fidelity (ctf) mutants isolated by Spencer et al. (1990). For ysm mutant genes that are not represented in the ctf collection, two approaches will be employed: (1) Complementation tests will be performed between ysm (MATα) mutants of interest and the yeast (MATa) deletion collection and (2) centromere-based yeast genomic plasmid suppressors of ysm mutants' YAC loss defect will be isolated.
The ysm mutant collection isolated by BIO106 students will continue to be a valuable resource to the BIO106 course, my own research laboratory, and the scientific community. Students who joined my lab upon completing the research course contributed to an ongoing project involving chromosome transmission. They further characterized the ysm mutant collection and initiated efforts to isolate genetic suppressors of a few ysm mutants' YAC loss defects. Following identification of the defective gene in each ysm mutant strain of interest, the mutant gene will be isolated by PCR and sequenced. In addition to revealing the nature of the mutation with respect to the ysm gene product, this work will lead to the proposal of hypothesis-driven studies concerning the protein's role in chromosome transmission. Ideally, this will promote collaborations with external scientists studying the same gene(s) and/or processes and will further reinforce students' appreciation that they are doing “real” science that is of interest to others.
Impact of the genetics research course:
Investigative laboratories teach students about the nature of science. The following student comments are representative of 29 responses to the question, “What did you learn about research or the nature of science as a consequence of taking this course?”
I found that organization was very important, and that science can be interesting. This was the only science lab I actually truly enjoyed.
The importance of thoroughly researching a topic (in fact understanding the concepts) before designing a research project.
Getting different results than you expected is not failing and sometimes it can be more helpful in determining an answer than getting the result that you predicted.
The course really helped me see the connections between the various tests that we did and allowed for a closer look at important techniques important to research. There was a lot to take in, but it made me respect the process and have patience in my research.
I learned that research can be fun!
It is a long process with many steps to get to a final picture. But each process is just as important as the next.
There is a lot of work that is put into a project and you have to adapt a lot from what you find out. You can't always follow lists of instructions.
There are many, many steps involved, lots of ways to make errors, skills do improve.
Patience is key—research takes time and effort, and must be done accurately and cleanly.
Small, project-based laboratories provide excellent opportunities for student learning. All of the students who participated in the “Research in Genetics” course had previous experiences with traditional, “cookbook” laboratory courses. When asked to compare their experiences in the two types of labs, students indicated that the inquiry-based approach was more beneficial for understanding genetics concepts and methods, for making connections between different concepts/experiments, and for appreciating that research is interdisciplinary (Table 4). Furthermore, students responded that, relative to a more traditional laboratory course, the concepts and technical skills gained while participating in “Research in Genetics” are/will be valuable in further studies at Drake and/or in a future career (Table 4).
A goal of project-based laboratory courses is to stimulate student interest and participation and to attract more students to biomedical research (National Research Council Committee on Undergraduate Biology Education to Prepare Research Scientists for the 21st Century 2003). Of the 25 students who took this course the first two semesters in which it was offered, 8 (32%) continued working on this same project as an independent research study in a later semester(s). Their efforts resulted in poster presentations at local, state, and national meetings (e.g., Fatland et al. 2004). Postcourse surveys of 29 students over the four semesters in which the research course was offered revealed that 22 (76%) did/would seek additional research experiences (a portion of this survey is included in Table 4). Selected student comments on this survey support the impact of the course in attracting students to research:
I think this course was the main reason I got involved in research at Drake and a part time research job. I would not have had the skills or confidence to pursue these opportunities without taking this course and it has definitely prepared me for the future since research is a great thing for undergraduates to take part in to help figure out what they are interested in for a career.
I think the critical thinking aspect of the course is important to developing a good handle on how science is practiced in real life and is important for those pursuing a career either in research or in a medical field since medicine requires the ability to be creative and think critically.
It is by far the best science lab course I took at Drake. It was arranged in a way that you can see how each of the different skills and experiments comes together and can be used in the real world, and is a great way to see if you might be interested in pursuing a career in research after graduation.
When asked on a postcourse survey whether they preferred the research course or a traditional laboratory course, 28 of 29 (97%) students chose the research course. Examples of justifications for this preference include the following statements:
… because I learn better when I know what I am doing is contributing to something beyond just getting a grade. This course gave me the opportunity to be invested in our research.
I really enjoyed thinking rather than just doing. We had to know what we were doing in order to correctly accomplish tasks.
… because you're focusing on one big picture rather than small, unrelated experiments. I also feel more satisfied completing a course like this.
Cookbook labs do not seem to have any bearing on any future work or any purpose except to get a grade.
This course has truly been “science.” Cookbook classes—I generally don't learn anything.
Cookbook courses only teach you how to read and perform (which we learned from BIO106) but BIO106 also taught us how to think and change experiments to make new discoveries. You can't teach that with cookbook experiments.
While most students who experienced the genetics research course prefer it over a traditional laboratory course, both types of experiences are valuable. Typically, a traditional laboratory course requires less planning and preparation by the instructor and, as a result, can accommodate more students. Also, a traditional laboratory course would likely allow for greater coverage of techniques as the research course would focus on repeated practice of techniques directly relevant to the research project. Both types of laboratory courses taken concurrently with a genetics “lecture” course would promote learning of methods (e.g., gel electrophoresis) by hands-on experience. An added advantage of a research course is that students gain first-hand experience with when and why to apply a particular method in addition to how to perform the method.
Implementation of the research course at other institutions:
The research course described here could be adapted to accommodate the circumstances of educators at other institutions. While meeting twice a week with Drake's BIO106 students is ideal with respect to project momentum, many project goals could also be accomplished by meeting once a week. In either case, a 3-hr class period is recommended for students to have sufficient time to complete relevant experimental methods. If needed, the weekly 50-min discussion section could be substituted by communicating with students electronically (this has been the case in two of the four semesters in which the course has been offered at Drake).
Equipment needed for the chromosome transmission project is listed in supplemental Table 7 at http://www.genetics.org/supplemental/. Modifications could be made on the basis of available resources. For example, educators without access to a UV irradiation chamber could chemically mutagenize yeast cells with ethyl methanesulfonate.
A research laboratory course involving undergraduates may be particularly useful for an educator with a heavy teaching load in a program that lacks a graduate program. The course described here could be modified to fit an educator's own research goals and interests. The primary literature is a great source of ideas for investigative laboratory courses. In fact, the design of BIO106's YAC stability in mitosis research project was inspired by published work of Spencer et al. (1990). Examples of other inquiry-based laboratory courses/modules involving yeast (Odom and Grossel 2002; Vallen 2002; Gammie and Erdeniz 2004), bioinformatics and human disease (Bednarski et al. 2005), apoptosis in cultured human cells (Dibartolomeis and Moné 2003), Chlamydomonas (Mitchell and Graziano 2006), and plants (Wenzel 2006) have been reported.
In summary, the “Research in Genetics” course allowed students to experience scientific inquiry. Surveys during the four semesters in which the research course was taught revealed that the course improved students' understanding of scientific concepts and technical skills, improved students' critical thinking skills, and helped students make connections between concepts. In addition, students gained an appreciation for the nature of science, had fun, and considered research in their future plans.
Acknowledgments
I am thankful to the 48 undergraduate students who contributed to the isolation and characterization of yeast mutants defective in chromosome segregation while they were enrolled in Drake University's BIO106: Research in Genetics course. I thank Bob Malone (University of Iowa) for providing yeast strain AHJ1-3-19B [IC4Y12a] and Drake University for providing equipment and reagents. I am grateful to Forrest Spencer for her initial publication of ctf mutants as this article (Spencer et al. 1990) was very influential in the design of the BIO106 research project. Moreover, Spencer has offered to provide ctf mutants for future complementation analyses. I am also grateful to Jerry Honts, Alesia Hruska-Hageman, and anonymous reviewers of this article for helpful suggestions.
References
- Alberts, B., A. Johnson, J. Lewis, M. Raff, K. Roberts et al., 2001. Molecular Biology of the Cell, Ed. 4. Garland Science, New York.
- Baetz, K. K., N. J. Krogan, A. Emili, J. Greenblatt and P. Hieter, 2004. The ctf13–30/CTF13 genomic haploinsufficiency modifier screen identifies the yeast chromatin remodeling complex RSC, which is required for the establishment of sister chromatid cohesion. Mol. Cell. Biol. 24: 1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarski, B. E., S. C. R. Elgin and H. B. Pakrasi, 2005. An inquiry into protein structure and genetic disease: introducing undergraduates to bioinformatics in a large introductory course. Cell Biol. Educ. 4: 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, E., 2001. The future of education in the molecular life sciences. Nat. Rev. Mol. Cell Biol. 2: 221–225. [DOI] [PubMed] [Google Scholar]
- Bjordahl, R., R. Davison, D. Hatz, K. Kostelc, R. Larew et al., 2005. Implementation of a genetic screen to elucidate proteins important for chromosome transmission. http://www.lib.drake.edu:8080/dspace/handle/2092/387.
- Bjorge, A., A. Bryant, M. K. Caniglia, D. Doty, E. Jarvis et al., 2007. Complementary approaches to identify genes important for chromosome segregation (http://www.lib.drake.edu:8080/dspace/handle/2092/544).
- Burke, D. T., G. F. Carle and M. V. Olson, 1987. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science 236: 806–812. [DOI] [PubMed] [Google Scholar]
- DiBartolomeis, S. M., and J. P. Moné, 2003. Apoptosis: a four-week laboratory investigation for advanced molecular and cellular biology students. Cell Biol. Educ. 2: 275–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake, J., 1970. The Molecular Basis of Mutation. Holden Day, San Francisco.
- Fatland, S., S. Leeson, D. Bellani, E. Scolaro, A. Wood et al., 2004. Isolation and characterization of yeast mutants defective in mitotic chromosome segregation. Yeast Genetics and Molecular Biology Meeting Abstracts (http://www.yeastgenome.org/community/meetings/yeast04/abshtml/317B.html).
- Frantz, K. J., R. L. DeHaan, M. K. Demetrikopoulos and L. L. Carruth, 2006. Routes to research for novice undergraduate neuroscientists. Cell Biol. Educ. 5: 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie, A. E., and N. Erdeniz, 2004. Characterization of pathogenic human MSH2 missense mutations using yeast as a model system: a laboratory course in molecular biology. Cell Biol. Educ. 3: 31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, V., T. McMiller, E. Jones and C. M. Johnson, 2003. Identifying novel helix-loop-helix genes in Caenorhabditis elegans through a classroom demonstration of functional genomics. Cell Biol. Educ. 2: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford, W. H., 2001. Teaching peer review and the process of scientific writing. Adv. Physiol. Educ. 25: 167–175. [DOI] [PubMed] [Google Scholar]
- Howard, D. R., and J. A. Miskowski, 2005. Using a module-based laboratory to incorporate inquiry into a large cell biology course. Cell Biol. Educ. 4: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt, M. A., T. Stearns and D. Botstein, 1990. Chromosome instability mutants of S. cerevisiae that are defective in microtubule-mediated processes. Mol. Cell. Biol. 10: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E. W., and G. R. Fink, 1982. Regulation of amino acid and nucleotide biosynthesis in yeast, pp. 181–299 in The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression, edited by N. N. Strathern, E. W. Jones and J. R. Broach. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Kouprina, N., A. Tsouladze, M. Koryabin, P. Hieter, F. Spencer et al., 1993. Identification and genetic mapping of CHL genes controlling mitotic chromosome transmission in yeast. Yeast 9: 11–19. [DOI] [PubMed] [Google Scholar]
- Lawrence, C. W., 2002. Classical mutagenesis techniques, pp.189–199 in Methods in Enzymology: Guide to Yeast Genetics and Molecular and Cell Biology, edited by C. Guthrie and G. R. Fink. Academic Press, San Diego. [DOI] [PubMed]
- McGrew, J. T., Z. X. Xiao and M. Fitzgerald-Hayes, 1989. Saccharomyces cerevisiae mutants defective in chromosome segregation. Yeast 5: 271–284. [DOI] [PubMed] [Google Scholar]
- Measday, V., K. Baetz, J. Guzzo, K. Yuen, T. Kwok et al., 2005. Systematic yeast synthetic lethal and synthetic dosage lethal screens identify genes required for chromosome segregation. Proc. Natl. Acad. Sci. USA 102: 13956–13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, B. F., and M. R. Graziano, 2006. From organelle to protein gel: a 6-wk laboratory project on flagellar proteins. CBE Life Sci. Educ. 5: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, W. R., and D. M. Fraga, 2007. All-or-nothing grading structure enhances student performance on laboratory reports. Mol. Biol. Cell 17(Suppl.): L157. [Google Scholar]
- National Research Council Committee on Undergraduate Biology Education to Prepare Research Scientists for the 21st Century, 2003. Bio2010: Transforming Undergraduate Education for Future Research Biologists. National Academies Press, Washington, DC. [PubMed]
- Odom, D. P., and M. J. Grossel, 2002. Using the two-hybrid screen in the classroom laboratory. Cell Biol. Educ. 1: 43–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouspenski, I. I., S. J. Elledge and B. R. Brinkley, 1999. New yeast genes important for chromosome integrity and segregation identified by dosage effects on genome stability. Nucleic Acids Res. 27: 3001–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge, K. W., and V. A. Zakian, 1993. Saccharomyces cerevisiae linear chromosome stability (lcs) mutants increase the loss rate of artificial and natural linear chromosomes. Chromosoma 102: 207–217. [DOI] [PubMed] [Google Scholar]
- Sleister, H. M., K. A. Mills, S. Blackwell, A. Killary, J. C. Murray et al., 1992. Construction of a human chromosome 4 YAC pool and analysis of artificial chromosome stability. Nucleic Acids Res. 20: 3419–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleister, H., C. Nelson and M. Bohorquez, 2004. Drake University conference on undergraduate research in the sciences. Am. J. Undergrad. Res. 3: v–viii. [Google Scholar]
- Smith, K. A., S. D. Sheppard, D. W. Johnson and R. T. Johnson, 2005. Pedagogies of engagement: classroom-based practices. J. Eng. Ed. 94: 87–102. [Google Scholar]
- Spencer, F., S. L. Gerring, C. Connelly and P. Hieter, 1990. Mitotic chromosome transmission fidelity mutants in Saccharomyces cerevisiae. Genetics 124: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallen, E., 2002. Analysis of protein localization and secretory pathway function using the yeast Saccharomyces cerevisiae. Cell Biol. Educ. 1: 173–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel, T. J., 2006. General chemistry: expanding the learning outcomes and promoting interdisciplinary connections through the use of a semester-long project. CBE Life Sci. Educ. 5: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, W. B., 2003. Inquiry-based undergraduate teaching in the life sciences at large research universities: a perspective on the Boyer Commission Report. Cell Biol. Educ. 2: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]