Abstract
We compared cortical folding patterns between patients with schizophrenia and demographically-matched healthy controls in prefrontal and temporal regions of interest. Using the Freesurfer (http://surfer.nmr.mgh.harvard.edu) cortical surface-based reconstruction methodology, we indirectly ascertained cortical displacement and convolution, together, by measuring the degree of metric distortion required to optimally register cortical folding patterns to an average template. An area within the pars triangularis of the left inferior frontal gyrus (Broca's area) showed significantly reduced metric distortion in the patient group relative to the control group (p = 0.0352). We discuss these findings in relation to the neurodevelopmental hypothesis and language dysfunction in schizophrenia.
Keywords: Schizophrenia, MRI, cortical folding, Broca's area, language
1. Introduction
During normal cortical development, neurons migrate from the ventricular zone to the cortical plate and begin to establish intrinsic and extrinsic connections around gestational week 20 (Chi et al., 1977; Marin-Padilla, 1992). This occurs with precise timing and is dependent upon a delicate balance of genetic regulation (Rakic, 1995). The connections formed during the pre-natal period eventually experience a dramatic period of plasticity in the pre-adolescent years during which pruning occurs (Lewis, 1997). Van Essen hypothesized that, as the brain grows and expands, the strength of connectivity between neighboring areas of cerebral cortex determines its adult folding patterns such that strongly connected areas form gyri and weakly connected areas form sulci (Van Essen, 1997).
Schizophrenia is a devastating psychiatric illness characterized by positive and negative symptoms as well as severe cognitive impairments across multiple domains including language, memory and executive function. Abnormal cortical development has been thought to be part of the etiology of schizophrenia (Murray and Fearon, 1999; Weinberger, 1987, 1996). Although the pathophysiology of the disease is not yet understood, it has been hypothesized that abnormal structural and/or functional connectivity may lead to its symptoms and cognitive impairments (Elvevag and Weinberger, 2001; Friston, 2005; Friston, 1996, 1998, 1999; Friston and Frith, 1995; Weinberger, 1987). Given Van Essen's hypothesis, it is possible that maldevelopment of intrinsic or extrinsic connections of the cerebral cortex in schizophrenia might manifest as abnormalities of cortical folding patterns in adult patients.
Cortical folding abnormalities in schizophrenia measured using the gyrification index (GI) methodology (Zilles et al., 1988) have been reported in post-mortem (Vogeley et al., 2000) and MRI studies (Harris et al., 2004a; Harris et al., 2004b; Kulynych et al., 1997; Sallet et al., 2003). Since the GI measures the ratio of inner and outer cortical surface contours, these studies were limited to analyses of brain slices in different lobes. Cortical folding abnormalities of asymmetry, complexity and variability in the frontal, temporal and parietal lobes of schizophrenia patients have been reported in MRI studies by Narr and colleagues (Narr et al., 2001; Narr et al., 2004). Using cortical surface matching based on major sulcus landmarks, Narr and colleagues found greater variability and deviations of gyral complexity asymmetry in the frontal areas and significant gyral asymmetries in the temporal and parietal regions of schizophrenia patients. All these studies motivated the present study to examine cortical folding within specific, functionally relevant areas of cortex.
We used the Freesurfer cortical surface-based reconstruction methodology (Dale and Sereno, 1993; Dale et al., 1999; Fischl et al., 2001; Fischl et al., 1999a; Fischl et al., 1999b; Segonne et al., 2004; Segonne et al., 2005) to examine cortical folding displacement and convolution, together, in patients with schizophrenia compared to healthy demographically-matched controls. The entire cortical surface of each individual participant was unfolded and the degree of cortical folding was examined within three selected prefrontal and temporal anatomical cortical ROI's: (1) the left inferior frontal gyrus [Broca's area, comprised of the pars orbitalis (pOr), pars triangularis (pTr) and pars opercularis (pOp)], and the corresponding regions in the right hemisphere, (2) the right and left dorsolateral prefrontal cortices (DLPFC) and (3) the right and left superior temporal gyri (STG). These cortical regions were selected on the basis of previous morphometric studies that have demonstrated reduced cortical thickness (Kuperberg et al., 2003) and volume (Shenton et al., 2001) in these regions in patients relative to controls, and on the basis of previous fMRI studies reporting that they show abnormal functional modulation and/or functional connectivity in patients relative to controls (Callicott et al., 2000; Jennings et al., 1998; Kuperberg et al., 2006b; Manoach et al., 2000; Manoach et al., 1999; Shenton et al., 1992).
The degree of cortical folding was operationalized as the degree of displacement (linear and/or rotational shift) and convolution (complexity of gyrification) of the cortical surface relative to an average template. The template was independently created from the cortical surfaces of a third group of 40 healthy participants (Desikan et al., 2006; Duvernoy, 1991; Ono et al., 1990). Displacement and convolution, together, were indirectly measured from the metric distortion as calculated by the Jacobian energy functional (Fischl et al., 1999a; Fischl et al., 1999b) required to align the cortical surface of of an individual to the template. We expected cortical surfaces for all individuals to undergo some metric distortion as each unique surface was mapped onto the template, and hypothesized that the degree of metric distortion would be greater in schizophrenia patients than control subjects within each of the a priori ROI's.
2. Experimental Methods
2.1. Participants
Written informed consent was obtained from all subjects before participation according to the established guidelines of the Massachusetts General Hospital Institutional Review Board and the Health Insurance Portability and Accountability Act (HIPAA) guidelines.
Twenty-five healthy volunteers (20 male, 5 female) with no history of neurological or psychiatric disorder were recruited by advertisement and 26 patients (22 male, 4 female) diagnosed with schizophrenia according to DSM-IV criteria (American Psychiatric Association 1990) and on a stable regimen of antipsychotic medication for at least 6 months were recruited from the Erich Lindemann Mental Health Center (Boston, MA). Handedness was measured using the modified Edinburgh Handedness Inventory (Oldfield, 1971; White and Ashton, 1976). Patients and controls were gender, age and handedness matched (see Table 1). Exclusion criteria included neurological disease or damage, head trauma with documented cognitive impairment or loss of consciousness greater than 5 minutes, medical disorders that could (American Psychiatric Association 1990) impair neurocognitive function, a history of substance abuse within three months prior to participation and specific exclusions for MRI.
Table 1.
Subject demographics. Subjects were gender, age and handedness matched. Data are expressed as mean (SEM) unless otherwise indicated. Abbreviations: males (M), females (F), right-handed (R), left-handed (L), Caucasian (C), African American (AA), Asian American (AsA), American Indian (AI), Mixed (M), Chlorpromazine (CPZ).
| Parameter | Controls (n = 25)* | Patients (n = 26)# |
|---|---|---|
| Age (years) | 45.68 (1.19) | 45.69 (1.32) |
| Age of onset (years) | 24.25 (2.22) | |
| Duration of illness (years) | 20.00 (2.28) | |
| Gender | 20 M, 5 F | 22 M, 4 F |
| Handedness‡ | 23 R, 2 L | 21 R, 5 L |
| Race/Ethnicity | 22 C, 1 AA, 1 AsA, 1 M | 17 C, 8 AA, 1 AI |
| Psychiatric Rating§ | ||
| PANSS Positive | 13.22 (1.19) | |
| PANSS Negative | 17.44 (1.67) | |
| PANSS General | 25.22 (1.45) | |
| PANSS Global | 55.89 (3.81) | |
| Medications | ||
| CPZ Equivalent | 608.80 (75.91) | |
| Anticholinergic | 1/26 | |
| Anti-anxiety | 5/26 | |
| Anti-depression | 9/26 | |
| Mood stabilizer | 2/26 | |
| Other | 7/26 |
Four subjects who did not complete the study are excluded from this count.
One subject who did not complete the study is excluded from this count.
Measured using the Edinburgh Handedness Inventory.
Data available from 18 (16 M, 2F) schizophrenic patients.
2.2. MRI acquisition
We acquired two data sets of T1-weighted MPRAGE images as part of a larger set of structural data that were acquired during a 1.5 hour scan session. The MPRAGE protocol was run in a Siemens 3T Trio scanner (Siemens Medical Solutions, Erlangen, Germany) using an 8-channel phased-array head coil: TR/TE/TI = 2530/3.45/1100 ms; flip angle = 7°; FOV = 256 mm; 1.3×1.0×1.3 voxels; 128 slices in the sagittal plane. Images covered the entire brain. Motion artifact was monitored throughout the scan session. The protocol for any image data set that exhibited excessive motion was re-run after verbally informing the participants.
2.3. Image reconstruction
Individual cortical surfaces were reconstructed using the Freesurfer analyses tools (http://surfer.nmr.mgh.harvard.edu) developed at the Martinos Center for Biomedical Imaging as described in detail previously (Dale and Sereno, 1993; Dale et al., 1999; Fischl et al., 2001; Fischl et al., 1999a; Fischl et al., 1999b; Segonne et al., 2004; Segonne et al., 2005). First, image intensity variations due to magnetic field inhomogeneities were normalized. Second, extra-cerebral voxels were removed, essentially “skull-stripping” the brain. Third, the resulting intensity normalized, skull-stripped brain images were segmented to classify voxels into gray and white matter regions. Fourth, cutting planes were computed to separate the cerebral hemispheres from each other and from the brainstem and cerebellum. Finally, the resulting hemisphere volumes were covered with a triangular tessellation and deformed to produce a representation of the gray/white matter interface for each subject. Any geometric inaccuracies were corrected manually and the reconstruction procedure was repeated until an accurate surface was achieved. The gray/white matter interface surface was inflated and transformed into a spherical representation (Figure 1).
Figure 1.
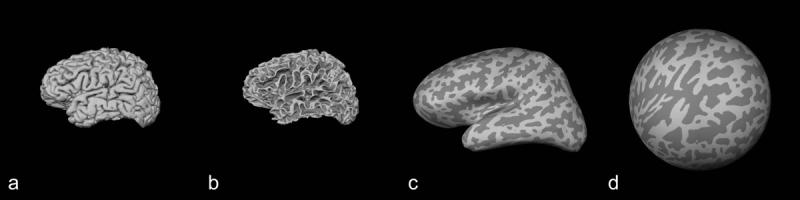
Cortical reconstruction. A given subject brain represented here by a 3D pial surface reconstruction (a) was transformed into gray matter/white matter interface surface (b), inflated (c) and transformed again into a spherical surface (d). Gyri and sulci are represented in light gray and dark gray colors, respectively.
2.4. Measuring metric distortion
Each individual subject's spheres were non-linearly registered to an average template sphere created from 40 independent control subjects (see example in Figure 2) (Fischl et al., 1999a). A spherical coordinate system based on the average template was translated back to the subject spheres such that every coordinate corresponded across all subjects, and thus every participant's brain was registered into a common coordinate system.
Figure 2.
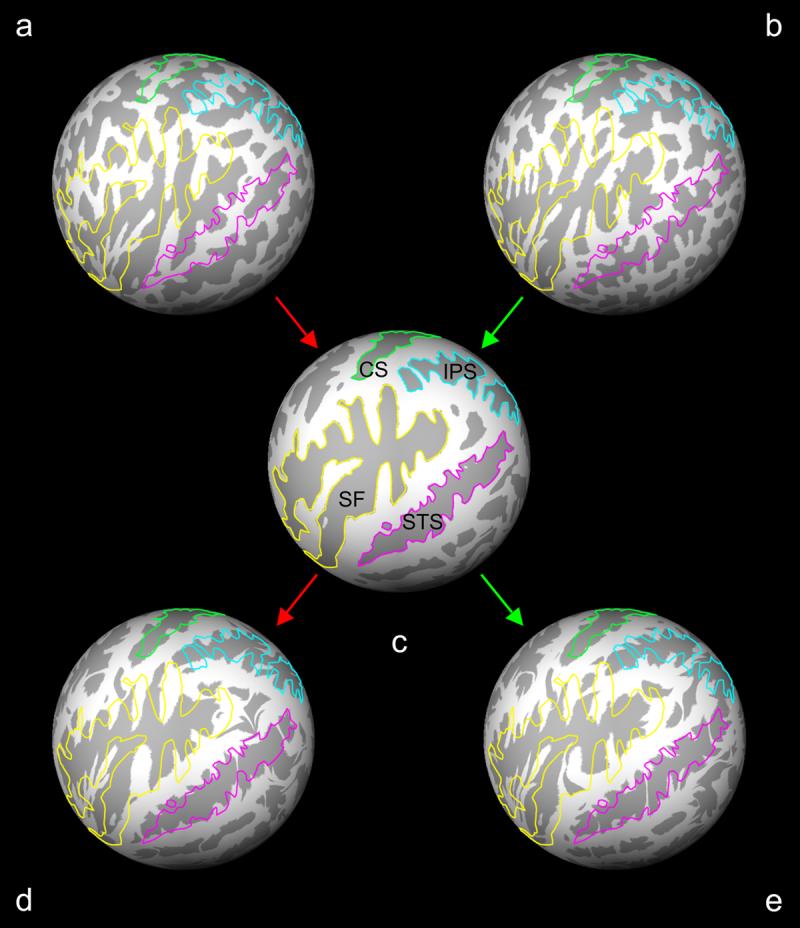
Spherical registration and metric distortion. Spheres (a, d) and (b, e) are reconstructed brain surfaces from a control subject and a schizophrenia patient, respectively. A subject sphere was registered to the average subject template sphere (c) using a non-linear warping algorithm. The metric distortion required to correctly align gyri (light gray) and sulci (dark gray) was a direct reflection of the cortical folding distortions and convolutions between a subject sphere and the template average. Outlines of the central sulcus (CS, light green), intraparietal sulcus (IPS, light blue), Sylvian fissure (SF, yellow) and superior temporal sulcus (STS, magenta) based on the average subject template sphere (c) are overlaid on the subject spheres before and after registration to illustrate the process.
In order to achieve the registration of individual spheres into the common coordinate system, the triangular tessellation used to represent the gray/white matter interface and its spherical representation was necessarily deformed. The deformation of a particular tessellation vertex was its metric distortion, which was calculated using the following general equation:
We measured the metric distortion required to achieve optimal spherical registration of gyri and sulci (at each tesselation vertex) across individual cortical surfaces (Fischl et al., 1999b). We restricted statistical analysis within three ROI's that were generated on each subject's gray/white matter interface surface during the Freesurfer reconstruction stream (see Figure 3). [These ROI's were validated previously (Desikan et al., 2006; Duvernoy, 1991; Ono et al., 1990)]. The ROI's included: (1) The inferior frontal gyri [pars orbitalis (pOr), pars triangularis (pTr) and pars opercularis (pOp) gyri, comprising Broca's area on the left] operationally defined as the three adjacent gyri rostral to the precentral gyrus (Desikan et al., 2006; Duvernoy, 1991; Ono et al., 1990); (2) bilateral dorsolateral prefrontal cortices (DLPFC), operationally defined as the middle third of the middle frontal gyrus (Rajkowska and Goldman-Rakic, 1995); (3) bilateral superior temporal gyri (STG) operationally defined as the gyrus surrounded by the Sylvian fissure, superior temporal fissure and supramarginal gyrus (Desikan et al., 2006; Duvernoy, 1991; Ono et al., 1990).
Figure 3.
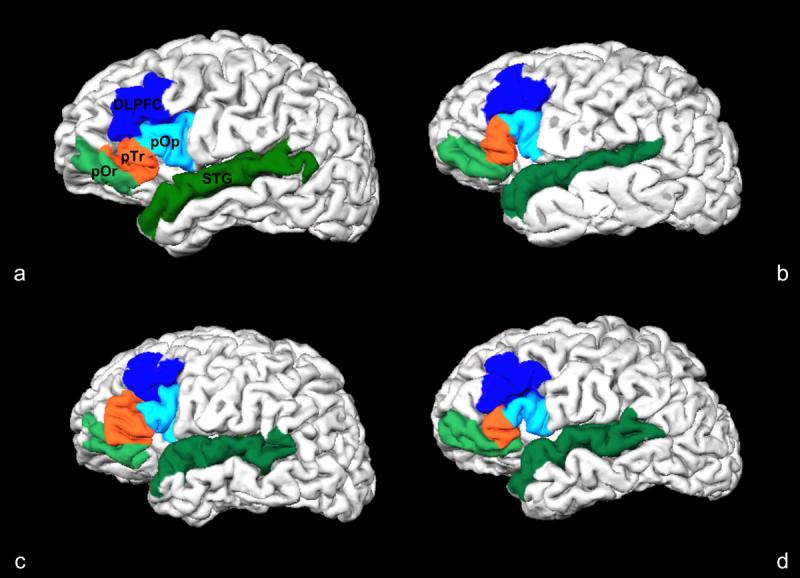
Cortical folding variability in Broca's area (pOr, pTr, pOp), dorsolateral prefrontal cortex (DLPFC) and superior temporal gyrus (STG). This illustrates the individual cortical folding variability of these ROI's between two control subjects (a, b) and two schizophrenia patients (c, d) in a pial surface reconstruction of brain image volumes. In the spherical registration of these subjects to an average subject template, the metric distortion required for optimal alignment is a reflection individual cortical displacement and convolution.
2.5. Statistical analysis of metric distortion
We used a General Linear Model (GLM) to calculate cohort differences in metric distortion at each tesselation vertex within the a priori ROI's (inferior frontal gyrus, DLPFC, STG) of both hemispheres. Monte Carlo permutation cluster analyses were then performed to correct for multiple comparisons using a cluster threshold of 0.0500. Finally, the mean metric distortion was calculated in any clusters that survived multiple comparisons analysis.
3. Results
In the left hemisphere, clusters that were significant (p < 0.05) before correction for multiple comparisons were located in pars triangularis (pTr) (maxima p = 0.0030) and pars operularis (pOp) (maxima p1 = 0.0039, p2 = 0.0481) (Table 2). No clusters were detected in the pars orbitalis (pOr), the left dorsolateral prefrontal cortex (L-DLPFC) or left superior temporal gyrus (L-STG). The only cluster surviving correction for multiple comparisons was located in pTr (630.18 mm2, clusterwise p = 0.0352). Metric distortion in this cluster was greater in schizophrenia patients relative to control subjects (Figure 4). Within the pTr cluster, we found the mean (SD) metric distortion in schizophrenia patients was 1.579 (0.269) and in control subjects was 1.377 (0.225).
Table 2.
Clusters of significant metric distortion between schizophrenia patients and control subjects. Maxima p-values for General Linear Model (GLM) analysis prior to Monte Carlo permutation correction for multiple comparisons correction are listed with corresponding clusterwise p-values after correction. Only the cluster located in the left pars triangularis (pTr) was found to be significant after multiple comparisons correction (bolded).
| a priori ROI | Distortion direction | Cluster Size (mm2) | Talairach Coordinates | Maxima p-value | Clusterwise p-value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Left Inferior Frontal Gyrus | |||||||
| Pars orbitalis | No clusters | ||||||
| Pars triangularis | Sz > C | 630.18 | −52.3 | 28.2 | 6.7 | 0.0030 | 0.0352 |
| Pars opercularis | Sz > C | 243.49 | −52.5 | 26.2 | 8.3 | 0.0039 | 0.1114 |
| Sz > C | 0.58 | −42.5 | 24.5 | 9.0 | 0.0481 | 0.3819 | |
| Right Inferior Frontal Gyrus | |||||||
| Pars orbitalis | C > Sz | 328.41 | 31.1 | 47.6 | −9.5 | 0.0071 | 0.0526 |
| Pars triangularis | Sz > C | 405.54 | 54.8 | 26.0 | 11.9 | 0.0041 | 0.0534 |
| Pars opercularis | Sz > C | 278.30 | 54.8 | 25.9 | 12.9 | 0.0042 | 0.0860 |
Figure 4.
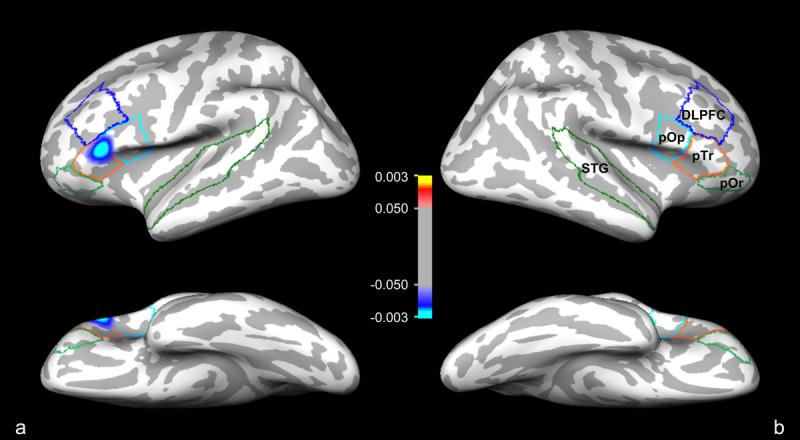
Composite surface representation of statistically significant clusters of metric distortion in the (a) left and (b) right hemispheres that survived multiple comparisons correction in the pars orbitals (pOr), pars triangulars (pTr), pars opercularis (pOp), dorsolateral prefrontal cortex (DLPFC) and superior temporal gyrus (STG) ROI's. One blue-cyan cluster in the left pTr survived multiple comparisons correction. Statistical analysis was performed using a General Linear Model (GLM) and corrected for multiple comparisons using the Monte Carlo permutation method. Color bar represents statistical significance for the maxima p-value of the coordinate vertices located within the significant clusters of metric distortion: control subjects > schizophrenia patients (0.1<p<0.003, red/yellow); schizophrenia patients > control subjects (0.1<p<0.003, blue/cyan). Refer to Table 2 for further details of the cluster in this figure and of the clusters that did not survive multiple comparisons correction.
In the right hemisphere, clusters that were significant before multiple comparisons correction were located in pOr (maxima p = 0.0071), pTr (maxima p = 0.0041) and pOp (maxima p = 0.0042) (Table 2). No clusters were found in the R-DLPFC or the R-STG. None of the clusters in the right hemisphere survived correction for multiple comparisons.
4. Discussion
4.1. Cortical development and schizophrenia
Previous studies have shown regional cortical folding abnormalities of gyrification (Harris et al., 2004a; Harris et al., 2004b; Kulynych et al., 1997; Sallet et al., 2003; Vogeley et al., 2000), asymmetry, complexity and variability (Narr et al., 2001; Narr et al., 2004) in patients with schizophrenia. These studies used a variety of methods to compare the spatial distribution of entire gyri and sulci. Their results motivated the present study to measure metric distortion at each point on the tessellated surface of the left inferior frontal gyrus, dorsolateral prefrontal cortex (DLPFC) and superior temporal gyrus, thereby providing an analysis of folding abnormalities within these cortical areas.
We have thus demonstrated an area within the left pars triangularis (pTr) of Broca's area in which metric distortion was significantly greater in schizophrenia patients relative to healthy, demographically-matched control participants. In the spherical registration algorithm of the Freesurfer analysis tools, the energy functional to generate accurate alignment includes a term for minimizing metric distortion while optimally aligning sulci and gyri (Fischl et al., 1999a; Fischl et al., 1999b). The magnitude of metric distortion, therefore, is an indirect measurement of cortical displacement and convolution, together, relative to the average subject template.
The cerebral cortex undergoes marked changes in its intrinsic and extrinsic connectivity over the life span (Chi et al., 1977; Lewis, 1997; Marin-Padilla, 1992; Rakic, 1995). Broca's area, specifically, is known to undergo substantial plasticity from birth to adulthood (Amunts et al., 2003; Etchepareborda and Lopez-Lazaro, 2005). The genetic process controlling cortical development may contribute to the onset and expression of schizophrenia (Woolf, 1997; Wyatt, 1996).
Volume measurement has been used as another index for assessing the integrity of gray matter and white matter. Bartzokis and colleagues made an interesting observation of white matter volume increase with concomitant gray matter volume decrease between the second and fourth decades of life in the frontal lobes of normal aging subjects (Bartzokis et al., 2001). However, within the same age range, they found an absence of white matter volume expansion accompanying gray matter volume reduction in the frontal lobes of patients with schizophrenia (Bartzokis et al., 2003). These studies together suggested that normal white matter myelination with increasing age is abnormal in patients with schizophrenia. White matter atrophy in the frontal lobes has been noted in other recent studies (Sanfilipo et al., 2000; Wible et al., 2001), but the discrepancy may be due to not taking the normal aging process into account.
Volume changes due to normal aging and the disease process of schizophrenia may affect the relative geometric surface patterns of sulci and gyri. Although Kuperberg and colleagues showed cortical thinning in specific areas of the frontal lobe, including Broca's area (Kuperberg et al., 2003), these changes in gray matter volume are unlikely to alter the relative position of sulci or gyri, which are more likely determined by the geometry of the underlying white matter (Van Essen, 1997). Aberrant white matter development between the second and fourth decades of life in schizophrenia, as observed by Bartzokis and colleagues (Bartzokis et al., 2003), would contribute most to abnormal cortical folding patterns. After the fourth decade, however, degeneration of white matter due to normal aging would contribute more to changes in cortical geometry. In this study, we measured metric distortion during the fourth decade of life (mean age of subjects and patients was approximately 45 years, see Table 1), which would be between the time gray matter and white matter development would be ending and normal aging degenerative processes would be beginning. Therefore, the results presented here may reflect the disease process of schizophrenia before degeneration of normal aging.
4.2. Functional significance of abnormalities in Broca's area
The precise functional significance of cortical folding abnormalities in Broca's area in schizophrenia is unclear. However, given that abnormal cortical folding may reflect abnormal structural and/or functional cortical connectivity, we can speculate on potential functional links between cortical folding abnormalities in this area and the symptoms and patterns of cognitive dysfunction that characterize the schizophrenia syndrome.
In the healthy brain, the left inferior prefrontal gyrus is a major component of the circuitry mediating both language comprehension and language output. Its precise role is debated but it has been implicated in aspects of semantic processing including semantic encoding (Demb et al., 1995; Kirchhoff et al., 2000; Wagner et al., 1998), semantic priming (Copland et al., 2003; Giesbrecht et al., 2004; Kotz et al., 2002; Kuperberg et al., 2006b; Matsumoto et al., 2005; Wheatley et al., 2005), controlled semantic retrieval (Wagner et al., 2001), and semantic selection (Fletcher et al., 2000; Moss et al., 2005; Thompson-Schill et al., 1997; Thompson-Schill et al., 1999). Broca's area has also been implicated in phonological processing (Poldrack et al., 1999), aspects of syntactic processing (Caplan, 2001; Caplan et al., 2000), syntactic working memory (Fiebach et al., 2005) and, more generally, in other aspects of verbal working memory function (Barde and Thompson-Schill, 2002), and in speech output (Blank et al., 2002).
Schizophrenia has long been characterized as a disorder of thought, language and communication (Andreasen, 1979; Bleuler, 1911/1950). Many of its core symptoms including verbal hallucinations and positive thought disorder with disorganized speech are characterized by language dysfunction (American Psychiatric Association 1990). Cognitively, language deficits have been identified in children at risk for schizophrenia (Cannon et al., 2002; Fuller et al., 2002; Ott et al., 2001) as well as in patients in their first episode of psychosis (Fuller et al., 2002; Hoff et al., 1999). Numerous neuropsychological and psycholinguistic studies have established that patients with schizophrenia show selective language abnormalities as they monitor the source of verbal input (Ditman and Kuperberg, 2005; Johns et al., 2001), explicitly or implicitly retrieve information from semantic memory [e.g (Goldberg et al., 1998; Minzenberg et al., 2002)], select the appropriate meaning of semantically ambiguous words (Sitnikova et al., 2002; Titone et al., 2000), detect linguistic anomalies within sentences (Kuperberg et al., 2006a; Kuperberg et al., 1998; Kuperberg et al., 2000; Kuperberg et al., 2006b) and parse syntactically complex sentences (Condray et al., 1996) [for reviews, see (Kuperberg and Caplan, 2003; Kuperberg and Goldberg, 2006)]. Functional imaging studies report both abnormal increases and decreases in the recruitment of the left inferior prefrontal gyrus in schizophrenia during semantic tasks including encoding (Kubicki et al., 2003; Ragland et al., 2004), retrieval (Weiss et al., 2003) and priming (Kuperberg et al., 2007), although abnormal function in this region is not usually seen in isolation, but rather in association with abnormal modulation of other language regions including superior, inferior and medial temporal cortices.
It is possible that localized abnormalities in folding within Broca's area reflect abnormal cortical connectivity in this region and that this, in turn, leads to widespread cortical dysfunction during language processing in schizophrenia and to the clinical language disturbances that characterize this disorder. However, the current sample size was relatively small and, at uncorrected thresholds, other regions including the inferior parietal lobule and frontopolar region (not reported here) also showed abnormal folding. Future studies with larger samples will determine the degree to which cortical folding abnormalities are localized or generalized across the cortical ribbon, and whether these abnormalities predict the severity of specific symptoms or specific cognitive deficits in schizophrenia.
4.3. Caveats
The present study included 26 patients and 25 demographically-matched healthy controls, a sample size that would be adequate for statistical analysis of gross morphological changes. However, we measured cohort differences in metric distortion at several thousand tessellation vertices, even within a priori ROI's. Table 2 illustrates the stringent statistical analysis performed to avoid false-positive results. The most plausible solution leading to the discovery of more clusters of significant metric distortion that might be expected in other areas of the cortex responsible for language would be to add many more subjects.
The sample of patients for this study was limited to chronic, medicated patients. The present study cannot account for whether medication might also contribute to the metric distortions associated with the disease. An opportunity to measure metric distortion in non-medicated patients would be valuable to pursue.
5. Acknowledgements
Support for this research was provided in part by the Athinoula A. Martinos Foundation, GlaxoSmithKline, the National Cancer Institute (5T32 CA09502), the National Institute for Mental Health (RO1 MH071635, R01 MH067720), the National Center for Research Resources (P41-RR14075, R01 RR16594-01A1 and the NCRR BIRN Morphometric Project BIRN002, U24 RR021382), the National Institute for Biomedical Imaging and Bioengineering (R01 EB001550), the National Institute for Neurological Disorders and Stroke (R01 NS052585-01) as well as the Mental Illness and Neuroscience Discovery (MIND) Institute. Dr. Fischl is also part of the National Alliance for Medical Image Computing (NAMIC), which is funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant (U54 EB005149). Information on the National Centers for Biomedical Computing can be obtained from http://nihroadmap.nih.gov/bioinformatics.
The authors would like to thank Mary Foley, Michael Zussman Christine Portal and Stuart Wallace for their invaluable assistance with the imaging sessions, recruitment and management of patients. We would like to thank Jenni Pacheco for her helpful advice with the cortical surface reconstructions. Finally, we would like to thank Dr. Doug Greve for his statistical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- American Psychiatric Association . DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th Rev ed. American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- Amunts K, Schleicher A, Ditterich A, Zilles K. Broca's region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Thought, language and communication disorders: I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36:1315–1321. doi: 10.1001/archpsyc.1979.01780120045006. [DOI] [PubMed] [Google Scholar]
- Barde LH, Thompson-Schill SL. Models of functional organization of the lateral prefrontal cortex in verbal working memory: evidence in favor of the process model. J Cogn Neurosci. 2002;14:1054–1063. doi: 10.1162/089892902320474508. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 2003;53:412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJ. Speech production: Wernicke, Broca and beyond. Brain. 2002;125:1829–1838. doi: 10.1093/brain/awf191. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox, or the group of schizophrenias. International Universities Press; New York: 19111950. [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Caplan D. Positron Emission Tomographic Studies of Syntactic Processing. In: Shapiro L, Grodzinsky Y, Shapiro D, editors. The Neurology of Language. Academic Press; 2001. Academic Press. [Google Scholar]
- Caplan D, Alpert N, Waters G, Olivieri A. Activation of Broca's area by syntactic processing under conditions of concurrent articulation. Hum Brain Mapp. 2000;9:65–71. doi: 10.1002/(SICI)1097-0193(200002)9:2<65::AID-HBM1>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Condray R, Steinhauer SR, van Kammen DP, Kasparek A. Working memory capacity predicts language comprehension in schizophrenic patients. Schizophr Res. 1996;20:1–13. doi: 10.1016/0920-9964(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenery HJ. Brain activity during automatic semantic priming revealed by event-related functional magnetic resonance imaging. Neuroimage. 2003;20:302–310. doi: 10.1016/s1053-8119(03)00279-9. [DOI] [PubMed] [Google Scholar]
- Dale A, Sereno M. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ditman T, Kuperberg GR. A source-monitoring account of auditory verbal hallucinations in patients with schizophrenia. Harv Rev Psychiatry. 2005;13:280–299. doi: 10.1080/10673220500326391. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The Human Brain. Springer-Verlag; Vienna: 1991. [Google Scholar]
- Elvevag B, Weinberger DR. The neuropsychology of schizophrenia and its relationship to the neurodevelopmental model. The MIT Press; Cambridge, MA: 2001. [Google Scholar]
- Etchepareborda MC, Lopez-Lazaro MJ. [Cytoarchitectural structure of the areas of language.] Rev Neurol. 2005:S103–106. [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca's area in sentence processing: syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. “Sculpting the response space”--an account of left prefrontal activation at encoding. Neuroimage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Friston K. Disconnection and cognitive dysmetria in schizophrenia. Am J Psychiatry. 2005;162:429–432. doi: 10.1176/appi.ajp.162.3.429. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Theoretical neurobiology and schizophrenia. Br Med Bull. 1996;52:644–655. doi: 10.1093/oxfordjournals.bmb.a011573. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O'Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Camblin CC, Swaab TY. Separable effects of semantic priming and imageability on word processing in human cortex. Cerebral Cortex. 2004;14:521–529. doi: 10.1093/cercor/bhh014. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Aloia MS, Gourovitch ML, Missar D, Pickar D, Weinberger DR. Cognitive substrates of thought disorder, I: the semantic system. Am J Psychiatry. 1998;155:1671–1676. doi: 10.1176/ajp.155.12.1671. [DOI] [PubMed] [Google Scholar]
- Harris JM, Whalley H, Yates S, Miller P, Johnstone EC, Lawrie SM. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biol Psychiatry. 2004a;56:182–189. doi: 10.1016/j.biopsych.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Harris JM, Yates S, Miller P, Best JJ, Johnstone EC, Lawrie SM. Gyrification in first-episode schizophrenia: a morphometric study. Biol Psychiatry. 2004b;55:141–147. doi: 10.1016/s0006-3223(03)00789-3. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal neuropsychological follow-up study of patients with first- episode schizophrenia. Am J Psychiatry. 1999;156:1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- Jennings JM, McIntosh AR, Kapur S, Zipursky RB, Houle S. Functional network differences in schizophrenia: a rCBF study of semantic processing. Neuroreport. 1998;9:1697–1700. doi: 10.1097/00001756-199806010-00005. [DOI] [PubMed] [Google Scholar]
- Johns LC, Rossell S, Frith C, Ahmad F, Hemsley D, Kuipers E, McGuire PK. Verbal self-monitoring and auditory verbal hallucinations in patients with schizophrenia. Psychol Med. 2001;31:705–715. doi: 10.1017/s0033291701003774. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical-semantic network by auditory semantic priming: an event-related functional MRI study. Neuroimage. 2002;17:1761–1772. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Nestor PG, Huh T, Kikinis R, Shenton ME, Wible CG. An fMRI study of semantic processing in men with schizophrenia. Neuroimage. 2003;20:1923–1933. doi: 10.1016/s1053-8119(03)00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulynych JJ, Luevano LF, Jones DW, Weinberger DR. Cortical abnormality in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry. 1997;41:995–999. doi: 10.1016/S0006-3223(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Kuperberg G, Caplan D. Language dysfunction in schizophrenia. In: Schiffer RB, Rao SM, Fogel BS, editors. Neuropsychiatry. Lippincott Williams and Wilkins; Philadelphia: 2003. pp. 444–466. [Google Scholar]
- Kuperberg G, Deckersbach T, Holt D, Goff D, West WC. Increased temporal and prefrontal activity to semantic associations in schizophrenia. Archives of General Psychiatry. 2007:138–151. doi: 10.1001/archpsyc.64.2.138. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Goldberg T. Insights into semantics and language in schizophrenia. In: Wood S, Allen N, Pantelis C, editors. Handbook of Neuropsychology of Mental Illness. Cambridge University Press; 2006. [Google Scholar]
- Kuperberg GR, Kreher DA, Goff D, McGuire P, David A. Building up linguistic context in schizophrenia: Evidence from self-paced reading. Neuropsychology. 2006a:442–452. doi: 10.1037/0894-4105.20.4.442. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, David A. Reduced sensitivity to linguistic context in schizophrenic thought disorder: Evidence from online monitoring for words in linguistically-anomalous sentences. J Abnorm Psychol. 1998;107:423–434. doi: 10.1037//0021-843x.107.3.423. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, David AS. Sensitivity to linguistic anomalies in spoken sentences: a case study approach to understanding thought disorder in schizophrenia. Psychol Med. 2000;30:345–357. doi: 10.1017/s0033291700001744. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Goff D, Holcomb PJ. Making sense of sentences in schizophrenia: electrophysiological evidence for abnormal interactions between semantic and syntactic processing. Journal of Abnorm Psychol. 2006b;115:243–256. doi: 10.1037/0021-843X.115.2.251. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory. J Comp Neurol. 1992;321:223–240. doi: 10.1002/cne.903210205. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Iidaka T, Haneda K, Okada T, Sadato N. Linking semantic priming effect in functional MRI and event-related potentials. Neuroimage. 2005;24:624–634. doi: 10.1016/j.neuroimage.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Ober BA, Vinogradov S. Semantic priming in schizophrenia: a review and synthesis. Journal of the International Neuropsychological Society. 2002;8:699–720. doi: 10.1017/s1355617702801357. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, Tyler LK. Selecting among competing alternatives: selection and retrieval in the left inferior frontal gyrus. Cereb Cortex. 2005;15:1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Fearon P. The developmental 'risk factor' model of schizophrenia. J Psychiatr Res. 1999;33:497–499. doi: 10.1016/s0022-3956(99)00032-1. [DOI] [PubMed] [Google Scholar]
- Narr K, Thompson P, Sharma T, Moussai J, Zoumalan C, Rayman J, Toga A. Three-dimensional mapping of gyral shape and cortical surface asymmetries in schizophrenia: gender effects. Am J Psychiatry. 2001;158:244–255. doi: 10.1176/appi.ajp.158.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Kim S, Thompson PM, Szeszko P, Robinson D, Luders E, Toga AW. Abnormal gyral complexity in first-episode schizophrenia. Biol Psychiatry. 2004;55:859–867. doi: 10.1016/j.biopsych.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the Cerebral Sulci. Thieme Medical Publishers; New York: 1990. [Google Scholar]
- Ott SL, Allen J, Erlenmeyer-Kimling L. The New York High-Risk Project: observations on the rating of early manifestations of schizophrenia. Am J Med Genet. 2001;105:25–27. [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. J Abnorm Psychol. 2004;161:1004–1015. doi: 10.1176/appi.ajp.161.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Sallet PC, Elkis H, Alves TM, Oliveira JR, Sassi E, Campi de Castro C, Busatto GF, Gattaz WF. Reduced cortical folding in schizophrenia: an MRI morphometric study. Am J Psychiatry. 2003;160:1606–1613. doi: 10.1176/appi.ajp.160.9.1606. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000;57:471–480. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Grimson E, Fischl B. Genetic algorithm for the topology correction of cortical surfaces. IPMI. 2005:393–405. doi: 10.1007/11505730_33. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Sitnikova T, Salisbury DF, Kuperberg G, Holcomb PI. Electrophysiological insights into language processing in schizophrenia. Psychophysiology. 2002;39:851–860. doi: 10.1111/1469-8986.3960851. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Titone D, Levy DL, Holzman PS. Contextual insensitivity in schizophrenic language processing: evidence from lexical ambiguity. J Abnorm Psychol. 2000;109:761–767. doi: 10.1037//0021-843x.109.4.761. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Schneider-Axmann T, Pfeiffer U, Tepest R, Bayer TA, Bogerts B, Honer WG, Falkai P. Disturbed gyrification of the prefrontal region in male schizophrenic patients: A morphometric postmortem study. Am J Psychiatry. 2000;157:34–39. doi: 10.1176/ajp.157.1.34. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale A, Rosen B, Buckner RL. Building memories: remembering and forgetting verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14:1S–11S. doi: 10.1016/0893-133X(95)00199-N. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Schacter DL, Goff D, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A. Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cogn Neurosci. 2005;17:1871–1885. doi: 10.1162/089892905775008689. [DOI] [PubMed] [Google Scholar]
- White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, Levitt JJ, O'Donnell BF, Kikinis R, Jolesz FA, McCarley RW. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res. 2001;108:65–78. doi: 10.1016/s0925-4927(01)00109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CM. Does the genotype for schizophrenia often remain unexpressed because of canalization and stochastic events during development? Psychol Med. 1997;27:659–668. doi: 10.1017/s003329179700473x. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ. Neurodevelopmental abnormalities and schizophrenia. A family affair. Arch Gen Psychiatry. 1996;53:11–15. doi: 10.1001/archpsyc.1996.01830010013003. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl) 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]


