Abstract
The dynamic modification of nuclear and cytoplasmic proteins with O-linked β-N-acetylglucosamine (O-GlcNAc) by the O-linked N-acetylglucosaminyltransferase (OGT) is a regulatory post-translational modification that is responsive to various stimuli. Here, we demonstrate that OGT is a central factor for T- and B-lymphocytes activation. SiRNA-mediated knockdown of OGT in T cells leads to an impaired activation of the transcription factors NFAT and NFκB. This results in a reduction of IL-2 production consistent with prevention of T-cell activation. OGT is also required for the early activation of B cells mediated by stimulation of the B-cell receptor. Mechanistically, we demonstrate that NFκB as well as NFAT are glycosylated with O-GlcNAc after direct binding to OGT. Moreover, kinetic experiments show that O-GlcNAc modification prominently increased shortly after activation of lymphoid cells and it might be required for nuclear translocation of the transcription factors NFκB and NFAT.
Keywords: lymphocytes activation, OGT, O-GlcNAc
Introduction
O-linked N-acetylglucosaminyltransferase (OGT) is a glycosyltransferase that catalyzes the addition of a single β-N-acetylglucosamine (GlcNAc) to Ser/Thr residues of various nuclear and cytoplasmic proteins (Holt et al, 1987; Kreppel et al, 1997; Lazarus et al, 2006). The proteins targeted by O-linked β-N-acetylglucosamine (O-GlcNAc)-modification can be divided into groups according to their function. The main group includes proteins involved in transcription and translation (Love and Hanover, 2005). Accumulating evidence suggests that the O-GlcNAc modification of proteins plays a key role in regulating protein activity. In some cases, the site of O-GlcNAc and O-phosphate (Ser/Thr phosphorylation) was mapped to the same residues leading to the hypothesis of reciprocal regulation and competition of both modifications (Kelly et al, 1993; Chou et al, 1995; Medina et al, 1998; Cheng et al, 2000; Cheng and Hart, 2001; Federici et al, 2002). An enzyme with antagonistic function to OGT, cytosolic β-N-acetylglucosaminidase (O-GlcNAse), has been identified and it is ubiquitously expressed in all tissues (Gao et al, 2001).
Different splice variants of OGT are known, but their biochemical properties are not well characterized (Shafi et al, 2000). Knockout mice for OGT are embryonically lethal (Shafi et al, 2000), suggesting a critical function during mice development. Conditional knockout of OGT in T cells resulted in an increase of apoptotic T cells (O'Donnell et al, 2004). One study shows that the activation of lymphocytes with ConA or PMA/ionomycin leads to a rapid increase in nuclear and cytoplasmic glycoproteins (Kearse and Hart, 1991). Still only limited information is available on the role of O-GlcNAc modifications in immune cells.
In T cells, specific systems downstream of the T-cell receptor (TCR) complex lead to the activation of transcription factors that regulate a variety of activation-associated genes. Among these genes are cytokines and surface receptors that play an important role in coordinating the immune response (Crabtree and Clipstone, 1994). A proximal consequence of the engagement of TCR is the activation of PLCγ, which catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate in inositol 1,4,5-triphosphate and diacylglycerol. Whereas the latter mediates activation of the PKC family members and the Ras-MAPK pathway, inositol 1,4,5-triphosphate promotes an increase of intracellular Ca2+ concentration (Dower et al, 2000; Baier, 2003). The increase of Ca2+ activates calcineurin, a Ca2+/calmodulin-dependent protein phosphatase, which dephosphorylates the transcription factor NFAT, allowing its nuclear translocation (Dower et al, 2000). In activated T cells, NFAT is a critical factor for transcriptional induction of IL-2 as well as other genes, for example, the cytokines IL-4, IFNγ and TNFα (Wang et al, 1996; Kiani et al, 2001; Monticelli and Rao, 2002). The NFAT family of transcription factors includes NFAT1, NFAT2, NFAT3 and NFAT4. NFAT1 and NFAT2 are preferentially expressed in peripheral T cells (Lyakh et al, 1997; Masuda et al, 1998).
The transcription factor NFκB regulates the expression of genes crucial for innate and adaptive immune responses, cell growth and apoptosis (Ghosh and Karin, 2002). The NFκB dimer is retained in the cytosol by interaction with the inhibitor of κB protein (IκB) complex. Its nuclear translocation can be induced by a wide variety of stimuli, for example, TCR or B-cell receptor (BCR) activation (Hayden and Ghosh, 2004). TCR or BCR ligation leads to activation of the IκB kinase (IKK) complex, which consists of two catalytic subunits, IKKα and IKKβ, as well as a regulatory subunit, IKKγ/NEMO. Upon IκB phosphorylation, IκBs are degraded in a ubiquitin-dependent manner, allowing the nuclear translocation of NFκB (Hayden and Ghosh, 2004).
Here, we show that NFκB and NFAT are O-GlcNAc-modified by OGT by direct interaction. A large increase of O-GlcNAc-modified NFAT was observed shortly after T-/B-cell activation, which correlated well with its translocation to the nucleus. Moreover, silencing of OGT results in impaired activation of T and B lymphocytes. Overexpression of OGT as well as the use of inhibitors of the antagonistic enzyme O-GlcNAcase sensitized T and B cells toward activation.
Results
SiRNA-mediated downregulation of OGT results in impaired IL-2 production and reduced CD69 surface expression in T cells
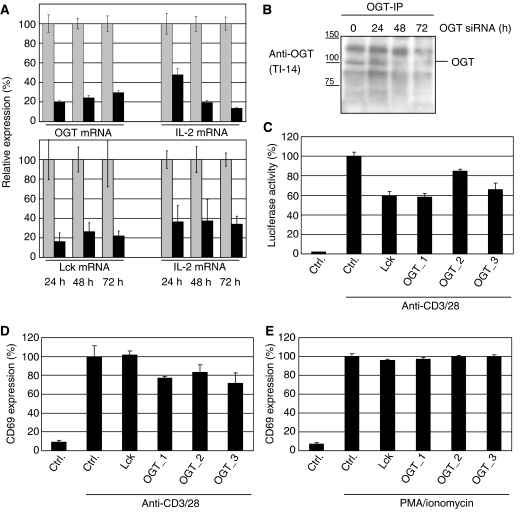
To analyze the role of OGT on TCR signaling, we took advantage of the siRNA technology to downregulate lymphocyte protein tyrosine kinase (Lck) as positive control or OGT. The efficiency of siRNA-mediated downregulation of OGT and Lck was analyzed using real-time PCR (Figure 1A). The amount of mRNA after treatment with siRNA (in percent) was compared with the amount of mRNA in cells transfected with a control siRNA (Alexa Fluor 488, QIAGEN) set as 100%. In parallel, the effect of the siRNA on the endogenous IL-2 transcription was measured as an indication of T-cell activation. OGT siRNA (OGT_1 was used in these experiments, but similar results were observed with OGT_2 and _3), control siRNA or siRNA specific for Lck were transfected into Jurkat cells. The cells were stimulated 24, 48 or 72 h after transfection with plate-bound anti-CD3/28 antibodies and mRNA was prepared. Real-time PCR experiments revealed efficient OGT downregulation, which was paralleled by markedly reduced IL-2 transcription (Figure 1A, upper panel); IL-2 mRNA was reduced up to 60–70%. Lck siRNA also showed efficient downregulation of the corresponding transcript and the mRNA for IL-2 (Figure 1A, lower panel). In summary, these data indicate that OGT affects TCR signaling, and downregulation of OGT results in impaired transcription of IL-2.
Figure 1.
SiRNA-mediated knockdown of OGT impairs early T-cell activation. (A) Jurkat cells were transfected with a negative control siRNA (Ctrl) or siRNAs specific for Lck or OGT (OGT_1). After transfection, the cells were transferred to fresh culture medium for 24, 48 and 72 h before stimulation with plate-bound anti-CD3 and anti-CD28 antibodies for 5.5 h and RNA was isolated. IL-2 mRNA as well as mRNAs for Lck or OGT were quantified by real-time PCR as described in Materials and methods. Experiments were performed in triplicates. Expression is given as the percentile of the amount of specific mRNA (black bars) compared to the amount in cells transfected with Ctrl (gray bars). (B) Jurkat cells were transfected as described in (A) and incubated for 24, 48 or 72 h prior lysis. OGT immunoprecipitations were performed and analyzed by Western blot using an antibody to OGT (TI-14). (C) Jurkat cells stably expressing a luciferase reporter plasmid under the control of the IL-2 promoter were transfected with a negative Ctrl or siRNAs specific for Lck or OGT (OGT_1, OGT_2, OGT_3). After transfection, the cells were transferred to fresh culture medium for 24 h before stimulation with plate-bound anti-CD3 and anti-28 antibodies for 5.5 h. Luciferase activity was quantified as described in Materials and methods. Experiments were performed in triplicates. Luciferase activity of the control transfection using nonsense siRNA is set to 100% and the luciferase activities after OGT and Lck downmodulation are given as percentage of the control. (D) Jurkat cells were treated as described in (C) and costimulated with plate-bound anti-CD3/28 antibodies for 16 h. After stimulation, CD69 surface expression was measured by Flow cytometry as described in Materials and methods. Experiments were performed in triplicates. For CD69 externalization, the control transfection using nonsense siRNA is set to 100% and the CD69 externalization after OGT and Lck downmodulation is given as percentage of the control. (E) Experiments were performed as described in panel (D), but cells were stimulated with PMA/Ionomycin. For CD69 externalization, the control transfection using nonsense siRNA is set to 100% and the CD69 externalization after OGT and Lck downmodulation is given as percentage of the control.
To visualize the siRNA-mediated downregulation of OGT at protein level, we immunoprecipitated OGT and analyzed the precipitates by Western blotting. Jurkat cells were transfected with siRNA specific for OGT and cultured for 24–72 h prior lysis and immunoprecipitation. Downregulation was detected already after 24 h of incubation and to a higher extent after 48 and 72 h (Figure 1B).
For further analysis of OGT's influence on T-cell activation, we took advantage of a luciferase reporter system that was under the control of the IL-2 promoter. Jurkat cells expressing the IL-2 reporter plasmid were transfected with either a control siRNA or siRNAs specific for Lck or OGT. The cells were incubated for 24 h to ensure downregulation of OGT by the siRNA. Transfection with a control siRNA did not affect TCR stimulation induced IL-2 promoter activity (Figure 1C). Lck silencing was again used as a positive control, since the well-established Lck plays an essential function in TCR signaling (Bijlmakers and Marsh, 2000). Silencing of Lck results in 40% decrease in activity of the IL-2 promoter. Similar results (up to 40% decrease of IL-2 promoter activity) were obtained for three independent siRNAs against OGT (Figure 1C), suggesting an important role of OGT in T-cell activation.
We further investigated the cell-surface expression of CD69, a marker of early T-cell activation. As visualized in Figure 1D, the downmodulation of OGT (three active siRNA for OGT were used) results in a reduced surface expression of CD69 upon TCR stimulation. CD69 surface expression upon stimulation with PMA/ionomycin (bypassing TCR signaling) was neither depending on OGT nor Lck (Figure 1E). In summary, our data suggest OGT as a critical factor involved in T-cell activation.
Modulation of OGT affects early activation of B and T lymphocytes
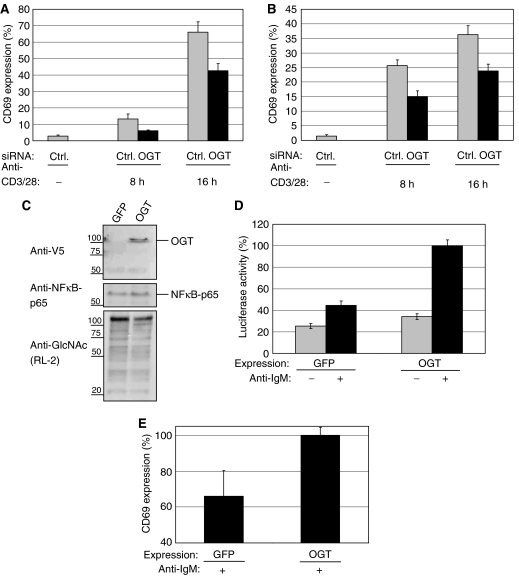
We examined if OGT also plays a critical role in the activation process of B lymphocytes. As for T cells, CD69 surface expression is a hallmark of early B-cell activation. Jurkat and BJAB cells were transfected with either a control siRNA or siRNA against OGT and cells were rested for 24 h. After TCR stimulation, Jurkat cells treated with OGT siRNA showed reduced surface expression of CD69 after 8 and 16 h of stimulation (Figure 2A). At 24 h after transfection, BJAB cells were stimulated with anti-IgM antibodies and like in T cells, a reduced surface expression of CD69 was detected (Figure 2B).
Figure 2.
OGT knockdown impairs, whereas overexpression sensitizes T-cell and B-cell toward early activation. (A) Jurkat cells were transfected either with a negative control siRNA (Ctrl) or siRNA specific for OGT (OGT_1). After transfection, the cells were transferred to fresh culture medium for 24 h before stimulation with plate-bound anti-CD3/28 antibodies for 8 or 16 h, respectively. After stimulation, CD69 surface expression was measured by Flow cytometry as described in Materials and methods. Experiments were performed in triplicates. CD69 externalization after OGT or Ctrl transfection is given as a percentage of the maximal externalization obtained by treating with PMA/ionomycin, set as 100%. (B) BJAB cells were either transfected with a negative Ctrl or siRNA specific for OGT (OGT_1). After transfection, the cells were transferred to fresh culture medium for 24 h before stimulation with anti-IgM antibodies for 8 or 16 h, respectively. After stimulation, CD69 surface expression was measured as in (A). (C) BJAB cells were transfected with plasmids encoding GFP as a control or OGT and transferred to fresh medium for 48 h. With an antibody to the V5 tag, total cellular lysates were analyzed for expression of OGT. NFκB-p65 was blotted as loading control. The overall amount of O-GlcNAc levels were blotted with antibodies to O-GlcNAc (RL-2). (D) BJAB cells were co-transfected with a luciferase reporter plasmid encoding a NFκB reporter together with either GFP (as a control) or full-length OGT. Cells were incubated for 24 h and stimulated with anti-IgM antibodies for additional 12 h (+) or left unstimulated (−). After lysis, the luciferase activity was measured as described in Materials and methods. Experiments were performed in triplicates. The maximal NFκB activity observed after OGT overexpression and anti-IgM stimulation was set to 100%. (E) BJAB cells were transfected with plasmids for the overexpression of GFP (as a control) or of the full-length OGT. Cells were transferred to fresh medium for 48 h and stimulated with anti-IgM antibodies for 12 h. The maximal CD69 externalization observed after OGT overexpression and anti-IgM stimulation was set to 100%.
In parallel, we evaluated the effect of overexpression of OGT. Cells were transfected using the Amaxa method with a plasmid containing the full-length OGT or GFP as a control, cultured for 48 h and then lysed. OGT overexpression was confirmed by Western blotting (Figure 2C). The lysates were also blotted for O-GlcNAc levels which are not increased upon overexpression of OGT, most likely because the levels of OGT's substrate UDP-N-acetylglucosamine remain unchanged.
We next examined whether overexpression of OGT might sensitize BJAB cells toward activation. As a readout for activation, we used a luciferase NFκB reporter assay. A plasmid encoding a luciferase gene under the control of NFκB promoter was cotransfected with OGT or GFP. The overexpression of OGT leads to an enhanced BCR-dependent activation of NFκB (Figure 2D). We also checked for increased CD69 surface externalization. Cells were transfected as described before and cultured for 48 h. After 48 h, cells were stimulated with anti-IgM antibodies. Overexpression of OGT leads to a higher CD69 surface externalization than control-transfected cells (Figure 2E).
Whereas the downregulation of OGT in lymphocytes leads to an impaired activation, the overexpression rather sensitizes lymphocytes toward activation. To analyze whether the effects of OGT are specific for lymphocytes, similar luciferase reporter gene assays as described in Figure 2D were performed in HEK293, COS-1 and CHO cells. In none of these three cell lines, the expression of OGT showed a significant influence on NFκB activity (data not shown).
The TCR-induced activation of the transcription factors NFAT and NFκB is impaired upon OGT downregulation
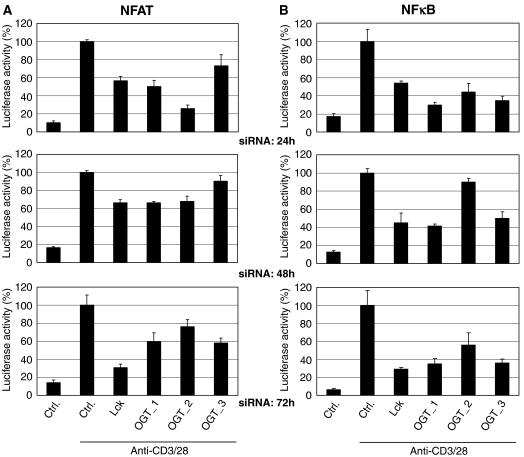
Among others, IL-2 mRNA transcription is dependent on the two transcription factors, NFAT and NFκB (Torgerson et al, 1998). To evaluate if the effect of OGT on IL-2 was dependent on NFAT and/or NFκB, Jurkat cells stably expressing a luciferase reporter plasmid for NFAT or NFκB were used. The cells were transfected with either a control siRNA or siRNAs for Lck or OGT. The cells were further cultured for 24, 48 or 72 h, respectively, to ensure optimal downregulation of Lck and OGT. After TCR stimulation of the cells, the activation of NFAT (Figure 3A) and NFκB (Figure 3B) was quantified by luciferase assay. The activity of both transcription factors upon TCR triggering was reduced by siRNA specific for Lck. Three independent siRNAs against OGT also led to impaired activation of NFAT (Figure 3A) and NFκB (Figure 3B).
Figure 3.
SiRNA-mediated knockdown of OGT impairs activation of NFAT and NFκB upon TCR stimulation. (A) Jurkat cells stably expressing a luciferase under the control of the NFAT promoter were transfected with either a negative control siRNA (Ctrl) or siRNAs specific for Lck or OGT (OGT_1, OGT_2, OGT_3). Cells were incubated for 24, 48 or 72 h and then costimulated with plate-bound anti-CD3/28 antibodies for 5.5 h. luciferase activity was quantified as described in Materials and methods. Experiments were performed in triplicates. (B) Jurkat cells stably expressing a luciferase under the control of the NFκB promoter were transfected with a negative Ctrl or siRNAs specific for Lck or OGT (OGT_1, OGT_2, OGT_3). Cells were treated as described in (A).
NFκB and NFAT are O-GlcNAc-modified
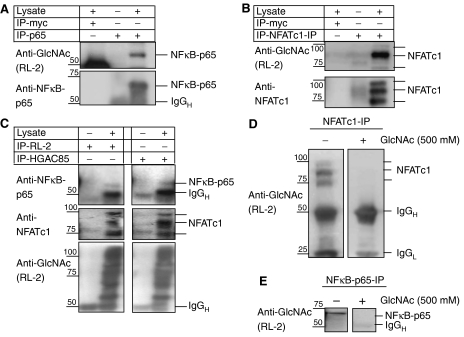
As OGT seems to be required for the transcriptional activity of NFκB and NFAT, we probed for O-GlcNAc modifications of NFκB and NFAT. NFκB was described to be O-GlcNAc-modified in rat mesangial cells (James et al, 2002). To confirm this observation in human T cells, we immunoprecipitated NFκB with an antibody specific for the p65 chain and probed for the presence of the O-GlcNAc residue (Figure 4A). As a control immunoprecipitation, we used an antibody to c-myc as irrelevant antibody. O-GlcNAc modification of NFATc1 was analyzed in a similar manner. NFATc1 was immunoprecipitated and analyzed for O-GlcNAc modifications. These experiments show that also NFATc1 is specifically detected by an antibody raised against O-GlcNAc (Figure 4B). Immunoprecipitations with two antibodies to O-GlcNAc revealed the presence of modified proteins in lymphocyte lysates, among which NFκB-p65 and NFATc1 were identified (Figure 4C). The specificity of the anti-O-GlcNAc antibody used for detection of O-GlcNAc-modified NFκB and NFATc1 was verified in Western blots. Preincubation of the anti-GlcNAc antibody RL-2 with an excess of N-acetylglucosamine, completely competed the signal for O-GlcNAc-modified NFκB-p65 (Figure 4D) and NFATc1 (Figure 4E).
Figure 4.
NFATc1 and NFκB are O-GlcNAc-modified. (A) Western blot analysis of O-GlcNAc modifications of NFκB-p65 after immunoprecipitation (5 × 107 Jurkat cells each) using anti-NFκB antibodies against p65 subunits of NFκB and irrelevant antibodies (anti-c-myc) as a negative control. To visualize the background bands of the antibodies used for IP, we did IPs omitting the lysate. Positions of NFκB subunits as that of IgGH are indicated. (B) Western blot analysis of O-GlcNAc modifications after immunoprecipitation (5 × 107 Jurkat cells each) using an anti-NFATc1 antibody or irrelevant antibodies (anti-c-myc) as a negative control. To visualize the background bands of the antibodies used for IP, we did IPs omitting the lysate. Positions of NFATc1 proteins are indicated. (C) Western blot analysis of NFκB-p65, NFATc1 and O-GlcNAc modifications after immunoprecipitation (5 × 107 Jurkat cells each) using anti-O-GlcNAc antibodies (RL-2 and HGAC85). Positions of NFκB-p65, NFATc1 and IgGH are indicated at the right side of the panels. To visualize the background bands of the antibodies used for IP, we did IPs omitting the lysate. Antibodies used in the immunoprecipitation reactions are given at the top, whereas the antibodies used in the Western blots are at the left side. (D) Western blot analysis of O-GlcNAc modifications after immunoprecipitation (5 × 107 BJAB cells each) using an anti-NFATc1 antibody. The Western blot was developed with or without preincubation of the anti-O-GlcNAc antibody in 500 mM GlcNAc. (E) Western blot analysis of O-GlcNAc modifications after immunoprecipitation (5 × 107 BJAB cells each) using an anti-NFκB-p65 antibody. The Western blot was developed with or without preincubation of the anti-O-GlcNAc antibody in 500 mM GlcNAc.
OGT interacts with NFκB and NFATc1
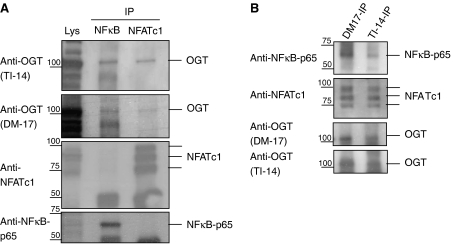
We were further interested in whether OGT might interact with NFκB and NFATc1. Immunoprecipitations of NFκB and NFATc1 were blotted for the presence of OGT, using the two antibodies TI-14 and DM-17. This experiment showed interaction of OGT to NFκB and NFATc1 (Figure 5A). Vice versa, OGT-IPs using two different antibodies were performed and co-precipitated NFκB and NFAT were detected in a Western blot, confirming the binding of OGT to both transcription factors (Figure 5B).
Figure 5.
OGT binds to NFATc1 and NFκB. (A) Western blot analysis of OGT, NFATc1 and NFκB-p65 after immunoprecipitation (5 × 107 Jurkat cells each) using antibodies to NFκB and NFATc1. (B) Western blot analysis of OGT, NFATc1 and NFκB-p65 after immunoprecipitation (5 × 107 Jurkat cells each) using antibodies to OGT (DM-17 and TI-14).
In summary, these data show that in lymphocytes NFκB as well as NFAT are O-GlcNAc-modified and the glycosylation is likely to occur after binding of OGT.
NFATc1 is O-GlcNAc-modified by OGT in vitro
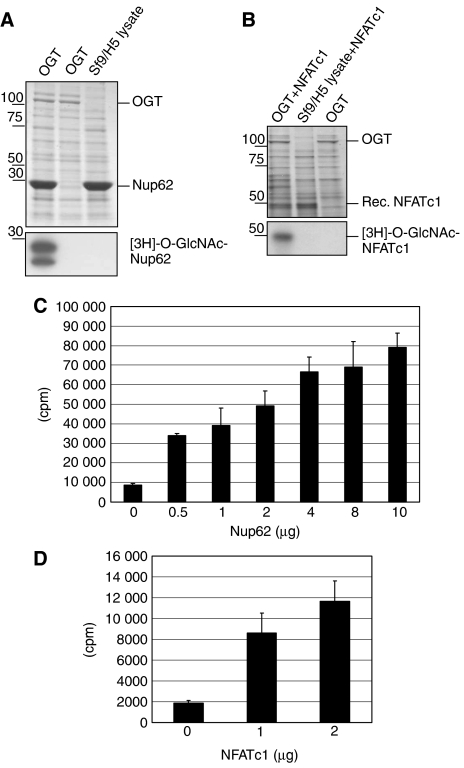
To confirm that NFATc1 is O-GlcNAc-modified by OGT, we established an in vitro glycosylation assay. OGT was overexpressed in baculovirus-infected Sf9/H5 cells and lysates enriched in OGT were used for the glycosylation assay. A lysate of uninfected cells was used as a control. A C-terminal fragment of the rat Nup62 expressed and purified in bacteria was used as a substrate for OGT (Marshall et al, 2003). The composition of the whole reaction mix was separated by SDS–PAGE and it is shown in Figure 6A after Coomassie brilliant blue staining (upper panel). Radioactive UDP-[3H]GlcNAc was added to the reaction mix, incubated for 30 min at 37°C, and the modification of Nup62 with [3H]GlcNAc was visualized after SDS–PAGE and autoradiography (Figure 6A, lower panel). The modification of Nup62 is only seen in the presence of OGT and Nup62 (left lane). No modification (radioactive band) was detected in either an assay performed in the absence of Nup62 (middle lane) or in the absence of OGT (right lane). A similar assay was performed using recombinant NFATc1 as a substrate for OGT (Figure 6B). The composition of the reaction mix was visualized after SDS–PAGE and a Coomassie brilliant blue staining (Figure 6B, upper panel). O-GlcNAc-modified recombinant NFATc1 was detected in the autography (lower panel, first lane). In the absence of either OGT (lane 2) or recombinant NFATc1 (lane 3), no radioactive band with the size of NFATc1 was detected.
Figure 6.
NFATc1 is O-GlcNAc-modified by OGT in vitro. An O-GlcNAc transferase assay was developed using a Sf9/H5 lysate containing enriched OGT expressed by baculovirus. Recombinant Nup62 was used as a control substrate for OGT. (A) Upper panel: Coomassie brilliant blue stained SDS–PAGE gel of whole reaction mix (Sf9/H5 lysates with or without OGT and Nup62). Lower panel: the gel (the portion containing Nup62 is shown) was processed to autoradiography after incubation of the lysate containing OGT or lysate from Sf9 cell infected with a control virus (Sf9 lysate) with UDP-[3H]GlcNAc. (B) Upper panel: Coomassie brilliant blue stained SDS–PAGE gel of whole reaction mix (Sf9/H5 lysates with or without OGT and recombinant NFATc1). Lower panel: the gel (the portion containing recombinant NFATc1 is shown) was processed to autoradiography after incubation with UDP-[3H]GlcNAc of the lysate containing OGT or lysate from Sf9 cells infected with a control virus (Sf9 lysate). (C) The O-GlcNAc transferase assay was performed as described in (A) with 0–10 μg purified Nup62. The reaction mixture was precipitated by TCA and the radioactivity associated with the pellet counted. The amount of radioactivity associated with the TCA precipitate correlates with the amount of Nup62 present in the transferase reaction. (D) An assay similar to that described in (B) was performed with 0, 1 and 2 μg of recombinant NFATc1. The reaction mixture was precipitated by TCA and the radioactivity associated with the pellet counted. The amount of radioactivity associated with the TCA precipitate correlates with the amount of NFATc1 present in the transferase reaction.
In parallel, the same reaction mixture was precipitated with TCA and counted in a liquid scintillation counter. The increase in radioactivity paralleled increasing concentrations of the substrate Nup62 (Figure 6C). The same assay was performed with 0–2 μg of recombinant NFATc1 as a substrate. The results strongly suggest that recombinant NFATc1 was O-GlcNAc-modified by OGT (Figure 6D).
NFATc1 is transiently modified shortly after lymphocytes stimulation
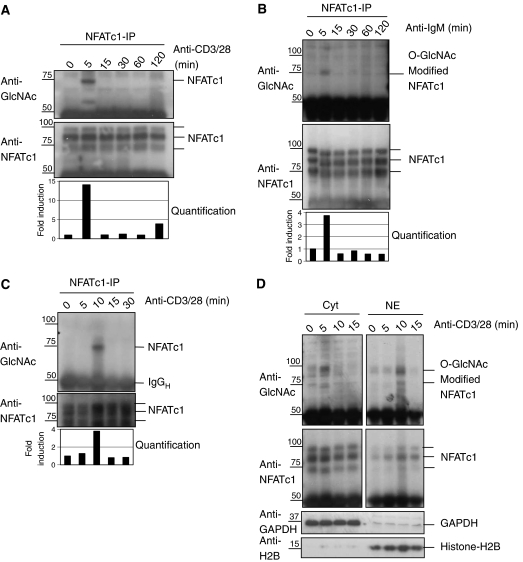
Next, we decided to analyze the kinetics of O-GlcNAc-modification of NFATc1 upon TCR stimulation. Jurkat cells were costimulated via the TCR and aliquots were retrieved at regular intervals. Immunoprecipitation of NFATc1 was performed and probed with an antibody recognizing O-GlcNAc-modified proteins (Figure 7A). As early as ∼5 min after stimulation, an increase of O-GlcNAc-modified NFATc1 was observed. The level of modified NFATc1 returned to base line levels by 15–20 min after stimulation. As OGT is also involved in B-cell activation, we evaluated time-dependent changes in O-GlcNAc modification of NFATc1 upon BCR stimulation. A large transient increase in the level of modified NFATc1 was observed shortly after IgM stimulation of BJAB cells (Figure 7B). Finally, the same type of experiment was carried out with primary human T lymphocytes. As in Jurkat and BJAB cells, a large transient increase in levels of modification of NFATc1 was observed after TCR stimulation (Figure 7C). Quantitation of Western blots revealed that after 5–10 min the amount of O-GlcNAc-modified NFATc1 increased ∼14-fold in Jurkat T cells and ∼4-fold in BJAB cells or primary human T cells (Figure 7A–C, lowest panels). The data show for the first time a large and transient OGT modification of NFATc1 in lymphocytes after stimulation of BCRs and TCRs.
Figure 7.
NFATc1 is O-GlcNAc-modified before entry into the nucleus. (A) Jurkat T cells were stimulated with a mixture of anti-CD3/anti-CD28 antibodies and total cellular lysates were prepared. NFATc1 immunoprecipitations were performed from these lysates. After separation by SDS–PAGE the immunoprecipitates were analyzed by Western blot using antibodies to O-GlcNAc (RL-2) and NFATc1. Quantification of the Western blots is shown in the lowest panel. (B) BJAB cells were stimulated with anti-IgM antibodies and total cellular lysates were prepared. NFATc1 immunoprecipitations were performed from these lysates and analyzed as described in (A). (C) Primary human PBMCs were costimulated with a mixture of anti-CD3/anti-CD28 antibodies and analyzed as described in the legend to panel (A). (D) Jurkat cells were stimulated for short times with anti-CD3/28 antibodies. Cytosolic and nuclear extracts were prepared. The cytosolic as well the nuclear fractions were used for NFATc1 immunoprecipitations. The purity of the cytosolic and nuclear extracts was controlled by doing Western blot of GAPDH (cytosolic fractions) and Histone H2B (nuclear fractions).
We then investigated if kinetics of O-GlcNAc modification might correlate with translocation of NFATc1 into the nucleus. Cytosolic as well as nuclear extracts were prepared from Jurkat cells stimulated for short periods of time via the TCR. Immunoprecipitations of NFATc1 coupled to Western blot analysis with an antibody to O-GlcNAc were performed (Figure 7D). In the cytosol, levels of O-GlcNAc-modified NFATc1 increased ∼5 min after TCR stimulation. In the nucleus, the bulk of O-GlcNAc-modified NFATc1 appeared by 10–15 min, with a shift of 5–10 min compared to the cytosol. The overall amounts of NFATc1 protein are reported in the lower panel of Figure 7D. Nuclear NFATc1 total protein concentration increased in parallel to the O-GlcNAc modification, while changes in the cytosol were much less prominent. Cytosolic and nuclear fractions were quantified by Western blots using the anti-GAPDH antibodies for cytosolic fractions and anti-Histone-H2B antibodies for nuclear fractions (Figure 7D).
In summary, the data suggest that OGT modification upon short-term stimulation of lymphocytes might be a prerequisite for nuclear translocation of NFATc1. In primary human T cells, the lowest of the three NFATc1 bands (likely the dephosphorylated form of NFATc1) seems to be preferentially modified by O-GlcNAc. It can be speculated that dephosphorylated NFATc1 is the preferred substrate for modification with O-GlcNAc.
The inhibitor of O-GlcNAcase, PUGNAc, sensitizes toward early B-cell activation
O-GlcNAc modifications are controlled by the transferase OGT and by a protein with antagonistic function, O-GlcNAcase, which removes this sugar residue from proteins (Hanover, 2001). We used PUGNAc (O-(2-Acetamido-2-deoxy-D-glucopyranosylidene)amino N-phenylcarbamate), a specific inhibitor of O-GlcNAcase, predicting that it should produce similar effects to those observed after overexpression of OGT (Vosseller et al, 2002). PUGNAc treatment should result in the increase of the level of O-GlcNAc modifications of NFκB and NFAT and therefore facilitate the activation of lymphocytes.
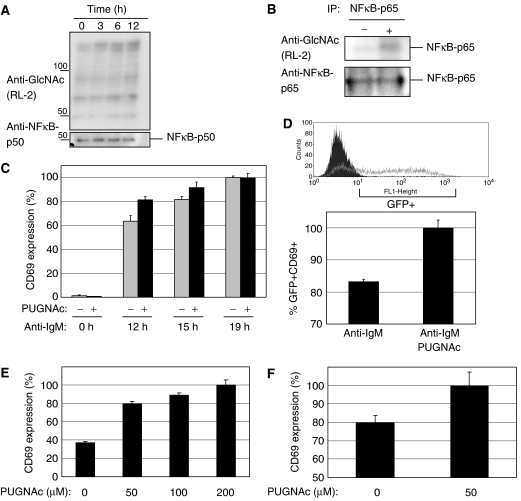
BJAB cells were treated with 100 μM PUGNAc for 0, 3, 6 or 12 h, respectively, and total cellular lysates were analyzed for the level of O-GlcNAc-modified proteins (Figure 8A). Western blot analysis of these lysates with antibodies against O-GlcNAc demonstrated a time-dependent increase of O-GlcNAc-modified proteins (Figure 8A). Upon treatment with PUGNAc, the level of O-GlcNAc-modified NFκB was also increased, whereas the overall amounts of immunoprecipitated NFκB was not influenced by PUGNAc (Figure 8B, lower panel). Flow cytometry analysis of the cells treated with 100 μM PUGNAc for up to 72 h did not affect proliferation of the cells (data not shown). We then examined the consequence of the inhibition of O-GlcNAcase with PUGNAc on early B-cell activation, by evaluating CD69 surface expression. BJAB cells were either left untreated or were treated for 5 h with the inhibitor before stimulation with anti-IgM antibodies for 0, 12, 15 or 19 h, respectively. Treatment of BJAB cells with PUGNAc accelerated CD69 externalization, whereas treatment with PUGNAc alone did not result in an increase in CD69 surface externalization (Figure 8C). To confirm that inhibition of O-GlcNAcase facilitated NFAT activation, BJAB.NFAT.GFP cells were used. These cells express GFP under the control of a NFAT promoter allowing activation of the NFAT promoter to be analyzed by Flow cytometry analysis. BJAB.NFAT.GFP cells were either left untreated or treated with PUGNAc for 5 h and subsequently stimulated with anti-IgM antibodies for 12 h. Prominent GFP expression can be observed upon stimulation by anti-IgM antibodies (example in upper panel Figure 8D). In the presence of PUGNAc, a prominent increase of double-positive cells, GFP+ and CD69+, is observed (Figure 8D, lower panel). Thus, pretreatment with PUGNAc leads to an increase in NFAT activation as well as CD69 surface externalization.
Figure 8.
The inhibitor of O-GlcNAcase (PUGNAc) sensitizes toward early lymphocytes activation. (A) Total cellular lysates of BJAB treated with 100 μM PUGNAc for the indicated periods of time were analyzed by Western blot using antibodies against O-GlcNAc (RL-2). A loading control was performed with an antibody to NFkB-p50 (SC-8414). (B) Western blot analysis of O-GlcNAc modifications of NFκB-p65 after immunoprecipitation (5 × 107 cells each) using anti-NFκB-p65 antibodies. The cells were either left untreated (−) or treated with 100 μM PUGNAc (+) for 12 h. (C) BJAB cells were treated with 100 μM PUGNAc for 5 h before stimulation with anti-IgM antibodies for 0, 12, 15 and 19 h, respectively. After stimulation, CD69 surface expression was measured by flow cytometry in triplicates. (D) BJAB.GFP.NFAT cells were treated with 100 μM PUGNAc for 5 h before a 12 h stimulation with anti-IgM antibodies. After stimulation, GFP expression was measured by Flow cytometry. Experiments were performed in triplicates. Upper panel shows a typical Flow cytometry trace for cells prior activation (dark trace) and after IgM stimulation (gray trace). (E) PBMCs were treated with the indicated amounts of PUGNAc for 12 h, stimulated with anti-IgM antibodies for 15 h and the activation of primary B lymphocytes was quantified using Flow cytometry. CD69 surface expression was measured for CD19 positive B cells. Experiments were performed in triplicates. (F) PBMCs were treated with the indicated amounts of PUGNAc for 12 h. Subsequently, cells were costimulated with plate-bound anti-CD3/28 for 15 h and the activation of primary human T lymphocytes was quantified using Flow cytometry. CD69 surface expression was measured for CD3 positive T cells.
The inhibitor of O-GlcNAcase, PUGNAc, sensitizes primary human lymphocytes toward activation
PUGNAc was used to test the effects of O-GlcNAc modification on the activation of primary human lymphocytes.
Human PBMCs were treated with PUGNAc, T and B cells in PBMCs were identified by anti-CD3 and anti-CD19 antibodies, respectively, and then analyzed for CD69 surface expression. The inhibition of O-GlcNAcase by PUGNAc leads to a concentration-dependent increase in activation of primary human B cells (Figure 8E) and primary human T cells (Figure 8F). In summary, an increase of O-GlcNAc modifications upon inhibition of O-GlcNAse leads to a sensitization of T as well as B cells toward TCR- and BCR-induced activation.
Discussion
This study demonstrates that the balance of O-GlcNAc modifications of the transcription factors NFAT and NFκB is decisive for lymphocytes activation. Proper levels of O-GlcNAc modifications are controlled by the O-GlcNAc transferase OGT and its counter-enzyme O-GlcNAcase. SiRNA-mediated downregulation of OGT leads to the impairment of T- and B-lymphocytes activation. Overexpression of OGT sensitizes lymphocytes toward activation similarly as the inhibition of O-GlcNAcase, the enzyme with antagonistic function to OGT. Mechanistic experiments showed that OGT interacts with NFAT as well as NFκB, thereby modifying both transcription factors. The effect of OGT on NFκB seems to be specific to lymphocytes, as we failed to reproduce a similar effect in other cells. This suggests that TCR-/BCR-mediated signaling is required for the functional effect of OGT on NFAT/NFκB transcription factors. Moreover, a transient increase of O-GlcNAc modification of NFAT occurs upon TCR/BCR stimulation in the cytosol of lymphocytes, that seems to precede its translocation into the nucleus. In summary, our study for the first time points toward OGT as a key signaling component downstream of the TCR and the BCR.
O-GlcNAc modifications of proteins have been described for the first time around 20 years ago (Torres and Hart, 1984). The slow progress in this field can be mostly due to a lack of efficient methods for detection of O-GlcNAc-modified proteins. The currently fast growing number of O-GlcNAc-modified proteins includes enzymes, kinases, transcription factors and proteasome components (Zachara and Hart, 2006). Nevertheless, the role of O-GlcNAc modifications in lymphocytes signaling is poorly understood. Tissue-specific deletion of the OGT gene in mice resulted in a decrease of total number of CD4 T cells, which was thought to be mediated by increased apoptosis. The same study demonstrated that OGT does not affect the development of immature CD4/CD8 thymocytes (O'Donnell et al, 2004). The results with these transgenic mice are consistent with the function of OGT during T-cell activation described in this manuscript. The lack of OGT results in an incomplete or improper activation of peripheral T cells, increasing their chance to move toward apoptosis. The role of OGT is likely restricted to T-cell activation, as no defect was observed in immature thymocytes, which have not yet needed TCR-dependent signaling for positive selection.
OGT-catalyzed modifications of proteins are described to play prominent roles in many signaling pathways. The level of O-GlcNAc modifications has a direct link to glucose homeostasis, implicating a role of the OGT/O-GlcNAcase system in diabetes (Roos et al, 1997; Deaciuc et al, 2004; Hanover et al, 2005). A putative role of O-GlcNAc in controlling the mitotic progression was also described (Slawson et al, 2005). Still limited information is available on the biochemical consequences of the O-GlcNAc modification of proteins, for example, the effects on the activity/function of target enzymes. Recently, it was reported that the modification with O-linked N-acetylglucosamine regulates the activity and stability of the tumor suppressor p53 (Yang et al, 2006). The O-GlcNAc modification of S149 leads to a decreased phosphorylation at T155. Lowering phosphorylation at this site results in decreased p53 ubiquitination/proteolysis and, thus, higher stability of the protein (Yang et al, 2006). Another study suggests that O-GlcNAc modification of the transcription factor Elf-1 is regulated by O-GlcNAc modification together with phosphorylation and that both modifications are needed for nuclear translocation (Juang et al, 2002). In the case of NFAT, O-GlcNAc modification is also likely to play a role in its nuclear translocation. Dephosphorylation of Ser/Thr residues by calcineurin upon TCR activation unmasks signals that allow NFAT to translocate into the nucleus. As O-GlcNAc modifications have been shown to compete for sites recognized by kinases, O-GlcNAc might prevent rephosphorylation in the cytosol, therefore, favoring the translocation of a ‘primed' state of NFAT. Or else, O-GlcNAc modifications promote a change in the conformation of NFAT that is prone to translocation. NFκB requires also phosphorylation/dephosphorylation events in order to be fully activated and to transduce into the nucleus. Upon phosphorylation, members of the IKK complex need to dissociate and to be degraded allowing NFκB to move to the nucleus. Enhanced dissociations might result from O-GlcNAc modification of NFκB. The detailed analysis of the underlying steps is a matter of future studies.
Interestingly, OGT is expressed at high levels in T cells and dendritic cells (data not shown). Despite being an essential gene for the survival of cells (Shafi et al, 2000), the high concentration of OGT suggests yet to be discovered additional and specific functions in T cells as well as dendritic cells. The novel role of OGT/O-GlcNAcase in lymphoid cells described above suggests that manipulation of this enzyme system might represent a novel way to therapeutic immunosuppression.
Materials and methods
Antibodies and reagents
The following antibodies were used: anti-NFκB p65 antibodies and anti-NFκB p50 antibodies (SC109 and SC-8414, Santa Cruz Biotechnology Inc); anti-NFATc1 antibodies (7A6, Alexis Biochemicals); anti-GlcNAc antibodies HGAC85 and RL-2 (ab2735 and ab2739, Abcam); anti-OGT antibodies (DM-17 and TI-14, Sigma-Aldrich); anti-V5 antibodies (R960-25, Invitrogen); anti-CD69-APC/anti-CD3-FITC, anti-CD19-PECy5 antibodies (BD Biosciences); anti-Histone-H2B (IMG-359, Imgenex); anti-GAPDH (6C5, Ambion); anti-myc (9E10, Roche). PUGNAc was from Toronto Research Chemicals. Secondary Horseradish peroxidase-conjugated antibodies were from Cell Signaling. All other chemicals used were highest analytical grade available and from Merck or Sigma-Aldrich.
Cell lines
The Jurkat T cell line (clone E6.1), the BJAB B lymphoblastoid cell line and primary PBMCs (prepared by Ficoll density centrifugation using Leucosep, Greiner) were maintained in RPMI 1640, 10 mM sodium pyruvate, nonessential amino acids, 50 μg/ml gentamicin (all purchased from Gibco Life Technologies) and 10% fetal calf serum (PAA Technologies) in 5% CO2, 37°C. Jurkat cells stably expressing the vector pGL2B-neo-IL-2 as part of the IL-2 promoter (kindly provided by P Wenner, Novartis Pharma AG) as well as the BJAB.NFAT.GFP cell line expressing GFP under a NFAT promoter were maintained in culture medium with addition of 0.5 mg/ml G418 (Gibco Life Technologies). Jurkat cells stably expressing the NFκB- and NFAT-reporter plasmids (kindly provided by J-P Evenou, Novartis Pharma AG) were maintained in culture medium with addition of 0.25 mg/ml Hygromycin (Gibco Life Technologies). Sf9/H5 cells were maintained in TNM-FH Insect Culture Medium with 10% FCS and 50 μg/ml gentamicin in a 27°C humidified incubator.
The IL-2-Luc Jurkat cell line was generated after stable transfection of Jurkat E6.1 with the vector pGL2-neo-IL-2. This vector was prepared by insertion of the IL-2 promotor region (−583 to +40 of human IL-2 promotor) in front (HindIII site) to the luciferase reporter gene in the vector pGL2-Basic (Promega). The G418 resistance gene was transferred from the vectors pMCIneoPolyA (Stratagene) into the XhoI–HindIII site. pNF-kB-Luc and pNFAT-Luc were purchased from Stratagene and used to prepare Jurkat stable cell lines. The NFAT-GFP construct used for the BJAB.GFP.NFAT cells was similar to the pNFAT-LUC available from Stratagene.
Protocols for activation of T and B cells
T cells were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies, B cells with 10 μg/ml anti-IgM antibodies (Dr Andreas Katapodis, Novartis Pharma AG) for 5.5 h at 37°C. Alternatively, cells were stimulated with 10 ng/ml PMA with 2.5 μM ionomycin. For coating, flat bottom 96-well microtiter plates (Microlite 2+, Thermo Electron Corp.) were incubated with 3 μg/ml goat anti-mouse IgG Fc antibodies (Dianova) for 2–3 h, washed with PBS and incubated with 20 ng/ml anti-TCRα/β antibodies (Becton Dickinson) and 600 ng/ml anti-CD28 antibodies (BD Biosciences) overnight at 4°C.
Western blot and immunoprecipitation
Western blot analysis as well as immunoprecipitations were performed as described by Laemmli (1970) and Golks et al (2006). PVDF membranes were used according to the manufacturer's protocol (Biorad). The antibodies were diluted as follows: anti-NFκB p65 and p50 antibodies (1:50); anti-NFATc1 antibodies (1:100); anti-GlcNAc antibodies HGAC85 or RL-2 (1:100); anti-OGT antibodies DM-17 or TI-14 (3 μg/ml); anti-myc (2 μg/ml). In case of preincubation of the antibody to O-GlcNAc (RL-2) with N-acetylglucosamine, 500 mM GlcNAc were dissolved in PBS-T and the antibodies preincubated for 30 min at room temperature. Nuclear and cytosolic extracts were prepared according to the manufacturer's protocol using the Nuclear Extract Kit (ActiveMotif). Quantification of Western blots was performed using the GeneTools Analysis Software version 3.02.00.
Flow cytometry analysis
CD69 surface staining was performed on 106 cells. The cells were washed with PBS, then incubated for 20 min in blocking solution (2% FCS/PBS), and stained with 1 μl anti-CD69APC in 20 μl 2% FCS/PBS for 20 min. The cells were washed with an excess of 2% FCS/PBS and analyzed using Flow cytometry. To evaluate the percentile of T cells and B cells, PBMCs were stained with anti-CD3-FITC or anti-CD19-PECy5 antibodies, respectively.
Cloning and expression of OGT in insect cells
OGT was amplified from the vector pQL-OGT-r8-C1_7-1 (Dr Brian Latario, Novartis Pharma AG) using the following primer pairs: OGT_F: 5′-GGTCGACTGACCATGGCGTCTTCC and OGT_Eco_R: 5′-CGCCCTTAGTATAGCCATTC; OGT_R_NO: 5′-CAGCGGCCGCTTATGACTCAGTGACTTCAAC and OGT_Eco_F: 5′-GGAAAAGCAGCTGATCGCATC. The PCR fragments were digested with SalI–EcoRI and EcoRI–EcoRV and ligated into pENTR2b. The full-length OGT was transferred to the pDEST10 vector by gateway reaction and the generation of baculovirus and expression of OGT were performed according to the manufacturer's protocol (Invitrogen).
Cell disruption and enrichment of OGT
OGT protein was isolated from 2–3 × 107 infected Sf9/H5 insect cells for 48 h. The cells were washed once in PBS, then lysed in 10 mM Tris–HCl, pH7.5 (1 × 107 cells/ml). After addition of 1 mM DTT and protease inhibitors (Complete, Roche), cells were homogenized and diluted with one volume of 10 mM Tris–HCl, pH7.5, 20% sucrose, 300 mM KCl. The cell suspension was centrifuged for 45 min at 100 000 g at 4°C. The in OGT-enriched supernatant was used for biochemical assays.
Cloning and expression of Nup62 in bacteria
Nup62 (the N-terminal 260 amino acids) was cloned from rat brain cDNA by PCR amplification with the following primer pair: Nup_F 5′-CACCATGTCCCTCTCAGCTCCAGCGACAAC-3′ and Nup_R 5′-CTGCTACAGGGCAGAGCTCTGGTCCAC. After transfer into pENTR/D-TOPO, the Nup62 fragment was shuttled using a gateway reaction into pDEST17 vector according to the manufacturer's protocol (Invitrogen). N-terminal His-tagged Nup62 was expressed using BL21-AI bacteria. Cultures were grown at 37°C to mid-log phase and then induced with 0.2% of L-arabinose for 2 h. Purification of N-terminal Nup62 was performed as described by Marshall et al (2003).
O-GlcNAc transferase assay
O-GlcNAc transferase reactions were performed with 0–2 μg of purified recombinant NFAT (Bioscource) or Nup62 (0.5–10 μg) in the presence of 0.5 μCi UDP-[3H]GlcNAc, Sf9 lysate containing enriched OGT (see above) diluted 1:10 in 50 mM Tris–HCl, pH7.5, 1 mM DTT and 12.5 mM MgCl2 and incubated for 30 min at 37°C. Glycosylated products were detected by two independent methods: (1) O-GlcNAc transferase reaction mixtures were separated on standard SDS–PAGE, the gels were fixed for 30 min in isopropanol:water:acetic acid (5:13:2) and incubated with Amplify (Amersham Biosciences) for 30 min prior drying. An autoradiography was performed for detection. The TCA method was performed as a second method (Marshall et al, 2003). Radioactivity was measured after addition of 5 ml scintillation fluid (OptiPhase HiSafe 3, PerkinElmer) in a liquid scintillation counter (2000 CA Tricarb, Packard).
SiRNA-mediated knockdown of OGT and Lck
5 × 106 Jurkat or BJAB cells were transfected by HiPerfect (QIAGEN) with a negative control or siRNA oligonucleotides specific for human OGT or Lck (OGT_1: ID11253, OGT_2: ID:11254, OGT_3: ID670, Lck: 5019647, all purchased from Ambion). As a negative control, we used a nonsense siRNA, which is labeled with Alexa Fluor 488 (QIAGEN, 1022563). Alternatively, Amaxa transfections were performed using the solution V and program C16 according to the manufacturer's protocol (Amaxa Biosystems). Transfected cells were incubated at 37°C for 24, 48 or 72 h before stimulation and further analysis. Transfection efficacy was evaluated by FACS where an aliquot of the cells treated with the nonsense siRNA Alexa Fluor 488 (QIAGEN, 1022563) was analyzed. Under the conditions used, we normally detected more than 90% of the cells positive for fluorescent signal, while only a small portion of the cells were dead (<10%).
RNA isolation and real-time PCR
RNA extraction, cDNA synthesis and real-time PCR were performed using the ABI PRISM™ 6100 system, the High Capacity cDNA archive kit and the following primers according to the manufacturer's protocols (Applied Biosystems): Lck (Hs00178427m1); OGT (Hs00269228m1); IL-2 (Hs00174114m1); 18S rRNA (4308329).
Luciferase assays
A total of 500 ng of vector (GFP or OGT) was transfected together with 1000 ng of the NFκB-luciferase construct pLuc-4xNF-κB (Proksch et al, 2005) in 105 BJAB cells. Luciferase assays with Jurkat cell stably transfected with NFAT-, IL-2 promotor- and NFκB-reporter plasmid were performed with 105 cells per experiment.
The cells were stimulated as described in the legend to the Figures and subsequently lysed for 20 min at room temperature in 50 μl lysis buffer (passive lysis buffer; Promega), followed by centrifugation (10 000 g) for 20 min to sediment insoluble materials. A total of 5 μl of cell lysates was mixed with 50 μl of the luciferase assay mixture (470 μM Beetle Luciferin (Promega), 1.07 mM (MgCO3)4Mg(OH)2 × 5H2O, 20 mM N-Tris-(hydroxymethyl)-methylglycine, 2.67 mM MgSO4, 100 μM EDTA, 33.3 mM DTT, 270 μM CoA(OAc), and 530 μM ATP), and relative light units were measured with a duoluminomat (Victor, Perkin Elmer).
Acknowledgments
We thank Dr Inna Lavrik for critically reading the manuscript and Dr Min Li-Weber for kindly providing the NFκB-luciferase reporter plasmid.
Conflict of interest The authors have no conflicting financial interests.
References
- Baier G (2003) The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol Rev 192: 64–79 [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M (2000) Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck). Mol Biol Cell 11: 1585–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Cole RN, Zaia J, Hart GW (2000) Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry 39: 11609–11620 [DOI] [PubMed] [Google Scholar]
- Cheng X, Hart GW (2001) Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor beta: post-translational regulation of turnover and transactivation activity. J Biol Chem 276: 10570–10575 [DOI] [PubMed] [Google Scholar]
- Chou TY, Hart GW, Dang CV (1995) c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem 270: 18961–18965 [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Clipstone NA (1994) Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem 63: 1045–1083 [DOI] [PubMed] [Google Scholar]
- Deaciuc IV, D'Souza NB, Burikhanov R, Nasser MS, Voskresensky IV, De Villiers WJ, McClain CJ (2004) Alcohol, but not lipopolysaccharide-induced liver apoptosis involves changes in intracellular compartmentalization of apoptotic regulators. Alcohol Clin Exp Res 28: 160–172 [PubMed] [Google Scholar]
- Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC (2000) RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol 1: 317–321 [DOI] [PubMed] [Google Scholar]
- Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R (2002) Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 106: 466–472 [DOI] [PubMed] [Google Scholar]
- Gao Y, Wells L, Comer FI, Parker GJ, Hart GW (2001) Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem 276: 9838–9845 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109 (Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Golks A, Brenner D, Schmitz I, Watzl C, Krueger A, Krammer PH, Lavrik IN (2006) The role of CAP3 in CD95 signaling: new insights into the mechanism of procaspase-8 activation. Cell Death Differ 13: 489–498 [DOI] [PubMed] [Google Scholar]
- Hanover JA (2001) Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J 15: 1865–1876 [DOI] [PubMed] [Google Scholar]
- Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, Ashwell G, Krause M (2005) A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci USA 102: 11266–11271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18: 2195–2224 [DOI] [PubMed] [Google Scholar]
- Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW (1987) Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol 104: 1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LR, Tang D, Ingram A, Ly H, Thai K, Cai L, Scholey JW (2002) Flux through the hexosamine pathway is a determinant of nuclear factor kappaB- dependent promoter activation. Diabetes 51: 1146–1156 [DOI] [PubMed] [Google Scholar]
- Juang YT, Solomou EE, Rellahan B, Tsokos GC (2002) Phosphorylation and O-linked glycosylation of Elf-1 leads to its translocation to the nucleus and binding to the promoter of the TCR zeta-chain. J Immunol 168: 2865–2871 [DOI] [PubMed] [Google Scholar]
- Kearse KP, Hart GW (1991) Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proc Natl Acad Sci USA 88: 1701–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WG, Dahmus ME, Hart GW (1993) RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem 268: 10416–10424 [PubMed] [Google Scholar]
- Kiani A, Garcia-Cozar FJ, Habermann I, Laforsch S, Aebischer T, Ehninger G, Rao A (2001) Regulation of interferon-gamma gene expression by nuclear factor of activated T cells. Blood 98: 1480–1488 [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Blomberg MA, Hart GW (1997) Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 272: 9308–9315 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lazarus BD, Love DC, Hanover JA (2006) Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology 16: 415–421 [DOI] [PubMed] [Google Scholar]
- Love DC, Hanover JA (2005) The hexosamine signaling pathway: deciphering the ‘O-GlcNAc code'. Sci STKE 2005: re13. [DOI] [PubMed] [Google Scholar]
- Lyakh L, Ghosh P, Rice NR (1997) Expression of NFAT-family proteins in normal human T cells. Mol Cell Biol 17: 2475–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S, Duong T, Orbus RJ, Rumberger JM, Okuyama R (2003) Measurement of UDP-N-acetylglucosaminyl transferase (OGT) in brain cytosol and characterization of anti-OGT antibodies. Anal Biochem 314: 169–179 [DOI] [PubMed] [Google Scholar]
- Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N (1998) Signalling into the T-cell nucleus: NFAT regulation. Cell Signal 10: 599–611 [DOI] [PubMed] [Google Scholar]
- Medina L, Grove K, Haltiwanger RS (1998) SV40 large T antigen is modified with O-linked N-acetylglucosamine but not with other forms of glycosylation. Glycobiology 8: 383–391 [DOI] [PubMed] [Google Scholar]
- Monticelli S, Rao A (2002) NFAT1 and NFAT2 are positive regulators of IL-4 gene transcription. Eur J Immunol 32: 2971–2978 [DOI] [PubMed] [Google Scholar]
- O'Donnell N, Zachara NE, Hart GW, Marth JD (2004) Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol 24: 1680–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proksch P, Giaisi M, Treiber MK, Palfi K, Merling A, Spring H, Krammer PH, Li-Weber M (2005) Rocaglamide derivatives are immunosuppressive phytochemicals that target NF-AT activity in T cells. J Immunol 174: 7075–7084 [DOI] [PubMed] [Google Scholar]
- Roos MD, Su K, Baker JR, Kudlow JE (1997) O glycosylation of an Sp1-derived peptide blocks known Sp1 protein interactions. Mol Cell Biol 17: 6472–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD (2000) The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA 97: 5735–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW (2005) Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem 280: 32944–32956 [DOI] [PubMed] [Google Scholar]
- Torgerson TR, Colosia AD, Donahue JP, Lin YZ, Hawiger J (1998) Regulation of NF-kappa B, AP-1, NFAT, and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kappa B p50. J Immunol 161: 6084–6092 [PubMed] [Google Scholar]
- Torres CR, Hart GW (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem 259: 3308–3317 [PubMed] [Google Scholar]
- Vosseller K, Wells L, Lane MD, Hart GW (2002) Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 99: 5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IM, Blanco JC, Tsai SY, Tsai MJ, Ozato K (1996) Interferon regulatory factors and TFIIB cooperatively regulate interferon-responsive promoter activity in vivo and in vitro. Mol Cell Biol 16: 6313–6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW (2006) Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol 8: 1074–1083 [DOI] [PubMed] [Google Scholar]
- Zachara NE, Hart GW (2006) Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta 1761: 599–617 [DOI] [PubMed] [Google Scholar]