Abstract
In self-incompatibility, a number of S haplotypes are maintained by frequency-dependent selection, which results in trans-specific S haplotypes. The region of several kilobases (∼40–60 kb) from SP6 to SP2, including self-incompatibility-related genes and some adjacent genes in Brassica rapa, has high nucleotide diversity due to the hitchhiking effect, and therefore we call this region the “S-locus complex.” Recombination in the S-locus complex is considered to be suppressed. We sequenced regions of >50 kb of the S-locus complex of three S haplotypes in B. rapa and found higher nucleotide diversity in intergenic regions than in coding regions. Two highly similar regions of >10 kb were found between BrS-8 and BrS-46. Phylogenetic analysis using trans-specific S haplotypes (called interspecific pairs) of B. rapa and B. oleracea suggested that recombination reduced the nucleotide diversity in these two regions and that the genes not involved in self-incompatibility in the S-locus complex and the kinase domain, but not the S domain, of SRK have also experienced recombination. Recombination may reduce hitchhiking diversity in the S-locus complex, whereas the region from the S domain to SP11 would disfavor recombination.
NATURAL selection can influence the pattern of polymorphisms around a selected locus. This phenomenon is known as the hitchhiking effect (Kojima and Schaffer 1967; Maynard-Smith and Haigh 1974). Recombination plays an important role in determining the genomic range under the effects of natural selection. Recombination may accelerate the rate of adaptation and increase an efficacy of purifying selection, because recombination can enable natural selection to act independently on the different loci (Barton and Charlesworth 1998).
Balancing selection increases nucleotide diversity not only in a selected locus but also in the surrounding genomic regions (Wiuf et al. 2004; Charlesworth 2006). The major histocompatibility complex (MHC) in humans possesses high nucleotide diversity, which is much higher than the average of the whole genome (Gaudieri et al. 2000). In heteromorphic sex chromosomes of animals and plants, two types of chromosomes are maintained by frequency-dependent selection. Due to suppression of recombination between two chromosomes, high divergence is observed along the entire recombination-suppressed region (Lahn and Page 1999; Bergero et al. 2007).
The plant self-incompatibility locus (S locus) holds a large number of alleles within a population by frequency-dependent selection (Wright 1939; Fisher 1958). This system involves self- or non-self-pollen recognition in the reproductive process. Several self-recognition mechanisms have been discovered in multiple plant lineages. They are classified into two types of pollen-side recognition, i.e., gametophytic (haploidy recognition) and sporophytic (diploidy recognition) self-incompatibility (Takayama and Isogai 2005). Self-recognition reaction requires two genes—one for the female (pistil) side and the other for the male (pollen) side—and allele-specific interaction between female and male molecules prevents self-fertilization to avoid inbreeding depression. Recombination between the two genes should be suppressed because recombination may disrupt the allele-specific self-recognition ability.
Balancing selection on the S locus increases the nucleotide diversity in regions around the two self-incompatibility-related genes. Sequence surveys of the S-locus region have been performed in Brassica species and Prunus mume, which employ sporophytic and gametophytic self-incompatibility systems, respectively (e.g., Boyes et al. 1997; Casselman et al. 2000; Entani et al. 2003; Fukai et al. 2003; Shiba et al. 2003). These studies have revealed extremely high nucleotide diversity in >10 kb in Brassica rapa (Shiba et al. 2003) and in P. mume (Entani et al. 2003). It has been thought that high nucleotide variation may result from the suppression of recombination in the entire S-locus region. In Brassica, the highly polymorphic region from SP6 to SP2 is called the “S-locus complex” in this study, since this region contains the genes that are not involved in self-incompatibility.
Self-incompatibility in Brassicaceae, including B. rapa, Brassica oleracea, Raphanus sativus, and Arabidopsis lyrata, has been intensively studied. SRK and SP11/SCR (SP11 hereafter) have been identified as the recognition genes of the female side and the male side, respectively (Stein et al. 1991; Schopfer et al. 1999; Suzuki et al. 1999). SRK contains the S domain and the kinase domain, and interaction between SP11 and the S domain of SRK results in self-pollen rejection (Kachroo et al. 2001; Takayama et al. 2001), and co-evolution between SP11 and the S domain of SRK has been suggested (Sato et al. 2002). SLG, which is similar to the S domain of SRK, is also located in the S locus, but its role is unclear (Suzuki et al. 2000; Takasaki et al. 2000). Since these three genes are inherited by progeny as one genetic unit, the term “S haplotype” is used instead of the classical term “S allele” (Nasrallah and Nasrallah 1993). The same S haplotypes are distributed in different Brassica species (Kusaba et al. 1997; Sato et al. 2002). Trans-specific S haplotypes in Brassica have maintained the same recognition specificity, although a coalescent event should predate speciation of Brassica species. Such pairs of S haplotypes are called “interspecific pairs” (Kimura et al. 2002; Sato et al. 2003).
A number of trans-specific polymorphic sites between A. lyrata and the closely related self-compatible species Arabidopsis thaliana were observed in their S-locus complexes, indicating that recombination rate in the S locus should be low (Charlesworth et al. 2006). Although recombination could not be detected in the SRK of A. lyrata (Charlesworth et al. 2003), Awadalla and Charlesworth (1999) have suggested the occurrence of recombination within SLG and SRK in Brassica on the basis of the pattern of linkage disequilibrium. High similarity in SLG and the kinase domain of SRK has been observed between BrS-8 and BrS-46 in B. rapa and similar sequences are considered to be probably due to recombination (Kusaba and Nishio 1999). The possibility of recombination prompted us to investigate the sequences of the Brassica S-locus complex. We detected recombination in the S-locus complex using the sequences of S haplotypes in B. rapa and their interspecific pairs in B. oleracea. The effects of recombination on the patterns of hitchhiking diversity are discussed here.
MATERIALS AND METHODS
Plant materials and DNA extraction:
Seventeen S-tester lines, i.e., S homozygotes, in B. rapa, maintained at Tohoku University (Nou et al. 1993; Nishio et al. 2006) and 21 S-tester lines in B. oleracea provided by D. Astley (Ockendon 2000) were used (supplemental Table 1 at http://www.genetics.org/supplemental/). S haplotypes in B. rapa and B. oleracea are designated as BrS-XX and BoS-XX, respectively (XX represents a given allelic number). SRK, SP11, and SLG in a certain B. rapa S haplotype are referred to as BrSRK-XX, BrSP11-XX, and BrSLG-XX, respectively. The same type of nomenclature is applied to other genes in the S-locus complex. Genomic DNA was prepared from leaves by the CTAB method (Murray and Thompson 1980) or by the modified CTAB method (Escaravage et al. 1998).
Isolation of genomic clones of the S locus and sequencing:
The genomic DNAs of BrS-8 and BrS-54 homozygotes were partially digested by Sau3AI and ligated to the λFIXII vector (Stratagene, La Jolla, CA). Six overlapping genomic λ-phage clones of the S-locus region in BrS-8 and four overlapping genomic clones including SLG and four clones including SRK and SP11 in BrS-54 were isolated by plaque hybridization. SacI, SalI, and XbaI fragments of these genomic clones were subcloned into pBluescriptsSK(−). The PAC clone, designated as G28, of the S-locus region of BrS-46 (length ∼80 kb) has been isolated (Kimura et al. 2002). The nucleotide sequences of an 11.7-kb region covering SP11 and the S domain of SRK in G28 have been determined by Kimura et al. (2002) (accession no. AB070625), and a 9.3-kb region neighboring the 11.7-kb region, including the kinase domain of SRK, has been determined by Fujimoto et al. (2006) (accession no. AB180897). In this study, EcoRI and SpeI fragments from a 58.6-kb region of the rest of G28 were newly subcloned into pBluescriptsSK(−). The DNA sequences of the subclones were determined with a CEQ 2000XL DNA Analyzer (Beckman Coulter), and the data were combined using Sequencher (Gene Codes).
DNA amplification from genomic DNA by PCR:
The genes in the S-locus complex were amplified from four B. oleracea S haplotypes, i.e., BoS-7, BoS-12, BoS-28, and BoS-32. SLL2, SP2, and AtPPa were amplified by specific primers listed in supplemental Table 2 at http://www.genetics.org/supplemental/. The amplified products were cloned into pGEM-T Easy Vector (Promega, Madison, WI) and sequenced. Three independent clones for each gene were sequenced to correct the PCR errors.
RT–PCR:
Total RNA was extracted from immature pistils using ISOGEN (Nippongene, Tokyo). Reverse-transcription reaction was performed using a first-strand cDNA synthesis kit (GE Healthcare Bio-sciences). The S domain of SRK was amplified according to Sato et al. (2002). The kinase domain was reverse transcribed and amplified with specific primers (supplemental Table 2 at http://www.genetics.org/supplemental/). Three independent clones for each allele were sequenced.
Sequence analyses:
The genes in the S-locus complex were predicted by RiceGAAS (Rice Genome Automated Annotation System, http://RiceGAAS.dna.affrc.go.jp/; Sakata et al. 2002) and by BLAST (http://www.ddbj.nig.ac.jp/search/blast-j.html; Altschul et al. 1997). The sequence alignment was constructed using ClustalW, version 1.83 (Thompson et al. 1994). Sliding-window analysis was performed using two haplotypes among BrS-8, BrS-46, and BrS-54 with a window size of 1000 bp and a sliding width of 400 bp after alignment and excluding all gapped sites. Inversion of BrSP11-54 was observed, and therefore the coding region of BrSP11-54 was reversed to construct alignment with BrSP11-8 and BrSP11-46. The reversed sequence included only the coding region because the BLAST search did not detect any similar region around SP11 between the three S haplotypes, such as an untranslated region or a promoter sequence. Pairwise nucleotide diversity values in synonymous and nonsynonymous sites were estimated by the method of Yang and Nielsen (2000) using PAML, version 3.15 (Yang 1997). A gene genealogy was inferred with the neighbor-joining method (Saitou and Nei 1987) using MEGA, version 3.1 (Kumar et al. 2004). Bootstrap probabilities of 1000 trials were calculated (Felsenstein 1985). To test the incongruence of gene genealogies, the Shimodaira–Hasegawa test (SH test; Shimodaira and Hasegawa 1999) was performed using CONSEL, version 0.1i (Shimodaira and Hasegawa 2001).
Estimation of synonymous divergence between B. rapa and B. oleracea:
The synonymous divergence rate between B. rapa and B. oleracea was estimated using 31 putative orthologs between B. rapa and B. oleracea (e.g., Fourmann et al. 2002; supplemental Table 3 at http://www.genetics.org/supplemental/ for details). Sequences of these orthologs were assembled and aligned (a total of 4092 codons). Using Yang and Nielsen's method (2000), synonymous divergence per site (Ks) and nonsynonymous divergence per site (Ka) were estimated to be 0.0967 ± 0.0058 (SE) and 0.0178 ± 0.0014 (SE), respectively. This Ks value was used as an expected lower boundary of nucleotide differences at neutral sites among B. rapa S haplotypes, since the used S haplotypes may have emerged before speciation of B. rapa and B. oleracea. If the nucleotide differences among S haplotypes are significantly smaller than the interspecific divergence, given S haplotypes may have descended after the speciation.
RESULTS
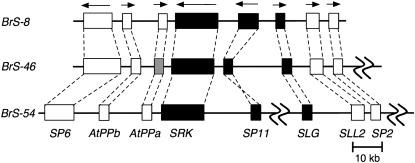
We determined the sequences of the S-locus complex, whose lengths are 51.1 kb in BrS-8 (accession no. AB257127), 79.6 kb in BrS-46 (accession no. AB257128), and 45.3 and 31.8 kb in BrS-54 (accession nos. AB298592 and AB298593, respectively). The sequences of BrS-54 were not assembled into a single contig, but rather into two contigs. Eight genes were predicted in the sequence of BrS-8 by RiceGAAS and BLAST. The three genes were self-incompatibility-related genes, i.e., SRK, SP11, and SLG, and the remaining genes were designated as SP6, AtPPb, AtPPa, SLL2, and SP2 according to previous studies (Figure 1) (Cui et al. 1999; Suzuki et al. 1999). These eight genes were also found in BrS-46, although BrAtPPa-46 was a pseudogene. A frameshift mutation due to a 2-bp insertion was found in the first exon of BrAtPPa-46. Partial sequences of BrS-54, in which a gap was located between BrSP11-54 and BrSLG-54, also possessed these eight genes (Figure 1). The sequences determined in this study covered the region from SP6 to SP2, which has been considered a recombination-suppressed region (Boyes et al. 1997; Casselman et al. 2000; Fukai et al. 2003; Shiba et al. 2003). The lengths of intergenic regions were highly variable (supplemental Table 4 at http://www.genetics.org/supplemental/). On the other hand, the gene order was conserved among the three S haplotypes (Figure 1). The orientations of the genes were also conserved, except for inversion of BrSP11-54 (Figure 1). Conservation of synteny led to a prediction that corresponding intergenic regions among these three S haplotypes may be homologous and allowed direct comparison and alignment.
Figure 1.—
Genomic organizations of the B. rapa S haplotypes BrS-8, BrS-46, and BrS-54. The solid boxes represent self-incompatibility-related genes. The open boxes represent genes not involved in self-incompatibility, and the shaded box denotes a pseudogene. The gene names are shown under the boxes, solid lines between boxes indicate intergenic regions, arrows show the orientations of genes, and dashed lines between boxes represent homologous genes.
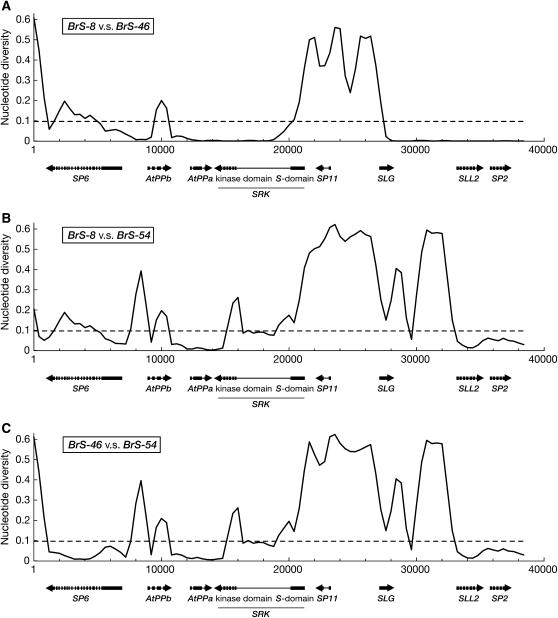
Sliding-window analysis of aligned sequences, in which all gapped sites were removed, was performed to investigate the pattern of nucleotide variations across the S-locus complex (Figure 2). The region from SRK to SLG, which was defined as the “S core region” (Fukai et al. 2003), was too highly diverged to construct a reliable alignment and the divergence of this region exceeded the estimated synonymous divergence value between B. rapa and B. oleracea (Ks = 0.0967, referred to as Dr-o). Coding regions in the other genes tended to have higher similarity than intergenic regions in all three combinations. However, there were two highly similar regions, >10 kb, between BrS-8 and BrS-46, including AtPPa, SP2, SLL2, and parts of SRK and SLG (Figure 2A), although the corresponding regions of BrS-54 were more diverged than Dr-o (Figure 2, B and C).
Figure 2.—
Sliding-window analysis among the three B. rapa S haplotypes with a window size of 1000 bp and a sliding width of 400 bp. The x-axes and y-axes represent the position in the S-locus complex and nucleotide diversity per site, respectively. The arrows indicate the genes and connecting thin lines within arrows indicate introns. The results of BrS-8 vs. BrS-46 (A), BrS-8 vs. BrS-54 (B), BrS-46 vs. BrS-54 (C) are shown.
Nucleotide diversity (π) values of the coding regions among the three S haplotypes were estimated. The π-values of eight genes are shown in Table 1. SP11 showed the highest nucleotide diversity and the other genes also showed relatively high π and synonymous diversity (πS) values. However, πS-values of the kinase domain of SRK, SLL2, and SP2 between BrS-8 and BrS-46 were zero (Table 1). The intergenic regions were also similar between BrS-8 and BrS-46 (Figure 2 and Table 2). Equation (10) in Hudson (1991) was employed for computing the probability distribution of the number of mutations (given by S) with a sample size of 2, conditional on θneu = 0.0967 (Dr-o), where θneu is the nucleotide diversity in a neutral site such as the synonymous site, intron, and intergenic region, for testing that the diversity is significantly <Dr-o. Probability of the observed number of mutations (Sob) is given by Pr(Sob), and P-value was obtained as Pr(S ≤ Sob). The significant P-values (P < 0.05) were obtained from the πS values of the kinase domain of SRK, SLL2, and SP2 and the diversity values of the intron (πInt) of SLL2 and SP2 between BrS-8 and BrS-46 (Table 1). The intergenic regions in two similar regions also showed significantly lower diversity (P < 0.05, Table 2). These results indicate a discrepancy of ancient origin of the S haplotypes under the assumption of recombination–suppression in the S-locus complex.
TABLE 1.
Nucleotide diversity among genes in the B. rapa S-locus complex
| Pair of S haplotypes | Total length (bp) | Exon (bp) | Intron (bp) | πa | πNb | πSc | πN/πS | πIntd |
|---|---|---|---|---|---|---|---|---|
| SP6 | ||||||||
| S8 vs. S46 | 5662 | 3159 | 2503 | 0.1909 | 0.0867 | 0.1882 | 0.4607 | 0.3040 |
| S8 vs. S54 | 6174 | 3645 | 2529 | 0.1521 | 0.0459 | 0.1405 | 0.3267 | 0.2768 |
| S46 vs. S54 | 5979 | 3105 | 2874 | 0.0896 | 0.0641 | 0.1444 | 0.4439 | 0.1033 |
| AtPPb | ||||||||
| S8 vs. S46 | 1204 | 786 | 418 | 0.0324 | 0.0367 | 0.0360 | 1.0194 | 0.0263 |
| S8 vs. S54 | 1184 | 786 | 398 | 0.0515 | 0.0336 | 0.0547 | 0.6143 | 0.0804 |
| S46 vs. S54 | 1775 | 786 | 989 | 0.1797 | 0.0072 | 0.0169 | 0.4260 | 0.3145 |
| AtPPae | ||||||||
| S8 vs. S46 | 1213 | NA | NA | 0.0701 | NA | NA | NA | NA |
| S8 vs. S54 | 1253 | 1059 | 194 | 0.012 | 0.0117 | 0.0216 | 0.5417 | 0.0000 |
| S46 vs. S54 | 1213 | NA | NA | 0.0816 | NA | NA | NA | NA |
| SRK (S domain) (first intron) | ||||||||
| S8 vs. S46 | 6647 | 1305 | 5342 | 0.0241 | 0.0780 | 0.1735 | 0.4496 | 0.0073 |
| S8 vs. S54 | 5587 | 1296 | 4291 | 0.1353 | 0.1393 | 0.2350 | 0.5928 | 0.1326 |
| S46 vs. S54 | 5624 | 1299 | 4325 | 0.1385 | 0.1464 | 0.2660 | 0.5504 | 0.1338 |
| SRK (kinase domain) (second to sixth intron) | ||||||||
| S8 vs. S46 | 1764 | 1269 | 495 | 0.0023 | 0.0011 | 0.0000* | NA | 0.0061 |
| S8 vs. S54 | 1767 | 1263 | 504 | 0.0113 | 0.0077 | 0.0145 | 0.5310 | 0.0159 |
| S46 vs. S54 | 1758 | 1263 | 495 | 0.0125 | 0.0065 | 0.0149 | 0.4362 | 0.0222 |
| SP11 | ||||||||
| S8 vs. S46 | 1445 | 213 | 1232 | 0.4215 | 0.2338 | 0.2100 | 1.1135 | 0.4602 |
| S8 vs. S54 | 506 | 204 | 302 | 0.5217 | 0.6062 | 0.9571 | 0.6334 | 0.5795 |
| S46 vs. S54 | 486 | 213 | 273 | 0.3663 | 0.6174 | 0.9170 | 0.6733 | 0.3114 |
| SLGf | ||||||||
| S8 vs. S46 | 1305 | 1305 | 0 | 0.0238 | 0.0167 | 0.0465 | 0.3591 | NA |
| S8 vs. S54 | 1305 | 1305 | 0 | 0.1379 | 0.1252 | 0.2526 | 0.4956 | NA |
| S46 vs. S54 | 1305 | 1305 | 0 | 0.1372 | 0.1269 | 0.2385 | 0.5321 | NA |
| SLL2g | ||||||||
| S8 vs. S46 | 2218 | 1011 | 1207 | 0.0000 | 0.0000 | 0.0000* | NA | 0.0000** |
| S8 vs. S54 | 2218 | 1011 | 1207 | 0.0266 | 0.0040 | 0.0691 | 0.0579 | 0.0323 |
| S46 vs. S54 | 2218 | 1011 | 1207 | 0.0266 | 0.0040 | 0.0691 | 0.0579 | 0.0323 |
| SP2g | ||||||||
| S8 vs. S46 | 1540 | 705 | 835 | 0.0000 | 0.0000 | 0.0000* | NA | 0.0000* |
| S8 vs. S54 | 1519 | 705 | 814 | 0.0592 | 0.0056 | 0.0939 | 0.0596 | 0.0885 |
| S46 vs. S54 | 1519 | 705 | 814 | 0.0592 | 0.0056 | 0.0939 | 0.0596 | 0.0885 |
*P < 0.05, **P < 0.01; computed from the probability distribution of the number of mutations using equation (10) in Hudson (1991) and assuming θneu = 0.0967.
Nucleotide diversity per site.
Nucleotide diversity per nonsynonymous site.
Nucleotide diversity per synonymous site.
Nucleotide diversity in intron per site.
Not applicable because BrAtPPa-46 is a pseudogene.
Not applicable because of a lack of an intron.
Not applicable because πS was zero.
TABLE 2.
Nucleotide diversity in the similar intergenic regions between BrS-8 and BrS-46
| Region | Aligned length (bp) | πa | P-valueb |
|---|---|---|---|
| AtPPa ∼ SRK | 414 | 0 | P < 0.05 |
| SLG ∼ SLL2 | 4277 | 0.00117 | P < 0.05 |
| SLL2 ∼ SP2 | 591 | 0 | P < 0.05 |
| SP2 ∼ | 2485 | 0.00161 | P < 0.05 |
Nucleotide diversity per site.
P-values were computed from the probability distribution of the number of mutations using equation (10) in Hudson (1991) and assuming θneu = 0.0967.
One intriguing finding is that the two low-diversity regions between BrS-8 and BrS-46 did not include the S domain of SRK and SP11, which are involved in a self-incompatibility reaction. One similar region including a part of SRK seemed to start at a site in the first intron, which is proximal to the S domain (Figure 2A). This may be interpreted as simply reflecting recombination between BrS-8 and BrS-46, which is consistent with previous findings (Awadalla and Charlesworth 1999; Kusaba and Nishio 1999). However, both π and πS values of SP6 and AtPPb were relatively higher than those of AtPPa and the kinase domain (Figure 2, Table 1). This is inconsistent with the theoretical prediction that nucleotide diversity and the distance from the selected region should negatively correlate under effects of recombination (Charlesworth 2006; see discussion).
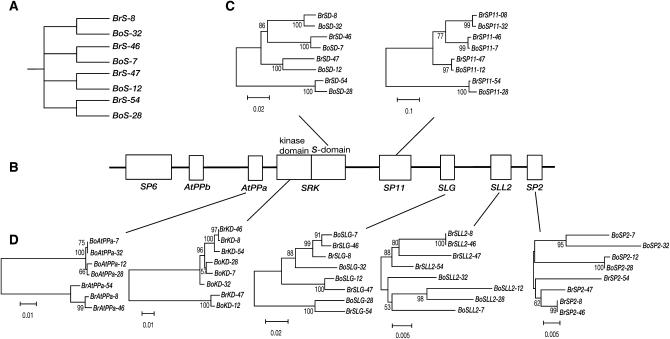
Gene genealogy analysis using four interspecific pairs, including BrS-8 and BrS-46, was performed to confirm the occurrence of recombination in the S-locus complex, since genealogies of interspecific pairs can be expected, as in Figure 3A. If recombination has occurred in the S-locus complex, the topology of the genes could be disrupted (Takahata 1990; Schierup et al. 2001b). Since intergenic regions and introns between interspecific pairs have been found to be highly variable (Fujimoto et al. 2006), only the exon sequences of the S-locus genes were used. AtPPa, SLL2, and SP2 in four B. oleracea S haplotypes in the interspecific pairs were successfully amplified by PCR and sequenced, although BrAtPPa-47 and BrAtPPb-47 were deleted (Fujimoto et al. 2006). The genealogies of the S domain of SRK and SP11 matched the expected topology (Figure 3, A and C) and suggested co-evolution of the S domain of SRK and SP11 (Sato et al. 2002). However, the topologies of the kinase domain of SRK and SLG were slightly disrupted and alleles of AtPPa, SLL2, and SP2 were divided into two species-specific clades (Figure 3D). Gene genealogies inferred only from synonymous divergence showed nearly the same topologies. The incongruence of gene genealogies of the kinase domain, AtPPa, SP2, and SLL2, was statistically significant using the S domain as a standard (SH test, P = 3×10−4 for kinase domain, P < 10−4 for the others), but that of SLG was not. We further surveyed the number of shared polymorphic sites between B. rapa and B. oleracea using a proportion of shared polymorphic sites in the S domain as a standard. These proportions in AtPPa, SLL2, and SP2, but not in SLG, and the kinase domain of SRK, were significantly lower than that in the S domain of SRK (Fisher's exact test; Table 3).
Figure 3.—
Gene genealogies of the genes in the S-locus complex. (A) Expected gene genealogy of the four interspecific pairs of S haplotype in B. rapa and B. oleracea. (B) The genomic organization of the S-locus complex, corresponding to Figure 1. (C and D) The gene genealogies of the genes in the S-locus complex inferred using the neighbor-joining method. More than 50% bootstrap values, estimated by 1000 time trials, are shown. SD in C and KD in D represent the abbreviations of the S domain and the kinase domain, respectively.
TABLE 3.
The proportion of shared polymorphic sites in the S-locus genes of B. rapa and B. oleracea
| Genesa | No. of polymorphic sites (S1)b | No. of shared polymorphic sites (S2)b | S2/S1 | P-valuec |
|---|---|---|---|---|
| SRK (S domain) | 307 | 221 | 0.720 | — |
| SRK (kinase domain) | 186 | 141 | 0.758 | NSd |
| SLG | 304 | 187 | 0.615 | NSd |
| AtPPa | 112 | 0 | 0.000 | P < 10−22 |
| SLL2 | 80 | 13 | 0.163 | P < 10−6 |
| SP2 | 57 | 6 | 0.105 | P < 10−7 |
SP11 was not included because lengths of SP11 were short (∼200 bp).
Number of polymorphic sites and number of shared polymorphic sites were counted in coding regions.
P-values were obtained by Fisher's exact test comparing the proportion of shared polymorphic sites of the S domain.
Not significant.
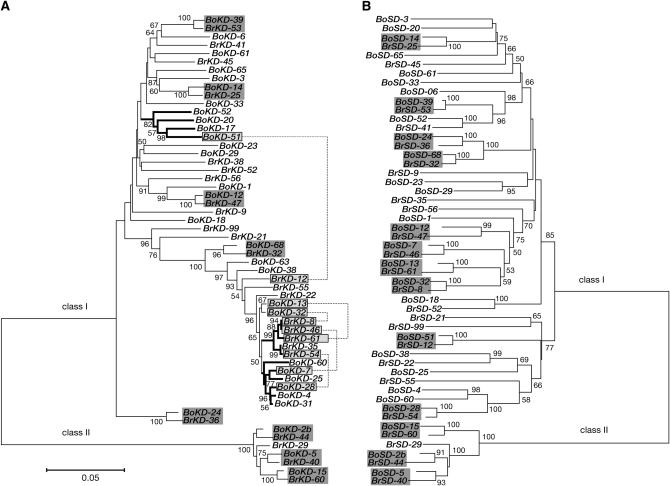
Our results suggested the recombination in the kinase domain of SRK, which is adjacent to the S domain. We inferred gene genealogy of the S domain and the kinase domain in B. rapa and B. oleracea to investigate the recombination in other S haplotypes. The kinase domain of 31 SRK alleles and the S domain of 13 SRK alleles were newly sequenced (AB257129, AB298875–AB298905). Including deposited sequences, we analyzed 25 and 31 kinase domain sequences from B. rapa and B. oleracea, respectively, and 24 and 28 S-domain sequences from B. rapa and B. oleracea, respectively (Figure 4; supplemental Table 5 at http://www.genetics.org/supplemental/). A phylogenetic tree of the kinase domain was divided into a clade of class I haplotypes (dominant in pollen) and a clade of class II haplotypes (recessive in pollen; Figure 4A) as shown in previous studies on SLG and the S domain of SRK (Figure 4B) (Kusaba et al. 1997; Sato et al. 2002). The tree also indicated that the kinase domain retained the pattern of trans-specific polymorphism as is the case with the S domain (shaded boxes in Figure 4). Ten interspecific pairs were predicted by the genealogy of the S domain (Figure 4B), but 5 pairs of the kinase domain sequences were disrupted and tended to be included in species-specific clusters (thick lines in Figure 4A). These results strongly suggest that recombination has occurred in the S-locus complex of B. rapa but outside the region from the S domain of SRK to SP11.
Figure 4.—
The difference of the gene genealogies between the kinase domain (A) and the S domain (B) of SRK. The darkly shaded boxes represent interspecific pairs. The lightly shaded boxes show the interspecific pairs with disrupted topology in the kinase domain. The disrupted interspecific pairs are connected by dashed lines. The thick lines indicate the clades including the kinase domains only from B. rapa or from B. oleracea.
DISCUSSION
Brassica self-incompatibility-related genes, i.e., SRK, SP11, and SLG, show trans-specific polymorphisms between B. rapa and B. oleracea and deep coalescent time may have resulted in the existence of interspecific pairs (Kusaba et al. 1997; Kimura et al. 2002; Sato et al. 2002, 2003). The strong balancing selection on the self-incompatibility-related genes increases the diversity in an adjacent region of ∼40–50 kb by the hitchhiking effect (Boyes et al. 1997; Casselman et al. 2000; Fukai et al. 2003; Shiba et al. 2003). Intergenic regions in the three S haplotypes used in this study were found to diverge up to 60% and to have a variation in lengths (Figure 2, supplemental Table 4 at http://www.genetics.org/supplemental/), whereas the coding regions tended to have relatively low diversity (Figure 2, Table 1).
Our analysis using the sequences of interspecific pairs detected recombination events in the S-locus complex, but not between the S domain of SRK and SP11. Recombination decreased the nucleotide diversity values at the neutral sites in some comparisons, which were significantly <Dr-o (Table 1). Especially, the lengths of the two recombined regions between BrS-8 and BrS-46 were >10 kb; such a long recombined region has not been reported in any self-incompatible species. However, high variability in the other intergenic regions may indicate the low recombination rate. Interspecific pairs are considered to be useful for detecting recombination because the S haplotypes in the pairs can be expected to be closely related (Figure 3) and disruption of topology could indicate recombination events (Takahata 1990; Schierup et al. 2001b). Although only exon sequences can be tested due to high variability in intergenic regions and introns between B. rapa and B. oleracea (Fujimoto et al. 2006), we successfully detected the recombination in the coding regions. The similarity of the coding regions, maintained by selective constraint, might lead to recombination or vice versa.
Suppression of recombination leads to the accumulation of deleterious mutations (reviewed in Uyenoyama 2005). Background selection working against deleterious mutation at linked genes reduces effective population size and results in stochastic accumulation of weak deleterious mutations (Charlesworth et al. 1993). Furthermore, reduction of effective population size would facilitate the process of Muller's ratchet (Muller 1964). Degeneration of nonrecombining Y chromosomes and haploid asexual species should be accelerated by these two processes (Charlesworth and Charlesworth 1997, 2000). Recombination rate in the S-locus complex has been considered to be very low. A small “effective population size” of each S haplotype (i.e., effective gene number; Takahata 1990) and an absence of recombination may lead to the S-haplotype-specific degeneration of linked genes (Uyenoyama 1997). The S-haplotype-specific reduction of fitness has been observed in the gametophytic self-incompatible species Solanum carolinense (Stone 2004). However, recombination in the Brassica S-locus complex outside of SP11 and the S domain of SRK may prevent the accumulation of detrimental mutations and moderate the effect of deleterious mutations.
Recombination is also important for facilitating the fixation of a new favorable mutation. When a favorable mutation spreads in a population, the hitchhiking effect may lead to fixation of linked deleterious mutations (Kojima and Schaffer 1967; Maynard-Smith and Haigh 1974). For example, in nonrecombining Y chromosomes and mitochondrial genomes, positive selection could affect all chromosomes or entire genomes, together with the simultaneous fixation of deleterious mutations and the reduction of genetic diversity (Rice 1987; Bachtrog 2004; Bazin et al. 2006). Recombination can prevent the hitchhiking effects. Our evidence of recombination in the Brassica S-locus complex prompted us to consider the possibility of a selective sweep in the AtPPa from the two observations: (i) The nucleotide diversity of the genes in the S-locus complex did not decrease linearly with distance from SRK and SP11 (Figure 2, Table 1), and (ii) a much more recent coalescent time was observed in AtPPa within B. rapa and B. oleracea than in the other genes (Figure 3). However, BrAtPPa-46 is a pseudogene (Figure 1) and AtPPa and AtPPb are not present in the S-locus complex of BrS-47 (Fujimoto et al. 2006). Therefore, the occurrence of selective sweep is unlikely and recombination may play a role only in decreasing hitchhiking diversity around AtPPa (Figure 3). Because SP6 and AtPPb in B. oleracea could not be amplified by PCR, we cannot test the occurrence of recombination in the two genes. The πS values in the two genes may indicate that they have a low recombination rate. The heterogeneity of the recombination rate in the S-locus complex may contribute to the difference in the degree of the effects on hitchhiking diversity.
Charlesworth et al. (2003) have pointed out the difference in recombination between Brassica and A. lyrata in SRK. Strong linkage disequilibrium is observed in the kinase domain of A. lyrata, but the kinase domain of B. rapa and B. oleracea shows disruption of allelic association between polymorphic sites (Awadalla and Charlesworth 1999; Charlesworth et al. 2003). Furthermore, recombination is suppressed in the genes in the A. lyrata S-locus complex (Charlesworth et al. 2006). Other theoretical analyses of the RNase-based self-incompatibility system also support a low recombination rate within S-RNase in Solanaceae, Rosaceae, and Scrophulariaceae (Vieira et al. 2003) and between S-RNase and a marker gene, 48A, in Nicotiana alata (Takebayashi et al. 2004). Therefore, Brassica species are worthy of attention with respect to recombination in the S-locus complex. One explanation is that the nucleotide diversity of SRK in Brassica is much lower than that in A. lyrata (Schierup et al. 2001a), which implies greater similarity among S haplotypes in Brassica species than among those in A. lyrata. The relatively low diversity might facilitate meiotic pairing, which results in recombination in Brassica. Recently, interspecific pairs have also been discovered between A. lyrata and Arabidopsis halleri (Bechsgaard et al. 2006), and further analysis may provide insights into the recombination in the S-locus complex of Brassicaceae.
It is interesting that the two recombined regions between BrS-8 and BrS-46 are very close to the self-recognition-related region, i.e., the S domain of SRK and SP11 (Figure 2A). The kinase domains of other S haplotypes also experienced recombination more frequently than the S domain (Figure 4). This would mean that the regions close to the selected region would tolerate recombination. A similar phenomenon has been observed in the MHC locus. The second exon, which corresponds to a peptide-binding region, is highly polymorphic, but diversity of the linked sites around the second exon decline due to recombination (Takahata and Satta 1998). On the other hand, A. thaliana is a fully self-fertile species, which results from the nonfunctionalization of SRK and SP11 (ψ-SRK and ψ-SCR). Sherman-Broyles et al. (2007) have suggested that the recombination in the region from ψ-SRK to ψ-SCR between different haplotypes occurred due to a relaxation of selective pressure after disruption of self-incompatibility.
Acknowledgments
We are grateful to Dave Astley for providing B. oleracea S-tester lines and to Hideki Innan, Kosuke M. Teshima, and the two anonymous reviewers for their helpful comments. We thank Dominique Brunel for providing information about the Brassica sequence database and Hidetoshi Simodaira for instruction on the CONSEL program.
References
- Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang et al., 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awadalla, P., and D. Charlesworth, 1999. Recombination and selection at Brassica self-incompatibility loci. Genetics 152: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtrog, D., 2004. Evidence that positive selection drives Y-chromosome degeneration in Drosophila miranda. Nat. Genet. 36: 518–522. [DOI] [PubMed] [Google Scholar]
- Barton, N. H., and B. Charlesworth, 1998. Why sex and recombination? Science 281: 1986–1990. [PubMed] [Google Scholar]
- Bazin, E., S. Glémin and N. Galtier, 2006. Population size does not influence mitochondrial genetic diversity in animals. Science 312: 570–572. [DOI] [PubMed] [Google Scholar]
- Bechsgaard, J. S., V. Castric, D. Charlesworth, X. Vekemans and M. H. Schierup, 2006. The transition to self-compatibility in Arabidopsis thaliana and evolution within S-haplotypes over 10 myr. Mol. Biol. Evol. 23: 1741–1750. [DOI] [PubMed] [Google Scholar]
- Bergero, R., A. Forrest, E. Kamau and D. Charlesworth, 2007. Evolutionary strata on the X chromosomes of the dioecious plant Silene latifolia: evidence from new sex-linked genes. Genetics 175: 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes, D. C., M. E. Nasrallah, J. Vrebalov and J. B. Nasrallah, 1997. The self-incompatibility (S) haplotypes of Brassica contain highly divergent and rearranged sequences of ancient origin. Plant Cell 9: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselman, A. L., J. Vrebalov, J. A. Conner, A. Singhal, J. Giovannoni et al., 2000. Determining the physical limits of the Brassica S locus by recombinational analysis. Plant Cell 12: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, B., and D. Charlesworth, 1997. Rapid fixation of deleterious alleles can be caused by Muller's ratchet. Genet. Res. 70: 63–73. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., and D. Charlesworth, 2000. The degeneration of Y chromosomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355: 1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, B., M. T. Morgan and D. Charlesworth, 1993. The effect of deleterious mutations on neutral molecular variation. Genetics 134: 1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, D., 2006. Balancing selection and its effects on sequences in nearby genome regions. PLoS Genet. 2: e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, D., C. Bartolomé, M. H. Schierup and B. K. Mable, 2003. Haplotype structure of the stigmatic self-incompatibility gene in natural populations of Arabidopsis lyrata. Mol. Biol. Evol. 20: 1741–1753. [DOI] [PubMed] [Google Scholar]
- Charlesworth, D., E. Kamau, J. Hagenblad and C. Tang, 2006. Trans-specificity at loci near the self-incompatibility loci in Arabidopsis. Genetics 172: 2699–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y., N. Brugière, L. Jackman, Y. M. Bi and S. J. Rothstein, 1999. Structural and transcriptional comparative analysis of the S locus regions in two self-incompatible Brassica napus lines. Plant Cell 11: 2217–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entani, T., M. Iwano, H. Shiba, F. S. Che, A. Isogai et al., 2003. Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 8: 203–213. [DOI] [PubMed] [Google Scholar]
- Escaravage, N., S. Questiau, A. Pornon, B. Doche and P. Taberlet, 1998. Clonal diversity in a Rhododendron ferrugineum L. (Ericaceae) population inferred from AFLP markers. Mol. Ecol. 7: 975–982. [Google Scholar]
- Felsenstein, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Fisher, R. A., 1958. The Genetical Theory of Natural Selection, Ed. 2. Dover, New York.
- Fourmann, M., P. Barret, N. Froger, C. Baron, F. Charlot et al., 2002. From Arabidopsis thaliana to Brassica napus: development of amplified consensus genetic markers (ACGM) for construction of a gene map. Theor. Appl. Genet. 105: 1196–1206. [DOI] [PubMed] [Google Scholar]
- Fujimoto, R., K. Okazaki, E. Fukai, M. Kusaba and T. Nishio, 2006. Comparison of the genome structure of the self-incompatibility (S) locus in interspecific pairs of S haplotypes. Genetics 173: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai, E., R. Fujimoto and T. Nishio, 2003. Genomic organization of the S core region and the S flanking regions of a class-II S haplotype in Brassica rapa. Mol. Genet. Genomics 269: 361–369. [DOI] [PubMed] [Google Scholar]
- Gaudieri, S., R. L. Dawkins, K. Habara, J. K. Kulski and T. Gojobori, 2000. SNP profile within the human major histocompatibility complex reveals an extreme and interrupted level of nucleotide diversity. Genome Res. 10: 1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. R., 1991. Gene genealogy and the coalescent process, pp. 1–44 in Evolutionary Biology, edited by D. Futoyma and J. Antonovics. Oxford Surveys, Oxford.
- Kachroo, A., C. R. Schopfer, M. E. Nasrallah and J. B. Nasrallah, 2001. Allele-specific receptor-ligand interactions in Brassica self-incompatibility. Science 293: 1824–1826. [DOI] [PubMed] [Google Scholar]
- Kimura, R., K. Sato, R. Fujimoto and T. Nishio, 2002. Recognition specificity of self-incompatibility maintained after the divergence of Brassica oleracea and Brassica rapa. Plant J. 29: 215–223. [DOI] [PubMed] [Google Scholar]
- Kojima, K., and H. E. Schaffer, 1967. Survival processes of linked mutant genes. Evolution 21: 518–531. [DOI] [PubMed] [Google Scholar]
- Kumar, S., K. Tamura and M. Nei, 2004. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 5: 150–163. [DOI] [PubMed] [Google Scholar]
- Kusaba, M., and T. Nishio, 1999. Comparative analysis of S haplotypes with very similar SLG alleles in Brassica rapa and Brassica oleracea. Plant J. 17: 83–91. [DOI] [PubMed] [Google Scholar]
- Kusaba, M., T. Nishio, Y. Satta, K. Hinata and D. Ockendon, 1997. Striking sequence similarity in inter- and intra-specific comparisons of class I SLG alleles from Brassica oleracea and Brassica campestris: implications for the evolution and recognition mechanism. Proc. Natl. Acad. Sci. USA 94: 7673–7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahn, B. T., and D. C. Page, 1999. Four evolutionary strata on the human X chromosome. Science 286: 964–967. [DOI] [PubMed] [Google Scholar]
- Maynard-Smith, J., and J. Haigh, 1974. The hitch-hiking effect of a favorable gene. Genet. Res. 23: 23–35. [PubMed] [Google Scholar]
- Muller, H., 1964. The relation of recombination to mutational advance. Mutat. Res. 1: 2–9. [DOI] [PubMed] [Google Scholar]
- Murray, M. G., and W. F. Thompson, 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8: 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah, J. B., and M. E. Nasrallah, 1993. Pollen-stigma signaling in the sporophytic self-incompatibility response. Plant Cell 5: 1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio, T., A. Izumida, H. Hanzawa and K. Sakamoto, 2006. Development of S tester lines of Brassica oleracea, Brassica rapa and Raphanus sativus as genetic resources. Acta Hortic. 706: 141–144. [Google Scholar]
- Nou, I., M. Watanabe, A. Isogai and K. Hinata, 1993. Comparison of S-alleles and S-glycoproteins between two wild populations of Brassica campestris in Turkey and Japan. Sex. Plant Reprod. 6: 79–86. [Google Scholar]
- Ockendon, D. J., 2000. The S-allele collection of Brassica oleracea. Acta Hortic. 539: 25–30. [Google Scholar]
- Rice, W. R., 1987. Genetic hitchhiking and the evolution of reduced genetic activity of the Y sex chromosome. Genetics 116: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou, N., and M. Nei, 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- Sakata, K., Y. Nagamura, H. Numa, B. A. Antonio, H. Nagasaki et al., 2002. RiceGAAS: an automated annotation system and database for rice genome sequence. Nucleic Acids Res. 30: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., T. Nishio, R. Kimura, M. Kusaba, T. Suzuki et al., 2002. Coevolution of the S-locus genes SRK, SLG and SP11/SCR in Brassica oleracea and B. rapa. Genetics 162: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y., R. Fujimoto, K. Toriyama and T. Nishio, 2003. Commonality of self-recognition specificity of S haplotypes between Brassica oleracea and Brassica rapa. Plant Mol. Biol. 52: 617–626. [DOI] [PubMed] [Google Scholar]
- Schierup, M. H., B. K. Mable, P. Awadalla and D. Charlesworth, 2001. a Identification and characterization of a polymorphic receptor kinase gene linked to the self-incompatibility locus of Arabidopsis lyrata. Genetics 158: 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierup, M. H., A. M. Mikkelsen and J. Hein, 2001. b Recombination, balancing selection and phylogenies in MHC and self-incompatibility genes. Genetics 159: 1833–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer, C. R., M. E. Nasrallah and J. B. Nasrallah, 1999. The male determinant of self-incompatibility in Brassica. Science 286: 1697–1700. [DOI] [PubMed] [Google Scholar]
- Sherman-Broyles, S., N. Boggs, A. Farkas, P. Liu, J. Vrebalov et al., 2007. S locus genes and the evolution of self-fertility in Arabidopsis thaliana. Plant Cell 19: 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba, H., M. Kenmochi, M. Sugihara, M. Iwano, S. Kawasaki et al., 2003. Genomic organization of the S-locus region of Brassica. Biosci. Biotechnol. Biochem. 67: 622–626. [DOI] [PubMed] [Google Scholar]
- Shimodaira, H., and M. Hasegawa, 1999. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 16: 1114–1116. [Google Scholar]
- Shimodaira, H., and M. Hasegawa, 2001. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17: 1246–1247. [DOI] [PubMed] [Google Scholar]
- Stein, J. C., B. Howlett, D. C. Boyer, M. E. Nasrallah and J. B. Nasrallah, 1991. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc. Natl. Acad. Sci. USA 88: 8816–8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, J. L., 2004. Sheltered load associated with S-alleles in Solanum carolinense. Heredity 92: 335–342. [DOI] [PubMed] [Google Scholar]
- Suzuki, G., N. Kai, T. Hirose, K. Fukui, T. Nishio et al., 1999. Genomic organization of the S locus: identification and characterization of genes in SLG/SRK region of S9 haplotype of Brassica campestris (syn. rapa). Genetics 153: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T., M. Kusaba, M. Matsushita, K. Okazaki and T. Nishio, 2000. Characterization of Brassica S-haplotypes lacking S-locus glycoprotein. FEBS Lett. 482: 102–108. [DOI] [PubMed] [Google Scholar]
- Takahata, N., 1990. A simple genealogical structure of strongly balanced allelic lines and trans-species evolution of polymorphism. Proc. Natl. Acad. Sci. USA 87: 2419–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata, N., and Y. Satta, 1998. Footprints of intragenic recombination at HLA loci. Immunogenetics 47: 430–441. [DOI] [PubMed] [Google Scholar]
- Takasaki, T., K. Hatakeyama, G. Suzuki, M. Watanabe, A. Isogai et al., 2000. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403: 913–916. [DOI] [PubMed] [Google Scholar]
- Takayama, S., and A. Isogai, 2005. Self-incompatibility in plants. Annu. Rev. Plant Biol. 56: 467–489. [DOI] [PubMed] [Google Scholar]
- Takayama, S., H. Shimamoto, H. Shiba, M. Funato, F. S. Che et al., 2001. Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413: 534–538. [DOI] [PubMed] [Google Scholar]
- Takebayashi, N., E. Newbigin and M. K. Uyenoyama, 2004. Maximum-likelihood estimation of rates of recombination within mating-type regions. Genetics 167: 2097–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. D., D. G. Higgins and T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyenoyama, M. K., 1997. Genealogical structure among alleles regulating self-incompatibility in natural populations of flowering plants. Genetics 147: 1389–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyenoyama, M. K., 2005. Evolution under tight linkage to mating type. New Phytol. 165: 63–70. [DOI] [PubMed] [Google Scholar]
- Vieira, C. P., D. Charlesworth and J. Vieira, 2003. Evidence for rare recombination at the gametophytic self-incompatibility locus. Heredity 91: 262–267. [DOI] [PubMed] [Google Scholar]
- Wiuf, C., K. Zhao, H. Innan and M. Nordborg, 2004. The probability and chromosomal extent of trans-specific polymorphism. Genetics 168: 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S., 1939. The distribution of self-sterility alleles in populations. Genetics 24: 538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13: 555–556. [DOI] [PubMed] [Google Scholar]
- Yang, Z., and R. Nielsen, 2000. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 17: 32–43. [DOI] [PubMed] [Google Scholar]