Abstract
The gravity response is an array of behavioral and physiological plasticity elicited by changes in ambient mechanical force and is an evolutionarily ancient adaptive mechanism. We show in Caenorhabditis elegans that the force of hypergravity is translated into biological signaling via a genetic pathway involving three factors: the degenerin/epithelial Na+ channel (DEG/ENaC) class of mechanosensory channels of touch receptor neurons, the neurotransmitter serotonin, and the FoxO transcription factor DAF-16 known to regulate development, energy metabolism, stress responses, and aging. After worms were exposed to hypergravity for 3 hr, their muscular and neuronal functions were preserved, but they exhibited DAF-16∷GFP nuclear accumulation in cells throughout the body and accumulated excess fat. Mutations in MEC-4/MEC-10 DEG/ENaC or its partners MEC-6, MEC-7, and MEC-9 blocked DAF-16∷GFP nuclear accumulation induced by hypergravity but did not affect DAF-16 response to other stresses. We show that exogenous serotonin and the antidepressant fluoxetine can attenuate DAF-16∷GFP nuclear accumulation in WT animals exposed to hypergravity. These results reveal a novel physiological role of the mechanosensory channel, showing that the perception of mechanical stress controls FoxO signaling pathways and that inactivation of DEG/ENaC may decouple mechanical loading and physiological responses.
THE physical force of gravity is a fundamental environmental parameter shaping the life of biological systems on earth, ranging from unicellular organisms to plants, animals, and men. Musculoskeletal systems, sensory networking, and metabolic machinery have evolved to counterbalance the earth gravitational force of 1G, enabling organisms to maintain posture, grow, and reproduce in the terrestrial environment. Acute or chronic exposure to microgravity (spaceflight) or hypergravity (centrifugation) generates mechanical stress, which has been shown to cause organisms to remodel their cellular and physiological processes, including the duration of exponential growth in the case of bacterium Escherichia coli, the growth rate of plants, the differentiation features of mammalian tissue cells, and the characteristics of the musculoskeletal system, endocrine system, body temperature, adiposity, and aging of animals (Le Bourg 1999; Macho et al. 2001; Fuller et al. 2002; Morey-Holton 2003; Soga et al. 2005). The phenotypes induced by changes in the gravitational force environment mostly disappear after the return to 1G, indicating that living organisms are hard wired to respond to the ambient physical force and that gravity response is a form of behavioral and physiological plasticity. Studies from a wide range of fields have identified many critical components involved in mechanotransduction, such as ion channels, cytoskeletal proteins, and signaling components (Ingber 2006; Orr et al. 2006). However, very little is known about how these components contribute to the pathway that translates mechanical stress into cellular mechanisms to generate physiological modifications in the context of a whole animal.
The FoxO (Forkhead-containing, O subfamily) transcription factors are the best-characterized regulators of stress responses in diverse organisms across phyla (Kops et al. 2002; Lee et al. 2003; Mcelwee et al. 2003; Murphy et al. 2003; Accili and Arden 2004; Hwangbo et al. 2004). The transcriptional activity of FoxO is controlled by subcellular localization. Signaling from insulin/insulin like-growth factor (IGF)-1 receptors induces phosphorylation of specific serine/threonine residues of FoxO, thereby sequestering FoxO in the cytoplasm. Conversely, aversive environmental and physiological cues or mutations abrogating insulin/IGF-1 receptor signaling cause FoxO nuclear accumulation, where they activate genes involved in development, energy metabolism, detoxification, and immunity, resulting in enhanced stress resistance. In Caenorhabditis elegans, inactivation of the insulin/IGF-1 receptor DAF-2 either by mutations or aversive factors promotes FoxO factor DAF-16 nuclear accumulation, causing developmental arrest, a shift of the metabolic profile favoring fat deposition, increased expression of a battery of homeostatic stress-response genes such as heat shock proteins and antioxidant enzymes, and extension of lifespan (Kimura et al. 1997; Lin et al. 1997; Ogg et al. 1997; Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001).
The signaling pathway from the DAF-2 insulin/IGF-1 receptor to DAF-16/FoxO is regulated by neuronal activity (Ailion et al. 1999; Apfeld and Kenyon 1999; Wolkow et al. 2000; Alcedo and Kenyon 2004). For example, worms bearing defective chemosensory neurons cannot sense the external chemical world, and these animals exhibit DAF-16 nuclear accumulation even under optimal growth conditions (Lin et al. 2001). Conversely, applying the neurotransmitter serotonin or the antidepressant fluoxetine, known to increase synaptic levels of endogenous serotonin, attenuates DAF-16 nuclear accumulation in wild-type (WT) animals under aversive conditions (Liang et al. 2006). These observations suggest that behavioral and physiological response to the environment reflects the interplay between neuronal signaling and the FoxO pathway and that changes in neuronal activity may modify the stress resistance of the animal.
In this study, we describe DAF-16/FoxO response to the mechanical stress of hypergravity and identify molecular mechanisms underlying this mechanosensory-to-FoxO transduction. Using a compact disc (CD)-based microfluidic cultivation system (Kim et al. 2007), we monitored the effects of hypergravity on neuronal function, muscular structure, and metabolic profiles in living worms and discovered that MEC-4/MEC-10 DEG/ENaC signaling is required for hypergravity force to induce DAF-16 nuclear accumulation. The mec-4 and mec-10 genes encode DEG/ENaC proteins and form a heteromeric, voltage-independent, amiloride-sensitive mechanosensory channel localized along the axons of six touch receptor neurons that sense gentle mechanical stimuli and mec-10 is additionally expressed in the PVD neurons that sense strong (harsh) mechanical stimuli (O'Hagan and Chalfie 2006). The sensory processes of these neurons extend along the length of the body. In vivo whole-cell recording has indicated that the MEC-4/MEC-10 channel can be activated by applying physical force against the body wall (O'Hagan et al. 2006). Conversely, mutations in either mec-4 or mec-10 render worms touch insensitive (Driscoll and Chalfie 1991; Huang and Chalfie 1994). Our study indicates that mechanosensation does not simply produce muscular reflex. Our results suggest that signaling from MEC-4/MEC-10 can influence a broad spectrum of physiological mechanisms, and inactivation of DEG/ENaC signaling of touch receptors can decouple mechanical loading on the body and the physiological mechanisms.
MATERIALS AND METHODS
Strains:
C. elegans strains used in this study were: WT variety Bristol strain (N2), TJ356(daf-16∷gfp), DR1808(daf-7∷gfp), GR1333 (tph-1∷gfp), RW1596 stEx30(Pmyo-3∷gfp), mec-4(u253), mec-4(e1611), mec-6(e1342), mec-7(u88), mec-9(e1494), mec-10(e1515), and SK4005: zdIs5[mec-4∷gfp; lin-15(+)]. Worms were maintained on nematode growth medium (NGM) at 20° with Escherichia coli OP50 as a food source (Brenner 1974).
100G paradigm:
The design and fabrication of the C. elegans CD cultivation system, including the materials, the microfluidic system, the dimensions of the chambers, and the spin-stand system used to apply centrifugal force have been described previously (Kim et al. 2007). The main fabrication material of the microfluidic platform is polydimethylsiloxane, which has the advantage of biocompatibility, high permeability to gases, chemically inert, and optical transparency down to 300 nm enabling observation of worms in the cultivation chamber. As depicted in Figure 1, the air vent channels further facilitate gas exchanges between the cultivation chamber and the environment. Each cultivation chamber can accommodate ∼1000 worms from three generations. On the basis of the criteria of growth, reproduction, and behavior, worms grown in the CD are indistinguishable from those under standard laboratory conditions (Kim et al. 2007).
Figure 1.—
Computer numeric control (CNC)-machined CD assembly used for C. elegans hypergravity experiments. (a) A photograph of the CD cultivation platform (12 cm in diameter and 4 mm thickness). Each CD contains three identical cultivation units. The CD can be fixed onto a spin-stand system, which is equipped with precise angular positioning and programmable rotations per minute (rpm) (Kim et al. 2007). The rotation speed for 100G is 1726 rpm. (b) A schematic illustration of the microfluidic structure of a C. elegans cultivation unit. It is composed of a nutrient chamber (1), a cultivation chamber (2), and a waste chamber (3). These chambers are connected by microchannels shown in yellow. Also shown in yellow, each chamber has a pair of air vent channels to facilitate gas exchanges. Notice that this microfluidic structure was designed for space applications that normally operate at low rotation speed to produce a 1G reference in spaceflight. In hypergravity experiments, food was directly added to the cultivation chamber. (c) A schematic depiction of fluidics in the cultivation chamber under 100G. Because of the high rotation speed, which overcomes the surface tension, liquid will flow into the waste chamber until the liquid level drops below the microfluidic channels connected to the waste chamber. Shown in blue, the equilibrium level has a volume of 70 μl. Among advantages of this CD over conventional culture tubes are the wide front edge of the cultivation chamber that prevents worms clumping together during high-speed centrifugation and the microchannels and air vent channels that provide better ventilation for the cultivation chambers. The temperature in the cultivation chambers under 100G was maintained between 23° and 24°.
The spin-stand system was originally designed for NASA to apply centrifugal force producing a 1G control in spaceflight. In the current study, this feature was used to generate hypergravity. Rotation of the CD on the spin-stand applies a force on the liquids in the CD. This “centrifugal force” can be precisely programmed and is identical for every cultivation chamber in the CD, allowing worms with different genetic backgrounds and drug treatments to be assayed in parallel. The force of 100G requires a rotation speed of 1726 rpm. At 100G, inward and outward flow of liquids in the cultivation chamber reach equilibrium within seconds, always leaving 70 μl of culture medium in the cultivation chambers (Figure 1). Because of the gravity force, worms are retained in the culture medium. The temperature in the cultivation chambers was measured using an EXTECH 470 Infrared Thermometer. It measures temperature utilizing a noncontact, infrared thermometer and includes a built-in laser beam to target area. The thermometer has 8:1 distance to target ratio, 0.95 fixed emissivity. On the basis of three independent trials, each measured five time points, 2 min for each time point, and the temperature in the cultivation chambers under 100G was maintained between 23° and 24°.
In the current study, food was added to the culture medium in the cultivation chambers. To prepare the food, a single colony of E. coli OP50 was inoculated into 250 ml of LB medium shaking overnight at 37°. The resulting bacteria were concentrated by centrifugation and the pellet was resuspended at the final concentration of 0.3 g/ml of S-basal. Twenty microliters of the bacterial suspension was added to 150 μl of S-medium in each cultivation chamber. For each experiment, about 15 1-day-old young adult animals were transferred into each cultivation chamber. The 1G controls are the same strain raised in parallel, transferred to the CD cultivation chambers, and rocked slowly on a rotator. All the experiments were performed at room temperature (∼22°). For drug experiments, the drugs were dissolved in water and the resulting solution was added to the medium to reach a final concentration of serotonin at 5 mg/ml and fluoxetine at 0.5 mg/ml.
To monitor food consumption in different gravitational environmental conditions, E. coli H115 DE3 expressing a red fluorescent protein (RFP) construct, pRSETB-mRFP, was used as a food source. The RFP-E. coli was prepared the same way used for OP50, except that ampicillin was added at a final concentration of 50 μg/ml LB medium to maintain the RFP plasmid. Worms were raised with OP50 as the food source, transferred to CD cultivation chambers containing RFP-E. coli, exposed to 100G or 1G for 15 and 30 min, and red fluorescence in the worms was visualized using a Texas red filter. Worms fed with RFP-E. coli on plates at 1G were observed as an additional control.
To test DAF-16∷GFP response to 100G in conventional test tubes, 8 –12 1-day-old adult animals were transferred to a 1.5-ml flat-bottom centrifugation tube (FastPlasmid Kit, Eppendorf) containing 150 μl S-medium and 20 μl of the bacterial suspension. The tubes were transferred to a fixed-angle rotor in an Eppendorf centrifuge (5810R) and spun at 1170 rpm (100G) for 3 hr at the temperature of ∼22°.
Fluorescence microscopy:
All GFP reporters used in this study are integrated into the chromosomes. The expression of GFP was observed using the Zeiss Axioplan II microscope and the Nikon Eclipse microscope, equipped with a fluorescence light source. The images were captured with a Zeiss AxioCam digital camera. To observe DAF-16 subcellular distribution in different genetic backgrounds, a stably integrated DAF-16∷GFP, zIs356 (Henderson and Johnson 2001) was crossed into individual genetic backgrounds. To observe GFP subcellular distribution, 8–10 animals were mounted to an agar pad containing 20 mm sodium azide and analyzed immediately (<10 min). Well-fed WT animals cultured under standard laboratory conditions were always assayed in parallel. For quantification of DAF-16∷GFP nuclear accumulation, animals were classified into three categories as depicted in Figure 2a.
Figure 2.—
Mechanical stress of 100G induces DAF-16∷GFP nuclear accumulation and excess fat storage. (a) DAF-16∷GFP subcellular distribution in WT animals. Under 1G, DAF-16∷GFP displayed a diffuse pattern in neuronal and nonneuronal cells throughout the body. The GFP expression in the hypodermis is shown. After 1 hr exposure to 100G, DAF-16∷GFP was distinctly enriched in the nuclei; the hypodermis and muscle cells are shown. After 3 hr exposure to 100G, DAF-16∷GFP was predominantly localized in the nuclei. We classified DAF-16∷GFP subcellular distribution into three categories: not localized, as exhibited by the animal under 1G control shown in the top photomicrograph; partial nuclear accumulation, as shown in the two middle photomicrographs; and predominant nuclear accumulation, as shown in the two bottom photomicrographs. (b) Kinetics of DAF-16∷GFP nuclear accumulation in worms exposed to 100G and recovery after they returned to 1G. Each bar represents the mean of at least three independent trials, 15–20 animals/trial. (c) Hypergravity causes increased fat storage. Fat content in animals exposed to 1G and 100G was detected by Sudan black staining. Three independent experiments were carried out including a blind test for animals exposed to 1G and 100G, 40–45 animals/treatment/trial. More than 50% of animals exposed to 100G for 12 hr showed increased fat accumulation on the basis of visual inspection, and the photomicrographs show the representative staining patterns. (d) Exogenous serotonin and fluoxetine suppress 100G-induced DAF-16∷GFP nuclear accumulation. WT animals were exposed to 100G in the presence or absence of a drug treatment, and the percentage of animals that exhibited predominant DAF-16∷GFP nuclear accumulation was scored. Each bar represents three independent trials ± SEM, each in triplicates. A total of 68–70 animals were assayed for each treatment.
To test DAF-16∷GFP response to heat shock, L4 animals were transferred to NGM plates seeded with OP50 food, and incubated at 20° for 24 hr. The plates were shifted to 35° for 2 hr, and the subcellular localization of DAF-16∷GFP in the animals was observed.
To quantify GFP intensity of tph-1∷gfp and daf-7∷gfp in individual neurons, GFP images were captured using a 40× lens at a fixed exposure time, and the fluorescence over a 25 × 25 pixel area within a neuron was quantified, using the Adobe Photoshop 6.0 software.
To observe the effect of aldicarb on body wall muscle sarcomeres, worms carrying a GFP-tagged myosin heavy chain protein MYO-3 (MYO-3∷GFP) transgene were incubated in the medium containing 1 mm aldicarb for 20 min, and the GFP patterns were observed.
Behavioral assays:
Touch avoidance response was assayed by touches at the shoulder and tail regions of individual worms. In general, worms move backward in response to a gentle touch at the head region and move forward when they are touched at the tail (Chalfie and Sulston 1981; Sze et al. 1997). Immediately following 3-hr exposure to 100G, individual worms were touched with a thin platinum wire 10 times alternately at the shoulder and tail regions, and the number of responses was scored. Such platinum wire touches have the potential to activate the touch receptor neurons and the PVD neurons (Goodman and Schwarz 2003; O'Hagan and Chalfie 2006).
Odor attraction assays were performed according to a standard protocol (Bargmann et al. 1993). The dilution of the odorant diacetyl in ethanol was 1:1000. The odortaxis index was calculated as [(number of worms at attractant) − (number of worms at solvent ethanol)]/(total number of worms moved).
Statistics:
Routine statistical analyses were performed using Minitab 12.1 (Minitab 1998). For comparisons between more than two groups an ANOVA (one-way) was used. When testing between more than two groups treated in two different ways a general linear model was used, followed by a Tukey's pair-wise multicomparison procedure. For comparisons between two test groups a student's t-test was carried out.
Fat storage assay:
We used Sudan black staining to visualize the fat storage in worms, using the Ogg-Ruvkun protocol (Ogg et al. 1997). Briefly, WT worms were exposed to 100G for 3 hr or 12 hr, fixed immediately in 1% paraformaldehyde in phosphate-buffered saline, frozen at −70°, thawed, washed, and then incubated overnight in saturated Sudan black solution.
RESULTS
The mechanical stress of hypergravity induces DAF-16 nuclear accumulation:
We have devised a CD-based microfluidic platform to enable the study of genetic, cellular, and physiological parameters in living C. elegans as a function of gravitation level (Figure 1). This CD system was designed for use in space as a 1G control to assess factors such as microgravity, radiation, and vibration on C. elegans behavior and gene expression (Kim et al. 2007). In this system, worms are maintained in cultivation chambers embedded in the CD, and the CD is fixed onto a spin-stand system. By programming rotational speed, a precise gravitational force is delivered to the cultivation chambers. Prior studies have established that worms cultivated in the CD exhibit the tempo of development, rate of population growth, and brood size as they grow under standard laboratory conditions (Kim et al. 2007). In this study, we used this CD cultivation system to test the effect of hypergravity on various genetic and cellular parameters in C. elegans.
Hypergravity paradigms have been used to complement studies on microgravity and as an experimental approach toward understanding the role of mechanical stress in biological systems (Le Bourg 1999; Tou et al. 2002; Morey-Holton 2003; Markin et al. 2004; Okaichi et al. 2004; Soga et al. 2005). The ability of a living system to withstand increased gravitational force is related to size. For example, rats can survive up to 15G, whereas single cells and nematodes can withstand 100,000G (Morey-Holton 2003). 100G is well within the range of centrifugation force commonly used in the laboratory for collecting living C. elegans from a solution or a liquid culture for subsequent behavioral assays and does not cause any significant detrimental effect on worms. We therefore used 100G as a hypergravity paradigm.
Because FoxO transcription factors regulate homeostatic stress response pathways in diverse organisms and because the subcellular distribution of the FoxO factor DAF-16 is known to be modulated by the environment and can be observed in living worms, we used DAF-16 as a tool to monitor physiological response to gravity. We compared the subcellular distribution of the DAF-16 protein tagged to a GFP (DAF-16∷GFP) in worms exposed to 1G and 100G (Figure 2a). As in worms raised under standard laboratory culture conditions (Ogg et al. 1997; Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001), animals cultivated in the CD at 1G expressed DAF-16∷GFP in most cell types, including muscles, intestine, hypodermis, and many neurons, and GFP expression appeared to be diffuse in the cytoplasm and nuclei. At the setting of 100G and over the course of 3 hr, worms retained their morphological integrity. However, they displayed progressive DAF-16∷GFP nuclear accumulation in neuronal and nonneuronal cells throughout the body. DAF-16∷GFP nuclear accumulation became evident after 1 hr and was further enhanced after 3 hr of 100G exposure. This 100G-induced DAF-16 nuclear translocation was reversed after the animals returned to 1G (Figure 2b). These observations revealed that the FoxO pathway responds to the mechanical loading of hypergravity.
Mechanical stress induces changes in metabolic profiles:
In response to aversive environment, C. elegans modifies its metabolic profiles. For example, under high growth temperature and starvation worms alter the activity of key metabolic enzymes, resulting in a shift of metabolic profiles favoring fat deposition, and this metabolic shift is in part regulated by the DAF-2 insulin/IGF-1 receptor signaling to DAF-16 (Ogg et al. 1997; Ashrafi et al. 2003). It has been shown in rats that spaceflight causes changes in insulin and glucose levels and alters the activity of enzymes involved in lipolysis (Macho et al. 2001). Also, hypergravity can cause weight increase and fat depositions (Smith 1976; Bouet et al. 2004; Markin et al. 2004). We therefore used fat storage as an assay to test the effect of hypergravity on metabolism. Worms exposed to 100G for 12 hr showed increased fat content in the intestine and hypodermis, as detected by Sudan black staining (Figure 2c). Increased fat storage became noticeable in worms exposed to 100G for 3 hr, although the differences were small (results not shown).
Hypergravity does not disturb feeding:
An implicit concern with worms exposed to 100G was that the physical loading might inhibit feeding, resulting in secondary changes in DAF-16 subcellular distribution and metabolism. We addressed this question by monitoring food ingestion in worms exposed to 100G. We fed worms with E. coli cells expressing an RFP and monitored the efficiency of red fluorescence to replace the colorless OP50 ingested prior to the experiment (Figure 3). Within 15 min red fluorescence can be detected in the intestine of the worms exposed to 1G or 100G and the intensity further increased after 30 min. These observations suggest that the function of the pharyngeal muscles was preserved, and 100G did not significantly disturb feeding.
Figure 3.—
Feeding behavior is preserved in worms exposed to 100G. Representative photomicrographs showing worms fed with E. coli expressing RFP at 1G and 100G. a, c, e, and g show RFP visualized by a Texas red filter, and b, d, f, and h show superposition of RFP and auto-fluorescence of the gut visualized by a FITC filter. These observations are based on 6–9 independent experiments, with 100G and 1G conditions tested in parallel, at least 10 worms/trial/gravity condition. All the animals shown are young adults, and the anterior is toward the left.
Hypergravity induces muscular relaxation but does not disturb the integrity:
To examine further the effects of hypergravity on the muscular system, we used MYO-3∷GFP as a reporter to examine the body wall muscles in animals exposed to 100G. There was no detectable difference in the organization and morphology of the muscle fibers between animals exposed to 100G for 3 hr and those at 1G. However, the muscle sarcomeres in worms exposed to 100G for 24 hr were elongated and the muscle fibers were more stretched apart compared to 1G controls (Figure 4, a and b). Unlike the acetylcholinesterase inhibitor aldicarb that causes body wall muscle hypercontraction (Mahoney et al. 2006) and irregular sarcomere organization (Figure 4c), the muscle fibers in animals exposed to 100G for 24 hr remained evenly organized in parallel rows (Figure 4b), demonstrating that the hypergravity force did not damage the structure of the body wall muscles. The sarcomere elongation is likely a result of muscular adaptation to the chronic mechanical loading imposed on the animal by the hypergravity force. It is noteworthy that this phenotype is distinctly opposite to muscle cell shrinkage, shortening of muscle fibers and sarcomeres, and muscle mass loss observed in rodents and humans under microgravity conditions (Vandenburgh et al. 1999; Ikemoto et al. 2001; Ohira et al. 2004).
Figure 4.—
The muscular structure is preserved in worms exposed 100G. Body wall muscle sarcomeres in living animals were observed using MYO-3∷GFP. One-day-old young adult animals were exposed to 100G for 3 hr or 24 hr and examined immediately afterwards. Exposure to 100G for 3 hr did not produce a detectable change (not shown). After 24-hr exposure, the sarcomeres were longer relative to the 1G controls (outlined by dashes), and the muscle fibers remained well organized. By contrast, age-matched animals under 1G treated with the cholinesterase inhibitor aldicarb, which causes muscle hypercontractions, displayed densely packed muscle fibers, and the muscle cells were shrunken and crenated, an antithesis of the stretched parallel striation seen in worms under 100G. These observations are based on three independent experiments with at least 10 worms/trial/condition.
Neuronal integrity is preserved in animals exposed to hypergravity:
We next tested the possibility that the physical force of 100G impairs neuronal functions that in turn affect physiology. In C. elegans the integrity of chemosensory neurons influences DAF-16 subcellular distribution, metabolism, and aging (Apfeld and Kenyon 1999; Alcedo and Kenyon 2004). The microtubule-based cilia at the dendritic tip of the amphid chemosensory neurons in the head are exposed to the external environment and express receptors and channels that sense chemical and physical stimuli, including odorants, soluble chemicals, food, temperature, osmotic strength, and mechanical touch at the nose (Ward et al. 1975; Bargmann and Mori 1997). Damaging the sensory structure of these neurons causes sensory deficits (Perkins et al. 1986) and DAF-16 nuclear accumulation (Lin et al. 2001).
We used GFP reporters to assess the effects of 100G on the structure of these chemosensory neurons. osm-6 is expressed in all the chemosensory neurons (Collet et al. 1998); 3-hr exposure to 100G did not produce a noticeable change in osm-6∷gfp expression (data not shown). We further examined the expression of specific genes in chemosensory neurons that are known to regulate DAF-16. The ADF chemosensory neurons are a pair of serotonergic neurons, and the pair of the ASI chemosensory neurons produces DAF-7/TGF-β signal. Reduction of daf-7 expression in ASI (Lin et al. 2001) or reduction in ADF of the tph-1 gene that encodes the serotonin-synthesizing enzyme, tryptophan hydroxylase (Liang et al. 2006), causes DAF-16∷GFP nuclear accumulation. Over the course of 3 hr, exposure to 100G did not cause a significant reduction of tph-1∷gfp or daf-7∷gfp expression in WT worms (Figure 5, a and b). Prior studies showed that this daf-7∷gfp reporter is significantly down-regulated under the conditions of starvation or high growth temperature (Ren et al. 1996; Schackwitz et al. 1996). The normal daf-7∷gfp expression level in animals exposed to 100G is another indication that 100G did not significantly alter growth temperature or disrupt feeding. Judged at the level of fluorescence microscopy, there was no detectable change in the cell position or dendritic morphology of ADF and ASI (Figure 5, a and b).
Figure 5.—
The integrity of the nervous system is preserved in worms at 100G for 3 hr. (a) Serotonergic neurons in the head region were visualized by a GFP reporter of the gene encoding the serotonin-synthesizing enzyme tryptophan hydroxylase (tph-1∷gfp). Over the course of 3 hr, GFP levels were not reduced in the ADF chemosensory neurons or the NSM secretory neurons (P > 0.05, one-way ANOVA). (b) The ASI chemosensory neurons were visualized by a GFP reporter of the daf-7/TGF-β gene (daf-7∷gfp). Unlike under starvation and high growth temperature (Ren et al. 1996; Schakwitz et al. 1996), the expression level of daf-7∷gfp was not significantly reduced in worms under 100G (P > 0.05, one-way ANOVA). Bars, mean ± SEM of at least three trials. All animals assayed were 1-day-old young adults. n, number of animals in which GFP was quantified. AU, arbitrary units. (c) Olfactory sensation to the attractant diacetyl. Worms were exposed to 100G for 3 hr and tested immediately afterwards. There was no significant difference in the olfactory sensitivity between worms exposed to 100G and the 1G controls (P > 0.05, Student's t-test). Bars, mean ± SEM of at least three trials, 24–30 animals/gravity condition/trial. One-day-old young adults were assayed.
We also conducted behavioral assays to assess the function of the chemosensory neurons. The activity of the AWA olfactory sensory neurons can influence DAF-16 (Alcedo and Kenyon 2004). AWA senses the attractive odorant diacetyl (Bargmann et al. 1993). Dysfunction of the AWA neurons, their postsynaptic targets, or muscular function prevents animals from responding to diacetyl. We used diacetyl sensation to test whether the physical force of 100G damages AWA. Worms assayed immediately following 3-hr exposure to 100G responded to diacetyl indistinguishably from their siblings kept under 1G (Figure 5c). Together, these results indicate that within this time frame the gross neuronal structure, the function of the amphid chemosensory neurons, and neuromuscular networking in C. elegans were preserved under 100G. Therefore, DAF-16 nuclear accumulation in animals exposed to 100G is unlikely to be a consequence of chemosensory damage.
The MEC-10 DEG/ENaC channel of touch receptor neurons couples hypergravity and DAF-16:
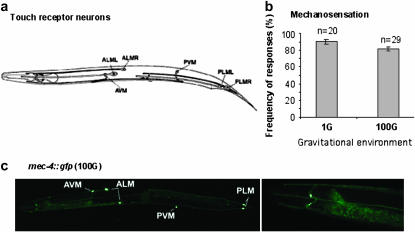
Having established that the hypergravity induced DAF-16 nuclear accumulation independently of starvation or neuronal and muscular damage, we sought for molecular mechanisms underlying this gravity response. In vertebrates, gravity is sensed by three classes of receptor cells: hair cells of the vestibular system, proprioceptors located in muscle joints, and mechanoreceptors located in various regions of the body. Mechanoreceptors are among the most conserved signaling components across phyla. In animals, mechanoreceptors are generally located in the epidermis and are covered by a connective tissue capsule. Stimuli, including touch, stretch, vibration, and pressure, deform mechanoreceptors or displace the attachment between ion channels of the mechanoreceptors and the extracellular matrix, which triggers signaling cascades to generate behavioral and physiological responses (Sukharev and Corey 2004). In C. elegans, a set of six touch receptor neurons senses mechanical stimuli along the body (Chalfie and Au 1989) (Figure 6a). Like mammalian mechanoreceptors, the processes of these touch receptor neurons are closely attached to the body wall, packed with specialized microtubules, and exhibit prominent extracellular matrix (Chalfie et al. 1985). In addition to connections to the locomotory circuitry, these neurons also have synapses connecting to many cells that are not directly involved in movement (Chalfie et al. 1985), suggesting that sensory perception of mechanical stimuli by these neurons could have a broad impact on the animal. We first asked whether 100G damages these touch receptor neurons. We used mec-4∷gfp (Driscoll and Chalfie 1991; Huang and Chalfie 1994) to visualize the touch receptor neurons in WT animals exposed to 100G and 1G. The morphology of the cell body and the processes of touch receptor neurons in animals exposed to 100G for 3 hr were indistinguishable from those seen in animals at 1G (Figure 6c). Assayed immediately following 3-hr exposure to 100G, worms responded instinctively to touches applied to anterior and posterior regions of the body (Figure 6b). Thus, 100G forces did not undermine the ability of the touch receptor neurons to sense and transmit sensory information.
Figure 6.—
Structure and function of the touch receptor neurons after 3 hr exposure to 100G. (a) A schematic representation of the touch receptor neurons. Adapted from Chalfie and Au (1989). (b) Touch response. The animals were assayed within 20 min after the return from 100G. Individual worms were touched with a thin platinum wire 10 times alternately at the shoulder and tail regions, and the number of responses was scored. Bars, mean ± SEM of at least two trials, 10 animals/trial. All animals assayed were 1-day-old young adults. While the six touch receptor neurons are required to respond to touches at the shoulder and tail regions, platinum wire touch could additionally activate the PVD neurons (Way and Chalfie, 1989) (c) The cell body and processes of touch receptor neurons visualized by mec-4∷gfp. The worms were exposed to 100G for 3 hr. The left photomicrograph shows the image of an entire animal at 5× magnification, and the right photomicrograph shows the ALM process extending to the head region at 20× magnification. The anterior is toward the left.
We next tested a hypothesis that these touch receptor neurons function to translate the mechanical stress of 100G into a biological signal inducing DAF-16 nuclear accumulation. Mechanosensation of these neurons involves a membrane protein complex of four gene products. The mec-4 and mec-10 genes encode DEG/ENaC proteins that form the pore of the mechanosensory channel and are expressed in the plasma membrane of the touch receptor neurons (Driscoll and Chalfie 1991; Huang and Chalfie 1994; Goodman and Schwarz 2003; O'Hagan and Chalfie 2006). The transmembrane paraoxonase-like protein MEC-6 interacts with MEC-4, localizing the mechanosensory channel to puncta clusters along the axon of the touch receptors (Chelur et al. 2002). The extracellular protein MEC-9 is secreted by the touch receptor neurons to provide an extracellular attachment point for the channel complex (Du et al. 1996). Signaling through the mechanosensory channel requires MEC-7, a β-tubulin that produces touch receptor-specific 15-protofilament microtubules (Savage et al. 1989). Dysfunction of any of these components causes worms to be insensitive to gentle touch along the body. To investigate the possibility of the DEG/ENaC channel as a primary transducer of gravity, we tested whether disruption of the channel function could decouple the physical force and DAF-16 subcellular distribution. We crossed the same DAF-16∷GFP transgene into worms bearing a loss-of-function mutation either in mec-4, mec-6, mec-7, mec-9, or mec-10 and observed GFP subcellular distribution in the mutant animals exposed to 100G and 1G. At 1G, there was no appreciable difference in DAF-16∷GFP subcellular distribution between WT and the Mec mutants. However, compared to WT animals assayed in parallel, every Mec mutation attenuated or blocked 100G-induced DAF-16∷GFP nuclear accumulation (Figure 7a). Interestingly, two mec-4 alleles conferred less inhibition of DAF-16∷GFP nuclear accumulation than mutations in the other mec genes. While all these mec genes are expressed in the six touch receptor neurons, mec-6, mec-7, mec-9, and mec-10, but not mec-4, are additionally expressed in the pair of PVD neurons. PVD senses harsh mechanical stimuli (Way and Chalfie 1989) and has the characteristics of the Drosophila multidendritic sensory neurons that mediate proprioceptive and nociceptive function (O'Hagan and Chalfie 2006). It is possible that the physical force of 100G activates the touch receptors as well as PVD to promote DAF-16 nuclear accumulation.
Figure 7.—
Mutations in the MEC-4/MEC-10 DEG/ENaC mechanosensory channel signaling block 100G-induced DAF-16 nuclear accumulation. (a and b) DAF-16∷GFP subcellular distribution in worms exposed to 100G in the CD platform (a) and a conventional centrifuge (b) for 3 hr. One-day-old young adults were assayed. WT and Mec mutants were always tested in parallel. Bars, mean ± SEM of at least three trials, 10–12 animals/genotype/trial, including a blind assay for genotypes. The percentage of WT displaying predominant DAF-16∷GFP nuclear accumulation was significantly higher than that of every Mec mutant strain examined in both hypergravity paradigms (P < 0.0001, general linear model). However, mec-4 mutants showed higher frequencies of partial DAF-16∷GFP nuclear accumulation. (c) Mechanosensory mutations do not affect DAF-16 response to heat stress. Like WT worms, mec-6, mec-9, and mec-10 mutants exposed to 35° for 2 hr exhibited DAF-16∷GFP nuclear accumulation. Each bar represents three independent experiments, each with 15 worms/condition. The frequency of mec-9 animals showing predominant DAF-16∷GFP nuclear accumulation appears to be lower than that of the other strains, but the difference between mec-9 and WT is not significant (P = 0.097, Student's t-test). The classification of DAF-16∷GFP nuclear accumulation is described in Figure 2.
To ensure that the DEG/ENaC channel complex indeed is required for DAF-16 response to the mechanical stress of hypergravity, we reproduced the same experiment in a more conventional laboratory setting. We placed worms in flat bottom culture tubes and spun the worms in a conventional centrifuge at the level of 100G. More than 60% of WT worms showed DAF-16∷GFP predominantly in the nuclei, whereas <10% of mec-9 and mec-10 mutants showed strong DAF-16∷GFP nuclear accumulation after 3 hr of chronic centrifugation (Figure 7b). The results confirmed our work with the CD. Both WT and Mec mutants incubated in the culture tubes for 3 hr at 1G or 100G showed a slight increase in DAF-16∷GFP nuclear accumulation relative to their siblings incubated in the CD chambers (Figure 7, a and b). One plausible explanation for the quantitative difference in the results could be inadequate oxygen in the conventional cultural tubes spun in a closed centrifuge, whereas the microchannels, air vent channels, and the open-air spin-stand provide better ventilation for the cultivation chambers in the CD. The results from both CD and the conventional lab setting suggest that signaling of the MEC-4/MEC-10 mechanosensory channel influences DAF-16 response to hypergravity.
In WT worms DAF-16 nuclear accumulation can be induced by another physical stress—heat (Henderson and Johnson 2001; Lin et al. 2001). The temperature in the cultivation chambers under 100G was between 23° and 24°. Because the optimal C. elegans growth temperature is 20°, we first tested the possibility of DAF-16∷GFP nuclear accumulation in WT being a result of the temperature elevation during the 100G treatment. There was no evident DAF-16∷GFP nuclear accumulation in WT animals incubated on NGM at 1G, 24° or 26°, for 3 hr (3 independent trials, 20 animals/temperature/trial). Thus, within this time frame mild heat stress alone would not be sufficient to induce DAF-16∷GFP accumulation.
We next tested whether defective DEG/ENaC signaling specifically decouples mechanical stress and DAF-16 or whether the mutations also obstruct DAF-16 response to other stress physical signals. We exposed well-fed WT and mec-6, mec-9, and mec-10 mutant animals to 35° heat shock and observed DAF-16∷GFP subcellular distribution. After exposure to the heat for 2 hr, both WT and Mec animals showed strong DAF-16∷GFP nuclear accumulation (Figure 7c). The percentage of mec-9 mutant animals exhibiting predominant DAF-16∷GFP nuclear accumulation appears to be slightly lower than that seen in the other strains, but the difference is not significant (P = 0.097, Student's t-test). These observations indicate that mechanical stress of hypergravity and thermal stress are sensed by distinctly different receptors to independently regulate DAF-16 activity. Our results are consistent with the model that the MEC-4/MEC-10 channel specifically regulates DAF-16 response to mechanical force. However, we do not exclude the possibility that 100G could produce some minor side effects that also promote DAF-16∷GFP nuclear translocation.
Drugs that enhance serotonin signaling confer resistance to mechanical stress:
The serotonergic system functions as a neuromodulator to control neuronal plasticity and physiological adaptation in both vertebrates and invertebrates (Azmitia 1999; Chaouloff et al. 1999). Drugs that target the serotonergic system are effective in the treatment of a wide variety of stress symptoms (Lucki 1998). Other work in our laboratory established that fluctuation of serotonin signaling modulates DAF-16 subcellular localization (Liang et al. 2006). As the first step toward identification of drugs that might be able to modify physiological response to changes in gravity, we tested whether an excess of serotonin can suppress hypergravity-induced DAF-16 nuclear accumulation. Applying serotonin or the selective serotonin reuptake inhibitor fluoxetine to WT worms during their exposure to 100G significantly attenuated DAF-16∷GFP nuclear accumulation compared to their untreated siblings (Figure 2d), showing that drugs that enhance serotonin signaling can mitigate the response elicited by the mechanical stress of hypergravity.
DISCUSSION
This article describes a paradigm for a systematic genetic, cellular, and physiological survey of the effects of an ambient physical force on living animals undergoing complex behavior. While a comprehensive appreciation of the effects of gravity on biology requires investigations into multiple generations under various levels of hypogravity and hypergravity, the ability of C. elegans to withstand the mechanical stress of 100G affords an opportunity to investigate molecular pathways that transduce mechanical forces into biological regulators and to identify genetic targets of mechanical stress in the context of a whole animal.
Our genetic analysis of mechanosensory mutants revealed a molecular mechanism by which a physical cue regulates metabolism and physiology. The phenotype of Mec mutants corroborates prior studies of chemosensory mutants (Lin et al. 2001), both suggesting that the perception of the environment regulates DAF-16, thereby influencing physiology. However, there is a major distinction between the roles of chemosensation and mechanosensation. In the case of chemosensation, mutants that cannot sense the chemical environment exhibit DAF-16 nuclear accumulation as WT animals exposed to aversive chemicals cues (Lin et al. 2001), suggesting that inactivation of these chemosensory neuronal signaling produces a perception of “stress” and that favorable chemical cues are necessary to suppress DAF-16 nuclear translocation. By contrast, Mec mutants exposed to 100G exhibited the DAF-16 subcellular distribution as if they were under the earth gravity of 1G, implying that in the absence of MEC-4/MEC-10 channel signaling mechanical loading cannot promote DAF-16 nuclear accumulation.
Importantly, Mec mutants that cannot sense hypergravity can vigorously respond to heat stress. While both heat and gravity are physical cues, temperature is a major determinant of all chemical action and interactions, whereas mechanical forces are generally considered lacking chemical information. Our data demonstrate that the MEC-4/MEC-10 channel selectively senses mechanical stress, and heat stress is sensed by an independent mechanism. The specific receptors transducing heat and chemical cues have not yet been reported; further studies are necessary to elucidate how individual sensory perceptions are integrated to modulate behavior and physiology.
The touch- or stretch-sensitive channels are among the most ancient signaling molecules conserved from bacterium to man (Sukharev and Corey 2004; Anishkin and Kung 2005; Ingber 2006), and FoxO and its downstream stress response pathways reflect an evolutionary conserved adaptive mechanism (Accili and Arden 2004). It has been shown in plants that blockade of stretch-activated channels by drugs prevents hypergravity-induced growth retardation (Soga et al. 2005). Hypergravity also induces the expression of a battery of homeostatic stress response genes that regulate growth, proliferation, antioxidant, and cell death in mammalian cell culture and humans (Markin et al. 2004; Okaichi et al. 2004) and causes lifespan extension in male Drosophila (Le Bourg 1999); all these phenotypes have been shown to be regulated by FoxOs. It will be interesting to determine whether mechanosensory channel activity also modulates FoxO signaling pathways in Drosophila and mammals.
Our pharmacological experiments showed that serotonin and fluoxetine can partially suppress DAF-16∷GFP nuclear accumulation in WT animals exposed to 100G. The precise role of serotonin in the touch receptor function is not clear. Serotonergic neurons are not connected to the touch receptors (White et al. 1986). Furthermore, applying serotonin and fluoxetine also inhibits DAF-16 response to starvation (Liang et al. 2006). One plausible explanation is that serotonin signaling produces a more general effect of inhibiting DAF-16 nuclear accumulation to alleviate stress responses. The development of the C. elegans hypergravity paradigm should enable a systematic analysis to identify genes and biochemical pathways that sense and respond to mechanical stress and potential drugs that may modify them.
Acknowledgments
We thank S. Sandmeyer for inspiring discussions, J. Hines and T. Ricco from NASA for their technical insights, and T. Stiernagle and the Caenorhabditis Genetics Center for worm strains. We specially thank J. Gargus for providing the facility where some of the experiments were carried out and for his critical reading of the manuscript. This work was supported by grants from NASA (to M.J.M.), a Marie Curie fellowship (to C.M.D.), and NIHMH (to J.Y.S.).
References
- Accili, D., and K. Arden, 2004. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117: 421–426. [DOI] [PubMed] [Google Scholar]
- Ailion, M., T. Inoue, C. I. Weaver, R. W. Holdcraft and J. H. Thomas, 1999. Neurosecretory control of aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 96: 7394–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo, J., and C. Kenyon, 2004. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41: 45–55. [DOI] [PubMed] [Google Scholar]
- Anishkin, A., and C. Kung, 2005. Microbial mechanosensation. Curr. Opin. Neurobiol. 15: 397–405. [DOI] [PubMed] [Google Scholar]
- Apfeld, J., and C. Kenyon, 1999. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402: 804–809. [DOI] [PubMed] [Google Scholar]
- Ashrafi, K., F. Y. Chang, J. L. Watts, A. G. Fraser, R. S. Kamath et al., 2003. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: 268–272. [DOI] [PubMed] [Google Scholar]
- Azmitia, E. C., 1999. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology 21: 33S–45S. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., and I. Mori, 1997. Chemotaxis and thermotaxis, pp. 717–737 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Bargmann, C. I., E. Hartwieg and H. R. Horvitz, 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527. [DOI] [PubMed] [Google Scholar]
- Bouet, V., R. J. Wubbels, H. A. A. de Jong and A. Gramsbergen, 2004. Behavioural consequences of hypergravity in developing rats. Dev. Brain Res. 153: 69–78. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie, M., and M. Au, 1989. Genetic control of differentiation of the caenorhabditis-elegans touch receptor neurons. Science 243: 1027–1033. [DOI] [PubMed] [Google Scholar]
- Chalfie, M., and J. Sulston, 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82: 358–370. [DOI] [PubMed] [Google Scholar]
- Chalfie, M., J. E. Sulston, J. G. White, E. Southgate and J. N. Thomson, 1985. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5: 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouloff, F., O. Berton and P. Mormede, 1999. Serotonin and stress. Neuropsychopharmacology 21: 28S–32S. [DOI] [PubMed] [Google Scholar]
- Chelur, D. S., G. G. Ernstrom, M. B. Goodman, C. A. Yao, L. Chen et al., 2002. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch cell degenerin channel. Nature 420: 669–673. [DOI] [PubMed] [Google Scholar]
- Collet, J., C. A. Spike, E. A. Lundquist, J. E. Shaw and R. K. Herman, 1998. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics 148: 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll, M., and M. Chalfie, 1991. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349: 588–593. [DOI] [PubMed] [Google Scholar]
- Du, H. P., G. Q. Gu, C. M. William and M. Chalfie, 1996. Extracellular proteins needed for C. elegans mechanosensation. Neuron 16: 183–194. [DOI] [PubMed] [Google Scholar]
- Fuller, P. M., T. A. Jones, S. M. Jones and C. A. Fuller, 2002. Neurovestibular modulation of circadian and homeostatic regulation: vestibulohypothalamic connection? Proc. Natl. Acad. Sci. USA 99: 15723–15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, M. B., and E. M. Schwarz, 2003. Transducing touch in Caenorhabditis elegans. Annu. Rev. Physiol. 65: 429–452. [DOI] [PubMed] [Google Scholar]
- Henderson, S. T., and T. E. Johnson, 2001. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 11: 1975–1980. [DOI] [PubMed] [Google Scholar]
- Huang, M., and M. Chalfie, 1994. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 367: 467–470. [DOI] [PubMed] [Google Scholar]
- Hwangbo, D. S., B. Gershman, M. P. Tu, M. Palmer and M. Tatar, 2004. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429: 562–566. [DOI] [PubMed] [Google Scholar]
- Ikemoto, M., T. Nikawa, S. Takeda, C. Watanabe, T. Kitano et al., 2001. Space shuttle flight (STS-90) enhances degradation of rat myosin heavy chain in association with activation of ubiquitin-proteasome pathway. FASEB J. 5: 1279–1281. [DOI] [PubMed] [Google Scholar]
- Ingber, D. E., 2006. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20: 811–827. [DOI] [PubMed] [Google Scholar]
- Kim, N. H., C. M. Dempsey, J. V. Zoval, J. Y. Sze and M. J. Madou, 2007. Automated microfluidic compact disc (CD) cultivation system of C. elegans. Sens. Actuators B 122: 511–518. [Google Scholar]
- Kimura, K. D., H. A. Tissenbaum, Y. Liu and G. Ruvkun, 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Kops, G. J., R. H. Medema, J. Glassford, M. A. Essers, P. F. Dijkers et al., 2002. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol. Cell. Biol. 22: 2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg, E., 1999. A review of the effects of microgravity and of hypergravity on aging and longevity. Exp. Gerontol. 34: 319–336. [DOI] [PubMed] [Google Scholar]
- Lee, R. Y., J. Hench and G. Ruvkun, 2001. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 11: 1950–1957. [DOI] [PubMed] [Google Scholar]
- Lee, S. S., S. Kennedy, A. C. Tolonen and G. Ruvkun, 2003. DAF-16 target genes that control C. elegans life-span and metabolism. Science 300: 644–647. [DOI] [PubMed] [Google Scholar]
- Liang, B., M. Mustapha, C. J. Kuan and J. Y. Sze, 2006. Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab. 4: 429–440. [DOI] [PubMed] [Google Scholar]
- Lin, K., J. B. Dorman, A. Rodan and C. Kenyon, 1997. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278: 1319–1322. [DOI] [PubMed] [Google Scholar]
- Lin, K., H. Hsin, N. Libina and C. Kenyon, 2001. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 28: 139–145. [DOI] [PubMed] [Google Scholar]
- Lucki, I., 1998. The spectrum of behaviors influenced by serotonin. Biol. Psych. 44: 151–162. [DOI] [PubMed] [Google Scholar]
- Macho, L., R. Kvetnansky, M. Fickova, I. A. Popova and A. Grigoriev, 2001. Effects of exposure to space flight on endocrine regulations in experimental animals. Endocr. Regul. 35: 101–114. [PubMed] [Google Scholar]
- Mahoney, T. R., S. Luo and M. L. Nonet, 2006. Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat. Protoc. 1: 1772–1777. [DOI] [PubMed] [Google Scholar]
- Markin, A., O. Juravlyova and V. Lukianuk, 2004. Lipid peroxidation and the system of antioxidant defence in humans after hypergravitational influence. J. Gravit. Physiol. 11: 69–70. [PubMed] [Google Scholar]
- McElwee, J., K. Bubb and J. H. Thomas, 2003. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2: 111–121. [DOI] [PubMed] [Google Scholar]
- Morey-Holton, E. R., 2003. Gravity, pp. 143–183 in Evolution on Planet Earth, edited by L. Rothschild and A. Lister. Academic Press, London.
- Murphy, C., S. A. McCarroll, C. I. Bargmann, A. Fraser, R. S. Kamath et al., 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–283. [DOI] [PubMed] [Google Scholar]
- Ogg, S., S. Paradis, S. Gottlieb, G. I. Patterson, L. Lee et al., 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999. [DOI] [PubMed] [Google Scholar]
- O'Hagan, R., and M. Chalfie, 2006. Mechanosensation in Caenorhabditis elegans. Int. Rev. Neurobiol. 69: 169–203. [DOI] [PubMed] [Google Scholar]
- O'Hagan, R., M. Chalfie and M. B. Goodman, 2006. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 8: 43–50. [DOI] [PubMed] [Google Scholar]
- Ohira, Y., F. Kawano, J. Stevens, X. Wang and A. Ishihara, 2004. Load-dependent regulation of neuromuscular system. J. Gravit. Physiol. 11: 127–128. [PubMed] [Google Scholar]
- Okaichi, K., M. Ide, A. Usui and Y. Okumura, 2004. Hypergravity induces phosphorylation of p53 at serine 15, but not an expression of p53-downstream genes. J. Radiat. Res. 45: 399–403. [DOI] [PubMed] [Google Scholar]
- Orr, A. W., B. P. Helmke, B. R. Blackman and M. A. Schwartz, 2006. Mechanisms of mechanotransduction. Dev. Cell 10: 11–20. [DOI] [PubMed] [Google Scholar]
- Perkins, L. A., E. M. Hedgecock, J. N. Thomson and J. G. Culotti, 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117: 456–487. [DOI] [PubMed] [Google Scholar]
- Ren, P., C. S. Lim, R. Johnsen, P. S. Albert and D. Pilgrim, 1996. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science 274: 1389–1391. [DOI] [PubMed] [Google Scholar]
- Savage, C., M. Hamelin, J. G. Culotti, A. Coulson and D. G. Albertson, 1989. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev. 3: 870–881. [DOI] [PubMed] [Google Scholar]
- Schackwitz, W., T. Inoue and J. Thomas, 1996. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17: 719–728. [DOI] [PubMed] [Google Scholar]
- Smith, A. H., 1976. Physiological changes associated with long-term increases in acceleration. Life Sci. Space Res. 14: 91–100. [PubMed] [Google Scholar]
- Soga, K., K. Wakabayashi, S. Kamisaka and T. Hoson, 2005. Mechanoreceptors rather than sedimentable amyloplasts perceive the gravity signal in hypergravity-induced inhibition of root growth in azuki bean. Funct. Plant Biol. 32: 175–179. [DOI] [PubMed] [Google Scholar]
- Sukharev, S., and D. P. Corey, 2004. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE 2004: re4. [DOI] [PubMed]
- Sze, J. Y., Y. Liu and G. Ruvkun, 1997. VP16-activation of the C. elegans neural specification transcription factor UNC-86 suppresses mutations in downstream genes and causes defects in neural migration and axon outgrowth. Development 124: 1159–1168. [DOI] [PubMed] [Google Scholar]
- Tou, J., A. Ronca, R. Grindeland and C. Wade, 2002. Models to study gravitational biology of mammalian reproduction. Biol. Reprod. 67: 1681–1687. [DOI] [PubMed] [Google Scholar]
- Vandenburgh, H., J. Chromiak, J. Shansky, M. Del Tatto and J. Lemaire, 1999. Space travel directly induces skeletal muscle atrophy. FASEB J. 13: 1031–1038. [DOI] [PubMed] [Google Scholar]
- Ward, S., N. Thomson, J. G. White and S. Brenner, 1975. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160: 313–337. [DOI] [PubMed] [Google Scholar]
- Way, J. C., and M. Chalfie, 1989. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 3: 1823–1833. [DOI] [PubMed] [Google Scholar]
- White, J. G., E. Southgate, J. N. Thomson and S. Brenner, 1986. The structure of the nervous system of the nematode C. elegans. Phil. Trans. R. Soc. Lond. B. 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Wolkow, C. A., K. D. Kimura, M. S. Lee and G. Ruvkun, 2000. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290: 147–150. [DOI] [PubMed] [Google Scholar]