Abstract
Nicotinic acetylcholine receptors (AChRs) are pentameric ligand-gated ion channels that mediate fast synaptic transmission at the neuromuscular junction (NMJ). After assembly in the endoplasmic reticulum (ER), AChRs must be transported to the plasma membrane through the secretory apparatus. Little is known about specific molecules that mediate this transport. Here we identify a gene that is required for subtype-specific trafficking of assembled nicotinic AChRs in Caenorhabditis elegans. unc-50 encodes an evolutionarily conserved integral membrane protein that localizes to the Golgi apparatus. In the absence of UNC-50, a subset of AChRs present in body-wall muscle are sorted to the lysosomal system and degraded. However, the trafficking of a second AChR type and of GABA ionotropic receptors expressed in the same muscle cells is not affected in unc-50 mutants. These results suggest that, in addition to ER quality control, assembled AChRs are sorted within the Golgi system by a mechanism that controls the amount of cell-surface AChRs in a subtype-specific way.
Keywords: C. elegans, intracellular trafficking, levamisole, nicotinic acetylcholine receptor
Introduction
Nicotinic acetylcholine receptors (AChRs) belong to a family of structurally related ligand-gated ion channels that includes γ-aminobutyric acid (GABA), serotonin (5-HT), and glycine receptors (Corringer et al, 2000). AChRs mediate fast synaptic transmission at the neuromuscular junction (NMJ) and modulate neurotransmitter release in the brain (Gotti and Clementi, 2004). Recently, AChRs have also been shown to be expressed in non-neuronal cells where they control important cell functions such as proliferation, adhesion, migration, secretion, survival, and apoptosis (Wessler et al, 2003; Gotti and Clementi, 2004). These receptors are built by the assembly of individual subunits into pentamers, which can be either homo- or heteromeric in nature (Unwin, 2005). To be functional, AChRs must be assembled into pentamers and trafficked to the postsynaptic plasma membrane. Malfunction or misexpression of AChRs is involved in neuro-pathological processes such as schizophrenia, epilepsy, and tobacco addiction (Ryan, 1999; Gotti and Clementi, 2004; Singh et al, 2004), emphasizing the importance of control checkpoints to ensure appropriate cell-surface display of properly folded and assembled receptors.
AChR subunits assemble and oligomerize within the endoplasmic reticulum (ER) in a sequential and ordered manner (Smith et al, 1987; Green and Millar, 1995). However, in mouse muscle cells, only about 30% of the synthesized subunits reach the cell surface, suggesting that the assembly process is tightly controlled (Merlie and Lindstrom, 1983). Newly synthesized AChR subunits are immediately bound by the ER chaperones calnexin and Bip, from which they detach upon further maturation and assembly (Forsayeth et al, 1992; Gelman et al, 1995). Correct pentameric assembly is monitored by an ER retention signal in the first transmembrane (TM) segment present in each of the subunits, which is masked upon assembly of the pentamer (Wang et al, 2002), and by recognition of ER export motifs present in the large TM3 to TM4 intracellular loop (Keller et al, 2001; Ren et al, 2005). AChR subunits that do not fold or assemble properly are retained within the ER, and are rapidly degraded by the ER-associated degradation (ERAD) machinery (Wanamaker et al, 2003; Christianson and Green, 2004). Although the maturation and assembly processes in the ER seem to be inefficient, about 80% of the cellular nAChR are found at the cell surface at steady state (Devreotes and Fambrough, 1975; Gu et al, 1989). Once AChRs have reached the cell surface, they are clustered and metabolically stabilized at the synapse by attachment to the postsynaptic scaffold (Wang et al, 1999; Sanes and Lichtman, 2001) or by extracellular interactions (Gally et al, 2004). Despite our knowledge of the early events during AChR assembly and ER retention, molecules required for AChR cell-surface transport have yet to be identified. This is intriguing since several reports indicate that cell-type-specific factors should exist that are necessary for AChR cell-surface expression (Sweileh et al, 2000)
Genetic screens performed in the nematode Caenorhabditis elegans have contributed to the identification of functional AChRs and molecules required for AChR assembly (Jones et al, 2005). ACh is the prominent excitatory neurotransmitter in C. elegans and is involved in most physiological functions such as locomotion, feeding, and mating. Genome sequencing revealed up to 42 genes potentially encoding AChR subunits (Jones and Sattelle, 2004). An AChR present at the C. elegans NMJ was first identified and characterized on the basis of its sensitivity to the nematode-specific nicotinic agonist levamisole (Lewis et al, 1980). This drug causes muscle hypercontraction and death at high concentration. A screen for resistance to levamisole identified mutations in the genes unc-29, unc-38 and unc-63 that encode obligatory subunits of the levamisole-sensitive AChR (Lev-AChR) expressed in body-wall muscles (Fleming et al, 1997; Culetto et al, 2004). In addition, the loss of LEV-1 or LEV-8 AChR subunits causes a partial resistance to levamisole, suggesting that these subunits are present in a subset of the levamisole receptors or are functionally redundant (Fleming et al, 1997; Towers et al, 2005). Mutants in Lev-AChRs exhibit a slow and uncoordinated movement, but are still able to move, since body-wall muscles express a second type of nicotinic AChR (Richmond and Jorgensen, 1999). acr-16 encodes an essential subunit of this receptor, which is likely to represent an α7-like homopentameric receptor (Ballivet et al, 1996; Francis et al, 2005; Touroutine et al, 2005).
Genetic strategies also identified proteins required for AChR synthesis or cell-surface expression. The gene ric-3 was isolated in a screen for suppressors of the neuronal degeneration caused by a gain-of-function mutation in the C. elegans AChR composed of the DEG-3 and DES-2 subunits (Halevi et al, 2002). ric-3 encodes an integral membrane protein localized in the ER, and is required for the maturation of all AChRs analyzed in C. elegans so far. Subsequently, mammalian homologs of RIC-3 have also been identified and shown to be involved in the functional maturation of different types of AChRs (Halevi et al, 2002, 2003). Another locus, unc-50, was identified in a screen for resistance to levamisole and was mapped to a genetic interval that did not contain genes potentially encoding AChR subunits (Lewis et al, 1980). However, unc-50 mutants displayed the same levamisole resistance and uncoordinated phenotype as mutations in the Lev-AChR subunits (Lewis et al, 1980). Furthermore, membrane extracts of unc-50 mutants were demonstrated to lack binding sites for labeled amino-levamisole in a ligand binding assay (Lewis et al, 1987). These data suggested that UNC-50 might be required for the synthesis of functional Lev-AChRs.
Here we demonstrate that UNC-50 belongs to an evolutionarily conserved protein family of integral membrane proteins (Fitzgerald et al, 2000; Chantalat et al, 2003). In C. elegans, UNC-50 is ubiquitously expressed and localized to the Golgi system. We show that UNC-50 is required in body-wall muscles for the trafficking of the assembled Lev-AChR to the NMJ. In unc-50 mutants, the Lev-AChR is rapidly degraded by the lysosomal system after receptor assembly. This late degradative pathway represents a novel regulatory step to control the biosynthesis of a specific subset of AChRs.
Results
unc-50 mutants lack Lev-AChRs at the cell surface
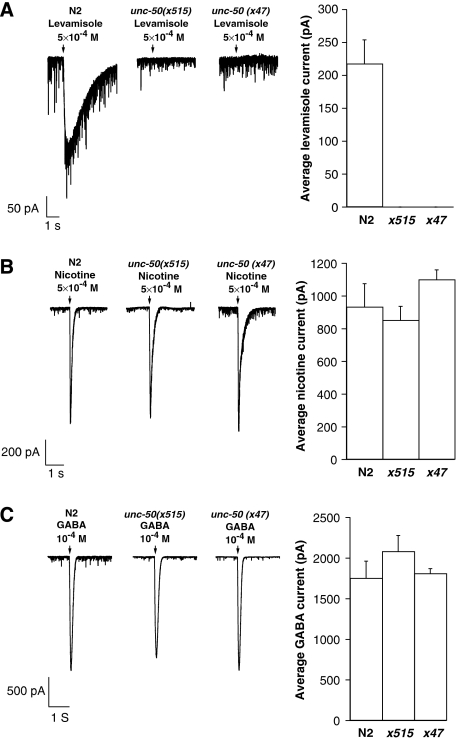
unc-50 mutants were initially isolated on the basis of impaired locomotion (Brenner, 1974), and were subsequently shown to be strongly resistant to the nicotinic agonist levamisole (Lewis et al, 1980). These two phenotypes suggest that unc-50 mutants lack functional Lev-AChR at NMJs. To test this prediction we recorded the electrophysiological response of body-wall muscles to pressure-ejected levamisole in the wild type and unc-50 mutants. In contrast to the wild type, unc-50 mutants (alleles x515 and x47) exhibited no response to levamisole (Figure 1A). The lack of response to levamisole was identical to that seen in mutants lacking the AChR subunits UNC-29, UNC-38 or UNC-63 (Richmond and Jorgensen, 1999; Culetto et al, 2004). Besides the Lev-AChR, a second AChR encoded by acr-16 is present at C. elegans NMJs. This ACR-16-containing receptor is insensitive to levamisole but sensitive to nicotine. To assess the effect of unc-50 mutations on this receptor, we recorded the response of body-wall muscles to nicotine and found no difference between wild-type and unc-50 mutant animals (Figure 1B). Analysis of ACR-16-dependent evoked response in muscle cells following nerve stimulation was similar in wild-type and unc-50 mutant animals (Supplementary Figure 1), hence demonstrating that UNC-50 is dispensable for expression and synaptic targeting of ACR-16-containing receptors. C. elegans muscles are also innervated by GABAergic motoneurons. At GABAergic NMJs, GABA activates an anionic GABAA receptor encoded by the unc-49 gene. Electrophysiological responses to GABA in wild type and in unc-50 mutants were similar (Figure 1C). Together, these results demonstrate that loss of UNC-50 function selectively eliminates the expression of functional Lev-AChRs, but does not affect the expression of other ligand-gated ion channels at the NMJ.
Figure 1.
Body-wall muscles of unc-50 mutants do not respond to levamisole, however the response to nicotine and GABA are unaffected. The electrophysiological responses of wild type (N2) and unc-50 mutant (alleles x515 and x47) body-wall muscles to pressure ejection of 500 μM levamisole (A), 500 μM nicotine (B), and 100 μM GABA (C) are shown. The arrow marks drug application. Results are presented as the means of independent experiments. Error bars represent the standard error of the mean (s.e.m.).
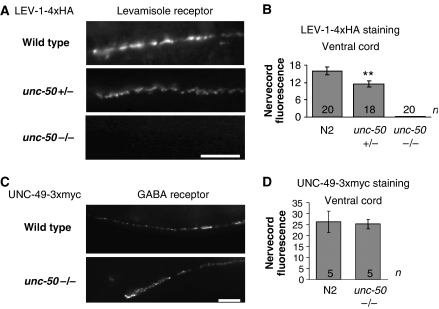
UNC-50 could either be required for cell-surface expression of the Lev-AChRs or for the function of these receptors at the cell surface. To distinguish between these two possibilities, we used a technique that allows the specific detection of cell-surface receptors in living C. elegans animals (Gottschalk et al, 2005) (see Supplementary data). Injection of fluorescently labeled antibody into wild-type animals expressing either levamisole or GABAA epitope-tagged receptor subunits generated a punctate staining pattern (Figure 2A and C, respectively), similar to the staining pattern of endogenous receptors at the NMJs (Gally and Bessereau, 2003; Gally et al, 2004). However, no signal was detected in unc-50 mutants expressing a tagged LEV-1 subunit, suggesting that no Lev-AChRs were present at the cell surface. In animals heterozygous for the unc-50 mutation, the signal of the tagged LEV-1 subunit was reduced by about 30% (Figure 2A and B). Consistently, electrophysiological recording of unc-50/+ animals showed a levamisole response reduced by approximately 50% (data not shown). This result suggests that UNC-50 might be a limiting factor for cell-surface expression of the Lev-AChR. In contrast, the GABAA receptor was expressed at wild-type levels at inhibitory NMJs in unc-50 mutants (Figure 2C and D). Therefore, the lack of UNC-50 specifically prevents the cell-surface expression of the Lev-AChR.
Figure 2.
unc-50 mutants do not display the Lev-AChR at the cell surface. Wild-type, unc-50(e306) heterozygote and unc-50(e306) mutant animals were engineered to express the C-terminally tagged LEV-1-4xHA Lev-AChR subunit (A, B). Similarly wild-type and unc-50(e306) mutant animals were engineered to express the UNC-49-3xmyc GABAA receptor subunit (C, D), and subsequently, fluorescently labeled antibodies against the respective epitopes were injected into the body cavity. After a recovery period of 6 h, in which the body cavity was cleared of unbound antibodies, the signal resulting from antibodies bound to the epitope-tagged receptors at the cell surface was measured and quantified. The signals originating from the ventral cord NMJs are shown in panels A and C, and quantified in panels B and D, respectively. The number of animals counted is indicated and the error bars represent the standard error of the mean (s.e.m.). **P<0.01, t-test. The scale bars correspond to 10 μm.
Lev-AChRs are not detectable in unc-50 mutants
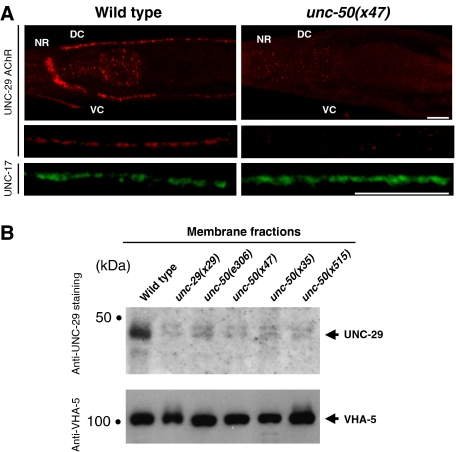
To determine the fate of Lev-AChRs in unc-50 mutants, we used an antibody directed against the AChR subunit UNC-29. In wild-type animals, clustered receptors were detected at NMJs along the ventral and dorsal nerve cords, and in the nerve ring (Figure 3A; Gally et al, 2004). In contrast, there was no staining detectable by immunofluorescence in unc-50 mutants, either at synaptic sites or in intracellular compartments, despite cell permeabilization during the staining procedure (Figure 3A). However, since we knew that endogenous Lev-AChRs escape detection if they are not clustered (Gally et al, 2004), this result did not rule out the possibility that receptors were diffusely distributed in intracellular membranes. To test this possibility, we analyzed isolated membrane fractions by Western blot experiments. In contrast to the wild type, there were no Lev-AChRs detectable in the membrane fractions of the unc-50 mutants (Figure 3B).
Figure 3.
No Lev-AChR can be detected in unc-50 mutants by immunostaining and Western blot analysis. (A) Wild-type (N2) and unc-50(x47) mutant young adult animals were freeze fractured, fixed in methanol/acetone and immunostained with polyclonal antibodies against the Lev-AChR subunit UNC-29. Antibodies to the ACh vesicular transporter UNC-17 were used to detect presynaptic terminals along the nerve cord. The positions of the nerve ring (NR), dorsal-, and ventral cord (DC and VC) NMJs are indicated. The scale bar represents 20 μm. (B) Total membrane fractions of staged L4 wild-type worms, unc-29, and unc-50 mutants were size fractionated by SDS gel electrophoresis and transferred onto a nitrocellulose membrane. The membrane was sequentially probed with antibodies against UNC-29 and VHA-5. The TM subunit of the vacuolar ATPase VHA-5 was used to normalize the membrane fractions.
To determine whether the lack of Lev-AChR expression was due to a transcriptional or post-transcriptional requirement for UNC-50, we analyzed the mRNAs of the Lev-AChR subunits by semi-quantitative RT–PCR. We could not detect significant differences in the expression of the subunits between wild type and unc-50 mutants (data not shown). Altogether, these data indicate that UNC-50 is required at a post-transcriptional step for expression of the levamisole-sensitive receptor in body-wall muscle cells.
UNC-50 acts after receptor assembly
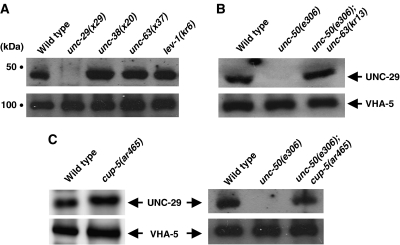
From the data obtained in the mammalian system, we know that the assembly of heteromeric AChRs is highly inefficient and active pathways exist at the ER level for their degradation (Green and Millar, 1995; Wanamaker et al, 2003; Christianson and Green, 2004). The lack of detectable Lev-AChR in unc-50 mutants could thus be due to a translational block of Lev-AChR subunit-encoding mRNAs, or a failure in receptor assembly, resulting in rapid receptor degradation. In order to determine the fate of partially assembled Lev-AChRs in C. elegans muscles, we analyzed the receptor levels in mutants in which one of the subunits, UNC-38, UNC-63 or LEV-1, was missing. Interestingly, unassembled or partially assembled Lev-AChRs could be stably detected by Western blot analysis in these mutants at levels comparable to those in the wild type (Figure 4A). Since all Lev-AChR subunits contain in their first TM domain an ER retention signal conserved in vertebrate AChR subunits (Wang et al, 2002), it is predicted that these unassembled receptors are retained within the ER. In agreement with this, we were never able to detect unassembled Lev-AChRs at the cell surface either by immunostaining of fixed animals or by in vivo antibody injections to label tagged receptors at the cell surface (data not shown).
Figure 4.
Total membrane fractions from staged L4 hermaphrodites of the indicated genotypes were size separated, blotted, and probed with antibodies against the Lev-AChR subunit UNC-29 and against the vacuolar ATPase subunit VHA-5 as a loading control. (A) The unassembled Lev-AChR is stable and can be detected in the mutants unc-38(x20), unc-63(x37), and lev-1(kr6) that lack one of the receptor subunits. While membrane extracts of unc-29 mutants exhibit no detectable UNC-29 staining (1.1±0.6% (n=4)), wild-type levels of staining are seen in unc-38 (115±18% (n=3)), unc-63 (101±11% (n=2)), and lev-1 (107±25% (n=2)) mutants. (B) UNC-50 acts after Lev-AChR assembly. The unassembled receptor can be detected in unc-50; unc-63 double mutants and not in unc-50 mutants. (C) The Lev-AChR is degraded by the lysosomal system. UNC-29 levels in cup-5 mutants (110±6% (n=2)) are comparable to wild-type N2 animals (100% (n=2)), but mutations in cup-5 stabilize the Lev-AChR in an unc-50 mutant background.
The fact that unassembled Lev-AChRs can be stably detected by Western blot experiments allowed us to test whether UNC-50 acts before or after receptor assembly. If UNC-50 is required before AChR assembly, impairing unc-50 in animals missing one of the levamisole AChR subunits should cause disappearance of unassembled receptors. Conversely, if UNC-50 is required for the transport of the Lev-AChR after assembly, mutating unc-50 should not affect the expression level of unassembled receptors. As shown in Figure 4B, the Lev-AChR subunit UNC-29 can be detected by Western blot analysis at similar levels in the membrane fractions of unc-63 and unc-63; unc-50 double mutants (72±20%, n=6). These results suggest that UNC-50 acts at a post-assembly step during trafficking of the Lev-AChR to the synapse.
Lev-AChRs are degraded by the lysosomal system in unc-50 mutants
Our results indicated that Lev-AChR subunits were expressed normally in unc-50 mutants, were able to assemble and potentially leave the ER, but were then mis-addressed and transported aberrantly within the cell. This inappropriate targeting within the cells would lead to the efficient degradation of the Lev-AChR in unc-50 mutants. It has been shown that TM proteins that leave the ER can be degraded by the lysosomal system (Blondel et al, 2004; Pizzirusso and Chang, 2004; Kim et al, 2005). To determine whether assembled Lev-AChRs are degraded by lysosomes in unc-50 mutants, we introduced a cup-5 mutation into the unc-50 mutant background. The C. elegans CUP-5 protein is required for the formation of lysosomes from endosomal–lysosomal hybrid organelles that are generated by late endosome to lysosome transport (Treusch et al, 2004). As a result, cup-5 mutants exhibit giant endosomal–lysosomal hybrid organelles that have reduced lysosomal activities, although the delivery of substrates to these structures occurs normally. Since complete loss of function of CUP-5 leads to embryonic lethality (Hersh et al, 2002), we used the viable cup-5(ar465) point mutation that is expected to retain low levels of CUP-5 activity. As shown in Figure 4C, the levels of UNC-29 are comparable between wild-type and cup-5(ar465) mutant animals. However, introduction of cup-5(ar465) in unc-50 mutants causes a reappearance of the Lev-AChR subunit UNC-29 (Figure 4C, 28±3% of wild-type levels, n=3 independent experiments), despite residual lysosomal function in cup-5(ar465). Despite the reappearance of detectable UNC-29 in Western blot experiments, unc-50; cup-5 double mutants remain as uncoordinated and levamisole resistant as unc-50 single mutants (data not shown), indicating that Lev-AChRs are not rerouted to the cell surface and are still delivered to lysosomes in a cup-5 mutant background. Together, these results suggest that in the absence of UNC-50 Lev-AChRs enter the lysosomal compartment and are efficiently degraded by the lysosomes.
unc-50 encodes an evolutionarily conserved integral membrane protein
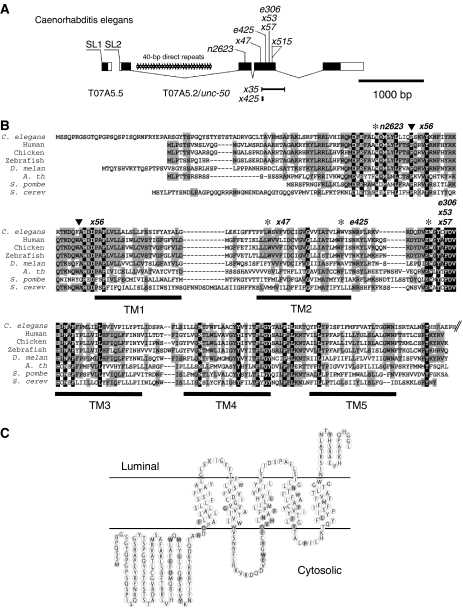
To gain insight into the role of UNC-50 in the trafficking of nicotinic receptors, we cloned the unc-50 gene (Supplementary Figure 2). unc-50 is the downstream gene of an operon, and corresponds to the ORF T07A5.2 on chromosome III (Figure 5A). Sequencing of two cDNA clones of unc-50 (provided by Y Kohara) revealed that unc-50 encodes a protein of 301 amino acids (Figure 5B) that is conserved in its gene structure and sequence between C. elegans and the related nematodes C. briggsae and C. remanei (data not shown). Nearly all unc-50 mutations found in T07A5.2 are nonsense mutations, deletions or insertions leading to frame shifts expected to severely impair UNC-50 function (Figure 5A and 5B; Supplementary Table 1).
Figure 5.
unc-50 encodes an evolutionarily conserved integral membrane protein. (A) Genomic organization of T07A5.2/unc-50. unc-50 is part of an operon, but all unc-50 alleles carry mutations in the gene T07A5.2. The black regions represent coding regions, while white parts represent the untranslated regions. Splice leaders are indicated. (B) ClustalX alignment of UNC-50 with its orthologs from yeast to humans. The locations of the predicted TM regions are indicated. Residues conserved between all species are highlighted in black and conserved residues between most species in gray. The positions of the mutations detected in unc-50 alleles are indicated above the protein sequence, asterisks correspond to stop mutations while the triangles mark the two point mutations found in the allele x56. The GenBank accession numbers for the sequences used can be found in the Supplementary Figure 3. The C-terminus of UNC-50 is truncated in this panel, but is shown in panel C. (C) Proposed membrane topology of UNC-50. The residues conserved between all species are underlined in gray.
The protein encoded by unc-50 is conserved throughout evolution from yeast to humans (Figure 5B). Orthologs of UNC-50 can be found in simple eukaryotic organisms like Entamoeba histolytica HM-1, but not in bacteria (Supplementary Figure 3). No other UNC-50-like protein could be identified in C. elegans. Interestingly, we were also unable to detect a second UNC-50-like protein in organisms such as Arabidopsis and zebrafish, in which a genome duplication has taken place. This suggests that UNC-50-like proteins provide a conserved and unique function in each organism.
Hydropathy analysis of UNC-50 and its orthologs in other organisms showed that this protein family contains five TM domains (Supplementary Figure 4). The N-terminus is predicted to reside in the cytoplasm, whereas the short C-terminus is located in a luminal compartment (Figure 5C). This topology is experimentally supported for the Saccharomyces cerevisiae homolog GMH1 by proteinase K protection assays of membrane fractions (Chantalat et al, 2003). Therefore, the largest domains of UNC-50, as well as the amino acids that are conserved among all species, are facing the cytoplasm (Figure 5C). This makes it likely that a conserved interaction surface is located at the cytosolic side of UNC-50.
UNC-50 is required in body-wall muscles for Lev-AChR trafficking
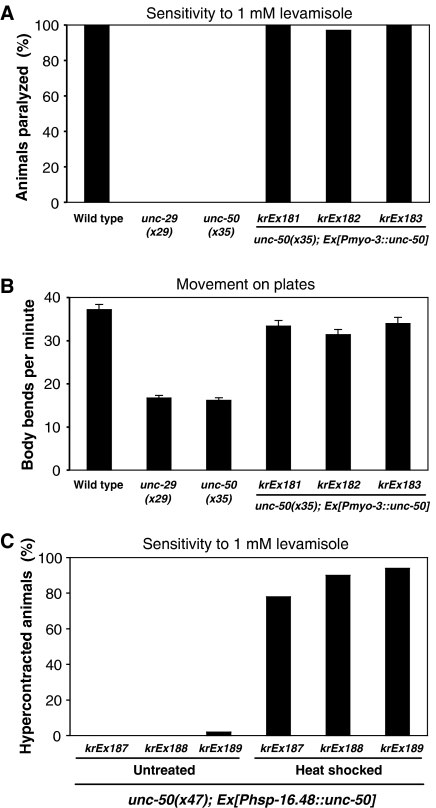
Based on unc-50∷gfp transcriptional and translational fusions, unc-50 is expressed ubiquitously from early embryogenesis to adulthood (Supplementary Figure 5). To determine whether UNC-50 is required only in body-wall muscles for Lev-AChR expression, we expressed UNC-50 under the control of the muscle-specific myo-3 promoter. unc-50 mutants exhibit uncoordinated slow movement and complete resistance to levamisole, identical to mutants expressing no Lev-AChR subunits (Figure 6A and B). Expression of the myo-3∷unc-50 construct was sufficient to completely rescue levamisole sensitivity (Figure 6A) and locomotory defects (Figure 6B). Immunostaining experiments performed on rescued unc-50 mutants indicated that Lev-AChRs were clustered at NMJs (data not shown). Therefore, UNC-50 is cell-autonomously required in body-wall muscles for transport of Lev-AChRs to the cell surface.
Figure 6.
Muscle-specific expression of unc-50 cDNA is sufficient to rescue the resistance to levamisole (A) and the uncoordinated movement phenotype (B) of unc-50 mutant animals. Transgenic unc-50 hermaphrodites express the unc-50 cDNA under the control of the muscle-specific myo-3 promoter from an extrachromosomal array. (C) Conditional expression of the unc-50 cDNA under the control of the heat-shock promoter is able to rescue the levamisole resistance after induction by heat shock in adult animals carrying the transgene.
However, these experiments did not rule out the possibility that UNC-50 might be required during development to generate structures required for the AChR trafficking rather than being necessary for its transport per se. To assess the temporal requirement of unc-50 function during muscle development, we placed the unc-50 cDNA under the control of the heat-shock-inducible promoter hsp-16.48. Transgenic unc-50 mutant animals raised to adulthood at 20°C without a heat shock exhibited the same uncoordinated movement and resistance to levamisole as unc-50 mutants (Figure 6C). These adult animals were then heat-shocked and assayed after 8 h for movement and levamisole sensitivity. Acute expression of UNC-50 in fully differentiated body-wall muscle was sufficient to rescue the uncoordinated movement and to render the transgenic animals sensitive to levamisole (Figure 6C). This experiment indicates that UNC-50 is a necessary factor for the cell-surface trafficking of the Lev-AChR in muscle and is not required for developmental processes within the muscle.
UNC-50 is localized to the Golgi system
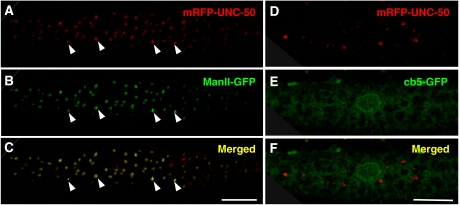
To gain further insight into how UNC-50 mediates the transport of the Lev-AChR, we determined its intracellular localization. UNC-50 was fused to different fluorescent protein tags and expressed in body-wall muscles. Fusions of fluorescent proteins to either the N- or C-terminus of UNC-50 were completely functional as they were able to rescue unc-50-associated phenotypes. In addition, N- and C-terminal UNC-50 fusion proteins showed the same vesicular staining pattern irrespective of the nature of the fluorescent protein tag (data not shown). As shown in Figure 7A, an mRFP-UNC-50 fusion protein expressed in body-wall muscles displayed a punctate distribution throughout the cytoplasm of the muscle cell. This staining is typical of the Golgi apparatus in Drosophila tissues or muscle myotubes (Gu et al, 1989; Rabouille et al, 1999). Indeed, a similar vesicular staining pattern is obtained with the medial Golgi marker α-Mannosidase II (Figure 7B). When confocal stacks were overlaid, mRFP-UNC-50 and Mannosidase II-GFP staining showed 91±3% (n=4) colocalization (Figure 7C). By contrast, no overlap was observed between mRFP-UNC-50 and the GFP-cytochrome b5 (cb5) fusion, which is ER resident. This demonstrates that at steady state UNC-50 is mainly localized to the Golgi system in C. elegans. However, UNC-50 does not seem to be required for the maintenance of the ER or Golgi structures, since the distribution of the GFP-cb5 and Mannosidase II-GFP are similar to wild type in unc-50 mutants (Supplementary Figure 6). Golgi residence has also been shown for the yeast and human ortholog of UNC-50 (Chantalat et al, 2003), suggesting that UNC-50-like proteins localize to the Golgi system in general.
Figure 7.
UNC-50 localizes to the Golgi system. (A–C) mRFP-UNC-50 fusion proteins display a vesicular staining throughout the cytoplasm of a body-wall muscle cell, as shown. This largely overlaps the staining of the Golgi-resident Mannosidase II-GFP fusion protein. Representative Golgi structures are marked by arrowheads. (D–E) The mRFP-UNC-50 fusion protein does not overlap with an ER-localized GFP-cb5 protein. (The scale bar=10 μm.)
UNC-50 can interact with the Sec7 domain-containing Arf-GEF Gea1
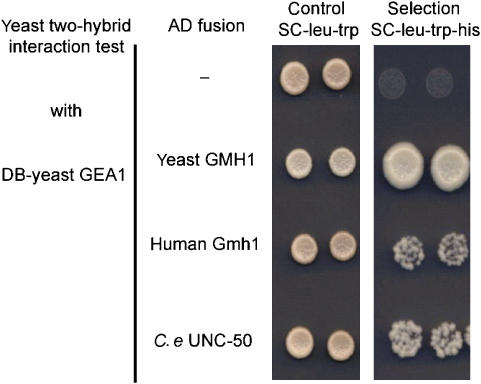
GMH1 is the S. cerevisiae ortholog of UNC-50. Yeast two-hybrid and immunoprecipitation experiments showed that GMH1 binds to Gea1/2p, the large Sec7 domain-containing guanine exchange factors (GEFs) of the small GTPase Arf (Chantalat et al, 2003). Gea1/2p, and their mammalian ortholog GBF1, associate with early components of the secretory pathway (Chantalat et al, 2003; Niu et al, 2005; Zhao et al, 2006). GBF1-dependent Arf activation might regulate the recruitment of the COPI coat protein complex which is necessary for anterograde and retrograde transport processes between ER and Golgi and within the Golgi system (Shima et al, 1999; Garcia-Mata et al, 2003). Since UNC-50-like molecules, Arf GTPases and Sec7 domain-containing GEFs are conserved between yeast and C. elegans (Jackson and Casanova, 2000; Chantalat et al, 2003), we hypothesized that the interaction between UNC-50 and Gea1/2p Arf GEFs might be conserved, as has been shown for the yeast and human UNC-50 orthologs (Chantalat et al, 2003). To test this possibility we fused UNC-50 to the GAL4 transcriptional activation domain and assayed its interaction with the yeast Arf GEF Gea1 using the yeast two-hybrid system. As shown in Figure 8, UNC-50, like the yeast Gmh1 and the human hGmh1, specifically interacted with the C-terminus of the yeast Arf GEF Gea1p. However, the interactions between C. elegans and human UNC-50 molecules and the yeast Gea1p were weaker than that seen between the yeast Gmh1p and the yeast Gea1p (Figure 8). Interactions were lost when using Gea1L862S, a mutation that was shown to strongly reduce the interaction with Gmh1p (Chantalat et al, 2003) (data not shown). This conserved interaction of UNC-50 with a class of large Sec7 domain-containing Arf GEFs suggests that UNC-50 is also likely to be involved in Arf activation at Golgi membranes, and subsequently in COPI-dependent transport processes.
Figure 8.
UNC-50 interacts with the SEC7 domain-containing Arf GEF Gea1p. Yeast two-hybrid interaction test of a yeast DB-GEA1 fusion protein with either the yeast GMH1, the human Gmh1, or C. elegans UNC-50 fused to the GAL4 activation domain (AD). A positive interaction allows yeast cells to grow on selective media lacking histidine.
Discussion
Trafficking receptors to and from synapses is an efficient way to regulate synaptic strength. General factors of the membrane protein synthesis machinery play a well-documented role in the assembly and intracellular transport of ionotropic receptors, but only few factors are known to specifically regulate the biosynthesis of a defined receptor. Using a mutant identified in a screen for resistance to the paralyzing and lethal effects of the nicotinic agonist levamisole in C. elegans, we identified and characterized an integral membrane protein, UNC-50, that is required specifically for the cell-surface trafficking of AChRs sensitive to levamisole. We have shown that UNC-50 is localized to the Golgi system and interacts with Arf-GEFs. In the absence of UNC-50, the assembled Lev-AChR is sorted to a lysosomal degradative pathway. Despite the enormous complexity of AChR repertoire that can be expressed in a cell, regulation of the UNC-50-dependent pathway would provide a means to specifically regulate the biosynthesis of one defined receptor subtype.
UNC-50 might act as a membrane receptor for large Sec7 domain-containing Arf-GEFs
UNC-50 belongs to an evolutionarily conserved protein family that is found in all eukaryotic organisms. Initial transfection experiment using the rat UNC-50 ortholog named UNCL suggested that UNCL localized to the ER and the nuclear membrane in SaOS-2 osteosarcoma cells (Fitzgerald et al, 2000). However, in C. elegans we observed that tagged UNC-50 proteins able to rescue the unc-50 mutant phenotypes localized to the Golgi at steady state. Results similar to ours have recently been reported for yeast and human UNC-50 orthologs. The yeast ortholog of unc-50, GMH1, was initially identified in a multicopy suppressor screen of growth defects caused by mutation of the genes GEA1/2. These genes encode large Sec7 domain-containing Arf-GEFs localized in the Golgi (Peyroche and Jackson, 2001; Garcia-Mata et al, 2003). A physical interaction between Gmh1p and Gea1/2p was further documented by yeast two-hybrid and immunoprecipitation experiments. We show that this interaction is conserved between UNC-50 and Gea1p, similar to the interaction between the human GMH1 and Gea1p.
How might UNC-50/GMH1 function in the trafficking of membrane proteins through the Golgi? The Gea1/2p Arf-GEFs are required for activation of the small GTPase Arf, which in turn is stably associated with Golgi and ER–Golgi intermediate compartment (ERGIC) membranes (Peyroche et al, 1996; Garcia-Mata et al, 2003). The active Arf molecule then recruits the COPI coat complex to these membranes, which leads to a further differentiation of these membranes. COPI binding to ERGIC membranes is required to polarize these membranes into domains of anterograde and retrograde cargo concentration (Shima et al, 1999). COPI function is also required to initiate the microtubule-dependent movement of the ERGIC toward the Golgi (Lippincott-Schwartz et al, 1998; Garcia-Mata et al, 2003). Furthermore, COPI-dependent processes are essential to maintain the integrity of the Golgi system by mediating intra-Golgi retrograde transport (Lippincott-Schwartz et al, 1998). It is believed that COPI-dependent processes are the main driving force for the maturation of Golgi stacks by sorting anterograde from retrograde cargo (Allan and Balch, 1999). In addition Arf activation through Arf GEFs regulates other coat protein assembly and sorting events. Clathrin-coated vesicle formation is also initiated by Arf-dependent recruitment of clathrin adaptors to Golgi subdomains (Nakayama and Wakatsuki, 2003). At this point we cannot exclude the possibility that UNC-50 also influences clathrin-dependent cargo sorting at the Golgi. However, it is unlikely that UNC-50 directly affects actin dynamics, which is also controlled by the Arf GEFs Gea1/2 in yeast (Zakrzewska et al, 2003), since we would expect that all receptors would be affected similarly.
The process of Arf activation, COPI coat recruitment, and subsequent GTP hydrolysis by the Arf molecules regulates the cargo concentration, sorting and uptake at specific Golgi membrane sites (Lanoix et al, 1999; Malsam et al, 1999). All these distinct coat-dependent processes are orchestrated by the activation of the small GTPases Arf through the action of Arf-GEFs. However, the Arf-GEFs do not contain any sequence for membrane attachment or TM domains. It is thus likely that integral membrane proteins like UNC-50 serve as membrane receptors to recruit Arf GEFs to distinct Golgi sites (Chantalat et al, 2003, 2004). Thus, recruitment of cargo proteins by an UNC-50-containing complex would provide a means to sort cargos into specific membrane domains dedicated to specific transport routes.
UNC-50 specifically affects the Lev-AChR transport
The specific sorting defect of one AChR subtype is a striking feature of the unc-50 mutant phenotype in C. elegans. The C. elegans genome encodes up to 42 different ionotropic AChR subunits, the largest number reported in a metazoan (Jones and Sattelle, 2004). The specificity of UNC-50 for the Lev-AChR is particularly intriguing, since RIC-3, the only known factor that is required for AChR assembly within the ER, has been shown to affect the functional expression of all AChR subtypes in C. elegans tested so far (Halevi et al, 2002). RIC-3 is necessary for cholinergic transmission mediated by the neuronal DEG-3/DES-2 AChR, the body-wall muscle levamisole- and nicotine-sensitive receptors, and the pharyngeal muscle AChR EAT-2 (Halevi et al, 2003). Therefore, RIC-3 most likely represents a general factor important for AChR maturation. This is in clear contrast to the AChR subtype specificity displayed by UNC-50, which does not affect the EAT-2 and DES-2/DEG-3 AChRs (data not shown) or the ACR-16-containing AChRs in muscle. Such specificity makes it highly unlikely that UNC-50 is affecting general transport in an unspecific manner that would impair AChR expression indirectly.
How is such specificity achieved? UNC-50 is conserved in organisms such as plants and yeast that do not express AChRs. In C. elegans, unc-50 is ubiquitously expressed in cells outside of the nervous system that most likely do not express AChRs. This suggests that other substrates for UNC-50 may exist. However, apart from impaired movement due to the loss of the levamisole receptor, C. elegans unc-50 mutants exhibit only marginal phenotypes including a slightly smaller body size and a low penetrance gonad migration defect (S Eimer and J-L Bessereau, unpublished observations). In line with these observations in C. elegans, S. cerevisiae deletion mutants of GMH1, the yeast ortholog of UNC-50, are completely viable and display no obvious abnormal phenotype (Chantalat et al, 2003). This further suggests that trafficking pathways redundant to UNC-50 might exist on a cellular level. The Lev-AChR might exclusively use this UNC-50-dependent pathway and solely rely on UNC-50-dependent transport to reach the cell surface. For example, UNC-50 might be necessary to generate a subset of Golgi membrane domains required for cell-surface trafficking of Lev-AChRs. Alternatively, UNC-50 might be required to sort a factor that directly interacts with the Lev-AChR to promote its export to the cell surface. In this respect, it is interesting to note that mutations in the C. elegans gene unc-74 confer the same levamisole resistance as unc-50 mutants and cause the same complete disappearance of levamisole-binding sites in their membranes as seen in unc-50 (Lewis et al, 1987). The integration of an additional factor mediating AChR specificity would thus also partially explain why UNC-50 is evolutionary conserved, but the cargo affected is not present in every organism.
A novel mechanism for the control of ionotropic AChR expression
A tight system of control checkpoints exists to ensure that only properly folded and assembled AChRs are displayed at the cell surface. One of the best-documented control systems operates at the ER level. There, newly synthesized AChR subunits are immediately bound by the ER chaperones calnexin and Bip/Hsp70, from which they detach upon correct maturation and assembly (Forsayeth et al, 1992; Gelman et al, 1995). The association with the ER-resident chaperones retains the unassembled AChR subunits in the ER, metabolically stabilizes them, and supports their correct folding and maturation. In C. elegans, tandem affinity purification of tagged UNC-29 or LEV-1 subunits showed that unassembled AChRs co-purify with the ER chaperones HSP-1, HSP-3, and HSP-4, the C. elegans Bip/Hsp70 homologs. When the purification is designed to primarily isolate assembled AChRs, the association of LEV-1 and UNC-29 with Bip is dramatically reduced (Gottschalk et al, 2005). This suggests that Bip does bind unassembled Lev-AChR subunits in C. elegans, and that this interaction is lost during further folding and assembly of the receptor.
While the association with the ER resident chaperones may assist the correct folding of the individual subunits, a second ER-based control mechanism has been reported that ensures the pentameric assembly of the AChRs. It has been shown that unassembled AChRs are retained in the ER by a short sequence PL(Y/F)(F/Y)XXN present in the first TM domain of the α4 subunit that is buried upon full assembly of the pentamer (Wang et al, 2002). This sequence can also be found in all of the Lev-AChR subunits with minor alterations (S Eimer, unpublished observation). Therefore, it is likely that in C. elegans, unassembled Lev-AChR subunits and receptors are also retained within the ER. These unassembled receptors can be stably detected in membrane extracts of mutants in which one of the Lev-AChR subunits is not produced. This is in agreement with previous reports showing that endogenous ER-retained receptors are metabolically stable in mouse myotubes (Gu et al, 1989), as well as when one subunit is missing (Black et al, 1987). Additionally, an Arg–Lys motif in the large cytoplasmic loop of the subunits has been implicated in Golgi-to-ER retrieval of subunits and unassembled complexes that have escaped out of the ER (Keller et al, 2001; Ren et al, 2005). This work suggests that an additional control mechanism may exist that operates within the ER–Golgi intermediate compartment to prevent immature AChRs from reaching the cell surface.
Our analysis of C. elegans unc-50 mutants revealed a regulation of AChR biogenesis that operates after AChR assembly. The assembled Lev-AChR has the option either to be delivered to the cell surface or to enter the lysosomal route and be degraded. We showed here that UNC-50 function is required to sort the Lev-AChR to the cell surface and, at the same time, prevent the receptor from entering the lysosomal route and degradation. Since the yeast ortholog of UNC-50, Gmh1p, was demonstrated to cycle from the Golgi to the ER, we cannot exclude the possibility that in the absence of UNC-50, assembled Lev-AChRs bypass the Golgi and are directly sent to lysosomes. Alternatively, UNC-50 might achieve its function within the Golgi apparatus by supporting a COPI-dependent sorting event, which would be necessary to keep the Lev-AChR en route to the plasma membrane. Several mechanisms can be used to sort defective proteins for lysosomal degradation after they exit the ER (reviewed in Arvan et al, 2002). It was recently shown in tobacco that the chaperone BiP can exit the ER with a subpopulation of ligands and target them to the lytic vacuole (Pimpl et al, 2006). One possible scenario could be that UNC-50, by recruiting Arf-GEFs to a subset of Golgi membranes, would sort a factor away from the receptor into the COPI-dependent retrograde route that otherwise would tag the Lev-AChR for lysosomal degradation.
The analysis of AChR synthesis in unc-50 mutants has unmasked a previously unsuspected degradative pathway for nicotinic receptors. The existence of post-assembly sorting from a degradative pathway would be worth exploring for other ligand-gated ionotropic receptors, since shifting the destiny of assembled receptors toward either degradation or cell-surface delivery would provide a means to rapidly modify the number of receptors delivered at the cell surface under physiological or pathological conditions. Genetic strategies in C. elegans will provide a means to further dissect the mechanisms of UNC-50-dependent AChR trafficking.
Materials and methods
Strains and genetics
C. elegans strains were cultured as described previously (Brenner, 1974) and kept at 20°C, if not otherwise stated. The following mutations and combinations thereof were used in this study: LG I: unc-29(x29), unc-38(x20), unc-63(kr13, x37); LG III: cup-5(ar465), unc-50(e306, x35, x47, x515); LG IV: lev-1(kr6); LG X: lin-15(n765ts). All mutations are described in wormbase (www.wormbase.org), unless otherwise noted.
Expression constructs and transgenic animals are described in Supplementary data.
Electrophysiology
Electrophysiological methods were performed as described previously (Richmond and Jorgensen, 1999). Further details are provided in Supplementary data.
Antibodies and immunostaining
Antibodies against UNC-29 were generated and purified as described previously (Gally et al, 2004). For immunostaining experiments, mixed-stage worms were fixed with methanol/acetone and stained according to the freeze fracture protocol (Gally et al, 2004).
Protein extraction and Western blotting
Protein extracts were prepared and probed as described (Gally et al, 2004; see Supplementary data).
Test for levamisole sensitivity and movement
To test the sensitivity to levamisole, well-fed young adult hermaphrodites were transferred onto plates containing 1 mM levamisole and assayed for lack of hypercontraction and their ability to move after 1 h. To quantitatively assess the movement of wild-type and mutant animals, worms were transferred to unseeded plates and after a two 2-min interval of adjustment the body bends per minute (corresponds to a whole 360° sine wave) were counted over a 1-min time interval. For each animal tested, five consecutive intervals were averaged.
Two-hybrid analysis
The interaction test of yeast proteins GMH1 and GEA1 within the two-hybrid system have been described previously (Chantalat et al, 2003). The GEA1(aa 749–1408) GAL4 DNA-binding domain (DB) fusion construct was kindly provided by A Peyroche and S Chantalat. pGADT7 (Clontech) was used to construct the fusions of UNC-50, GMH1, and hGmh1 to the GAL4 activation domain. The constructs were transformed into strain AH109 (Clontech) and analyzed according the manufacturer's protocol. As a negative control, the L862S mutation was introduced into GEA1(aa 749–1408) GAL4DB construct and the interactions with UNC-50, GMH1, and hGmh1 were assayed again. The L862S mutation has been shown to strongly reduce the interaction between Gmh1 and Gea1 (Chantalat et al, 2003).
Supplementary Material
Supplementary data
Acknowledgments
We thank J Lewis for isolating some of the unc-50 strains, M Labouesse for the anti-VHA-5 antibodies, J Rand for the anti-UNC-17 antibodies and B Bamber and A Benham for the UNC-49∷3 × MYC strain. We are grateful to Anne Peyroche and Sophie Chantalat for providing reagents and discussions, to Yuji Kohara for providing unc-50 ESTs, to Daniel Williams and Erik Jorgensen for sharing unpublished results, and to Bernard Lakowski for his comments on the manuscript. Some nematode strains used in this work were provided by the CGC, which is funded by the NIH NCRR. SE was an EMBO Long-Term Fellow and funded by the Fondation pour la Recherche Medicale, AG was a Long-Term Fellow of the Human Frontier Science Program. MH was supported by a predoctoral fellowship from the National Science and Engineering Council of Canada. HRH was supported by National Institutes of Health Grant GM24663. HRH is the David H Koch Professor of Biology at MIT, and an Investigator of the Howard Hughes Medical Institute. WRS was funded by NIH grants, JR by the NIH grant RO1NS41477-03, JLB was supported by an INSERM Avenir grant and by the Association Française contre les Myopathies.
References
- Allan BB, Balch WE (1999) Protein sorting by directed maturation of Golgi compartments. Science 285: 63–66 [DOI] [PubMed] [Google Scholar]
- Arvan P, Zhao X, Ramos-Castaneda J, Chang A (2002) Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic 3: 771–780 [DOI] [PubMed] [Google Scholar]
- Ballivet M, Alliod C, Bertrand S, Bertrand D (1996) Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J Mol Biol 258: 261–269 [DOI] [PubMed] [Google Scholar]
- Black R, Goldman D, Hochschwender S, Lindstrom J, Hall ZW (1987) Genetic variants of C2 muscle cells that are defective in synthesis of the alpha-subunit of the acetylcholine receptor. J Cell Biol 105: 1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel MO, Morvan J, Dupre S, Urban-Grimal D, Haguenauer-Tsapis R, Volland C (2004) Direct sorting of the yeast uracil permease to the endosomal system is controlled by uracil binding and Rsp5p-dependent ubiquitylation. Mol Biol Cell 15: 883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S, Courbeyrette R, Senic-Matuglia F, Jackson CL, Goud B, Peyroche A (2003) A novel Golgi membrane protein is a partner of the ARF exchange factors Gea1p and Gea2p. Mol Biol Cell 14: 2357–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat S, Park SK, Hua Z, Liu K, Gobin R, Peyroche A, Rambourg A, Graham TR, Jackson CL (2004) The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci 117: 711–722 [DOI] [PubMed] [Google Scholar]
- Christianson JC, Green WN (2004) Regulation of nicotinic receptor expression by the ubiquitin–proteasome system. EMBO J 23: 4156–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP (2000) Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol 40: 431–458 [DOI] [PubMed] [Google Scholar]
- Culetto E, Baylis HA, Richmond JE, Jones AK, Fleming JT, Squire MD, Lewis JA, Sattelle DB (2004) The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J Biol Chem 279: 42476–42483 [DOI] [PubMed] [Google Scholar]
- Devreotes PN, Fambrough DM (1975) Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol 65: 335–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J, Kennedy D, Viseshakul N, Cohen BN, Mattick J, Bateman JF, Forsayeth JR (2000) UNCL, the mammalian homologue of UNC-50, is an inner nuclear membrane RNA-binding protein. Brain Res 877: 110–123 [DOI] [PubMed] [Google Scholar]
- Fleming JT, Squire MD, Barnes TM, Tornoe C, Matsuda K, Ahnn J, Fire A, Sulston JE, Barnard EA, Sattelle DB, Lewis JA (1997) Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci 17: 5843–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsayeth JR, Gu Y, Hall ZW (1992) BiP forms stable complexes with unassembled subunits of the acetylcholine receptor in transfected COS cells and in C2 muscle cells. J Cell Biol 117: 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Evans SP, Jensen M, Madsen DM, Mancuso J, Norman KR, Maricq AV (2005) The Ror receptor tyrosine kinase CAM-1 is required for ACR-16-mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron 46: 581–594 [DOI] [PubMed] [Google Scholar]
- Gally C, Bessereau JL (2003) GABA is dispensable for the formation of junctional GABA receptor clusters in Caenorhabditis elegans. J Neurosci 23: 2591–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally C, Eimer S, Richmond JE, Bessereau JL (2004) A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature 431: 578–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Szul T, Alvarez C, Sztul E (2003) ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum–Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol Biol Cell 14: 2250–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman MS, Chang W, Thomas DY, Bergeron JJ, Prives JM (1995) Role of the endoplasmic reticulum chaperone calnexin in subunit folding and assembly of nicotinic acetylcholine receptors. J Biol Chem 270: 15085–15092 [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F (2004) Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 74: 363–396 [DOI] [PubMed] [Google Scholar]
- Gottschalk A, Almedom RB, Schedletzky T, Anderson SD, Yates JR III, Schafer WR (2005) Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J 24: 2566–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WN, Millar NS (1995) Ion-channel assembly. Trends Neurosci 18: 280–287 [PubMed] [Google Scholar]
- Gu Y, Black RA, Ring G, Hall ZW (1989) A C2 muscle cell variant defective in transport of the acetylcholine receptor to the cell surface. J Biol Chem 264: 11952–11957 [PubMed] [Google Scholar]
- Halevi S, McKay J, Palfreyman M, Yassin L, Eshel M, Jorgensen E, Treinin M (2002) The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J 21: 1012–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M (2003) Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 278: 34411–34417 [DOI] [PubMed] [Google Scholar]
- Hersh BM, Hartwieg E, Horvitz HR (2002) The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc Natl Acad Sci USA 99: 4355–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Casanova JE (2000) Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol 10: 60–67 [DOI] [PubMed] [Google Scholar]
- Jones AK, Buckingham SD, Sattelle DB (2005) Chemistry-to-gene screens in Caenorhabditis elegans. Nat Rev Drug Discov 4: 321–330 [DOI] [PubMed] [Google Scholar]
- Jones AK, Sattelle DB (2004) Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays 26: 39–49 [DOI] [PubMed] [Google Scholar]
- Keller SH, Lindstrom J, Ellisman M, Taylor P (2001) Adjacent basic amino acid residues recognized by the COP I complex and ubiquitination govern endoplasmic reticulum to cell surface trafficking of the nicotinic acetylcholine receptor alpha-subunit. J Biol Chem 276: 18384–18391 [DOI] [PubMed] [Google Scholar]
- Kim Y, Lampert SM, Philpott CC (2005) A receptor domain controls the intracellular sorting of the ferrichrome transporter, ARN1. EMBO J 24: 952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix J, Ouwendijk J, Lin CC, Stark A, Love HD, Ostermann J, Nilsson T (1999) GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J 18: 4935–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JA, Elmer JS, Skimming J, McLafferty S, Fleming J, McGee T (1987) Cholinergic receptor mutants of the nematode Caenorhabditis elegans. J Neurosci 7: 3059–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JA, Wu CH, Berg H, Levine JH (1980) The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 95: 905–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Cole NB, Donaldson JG (1998) Building a secretory apparatus: role of ARF1/COPI in Golgi biogenesis and maintenance. Histochem Cell Biol 109: 449–462 [DOI] [PubMed] [Google Scholar]
- Malsam J, Gommel D, Wieland FT, Nickel W (1999) A role for ADP ribosylation factor in the control of cargo uptake during COPI-coated vesicle biogenesis. FEBS Lett 462: 267–272 [DOI] [PubMed] [Google Scholar]
- Merlie JP, Lindstrom J (1983) Assembly in vivo of mouse muscle acetylcholine receptor: identification of an alpha subunit species that may be an assembly intermediate. Cell 34: 747–757 [DOI] [PubMed] [Google Scholar]
- Nakayama K, Wakatsuki S (2003) The structure and function of GGAs, the traffic controllers at the TGN sorting crossroads. Cell Struct Funct 28: 431–442 [DOI] [PubMed] [Google Scholar]
- Niu TK, Pfeifer AC, Lippincott-Schwartz J, Jackson CL (2005) Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi. Mol Biol Cell 16: 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyroche A, Jackson CL (2001) Functional analysis of ADP-ribosylation factor (ARF) guanine nucleotide exchange factors Gea1p and Gea2p in yeast. Methods Enzymol 329: 290–300 [DOI] [PubMed] [Google Scholar]
- Peyroche A, Paris S, Jackson CL (1996) Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature 384: 479–481 [DOI] [PubMed] [Google Scholar]
- Pimpl P, Taylor JP, Snowden C, Hillmer S, Robinson DG, Denecke J (2006) Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell 18: 198–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzirusso M, Chang A (2004) Ubiquitin-mediated targeting of a mutant plasma membrane ATPase, Pma1-7, to the endosomal/vacuolar system in yeast. Mol Biol Cell 15: 2401–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Kuntz DA, Lockyer A, Watson R, Signorelli T, Rose DR, van den Heuvel M, Roberts DB (1999) The Drosophila GMII gene encodes a Golgi alpha-mannosidase II. J Cell Sci 112 (Part 19): 3319–3330 [DOI] [PubMed] [Google Scholar]
- Ren XQ, Cheng SB, Treuil MW, Mukherjee J, Rao J, Braunewell KH, Lindstrom JM, Anand R (2005) Structural determinants of alpha4beta2 nicotinic acetylcholine receptor trafficking. J Neurosci 25: 6676–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM (1999) One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci 2: 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SG (1999) Ion channels and the genetic contribution to epilepsy. J Child Neurol 14: 58–66 [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 2: 791–805 [DOI] [PubMed] [Google Scholar]
- Shima DT, Scales SJ, Kreis TE, Pepperkok R (1999) Segregation of COPI-rich and anterograde-cargo-rich domains in endoplasmic-reticulum-to-Golgi transport complexes. Curr Biol 9: 821–824 [DOI] [PubMed] [Google Scholar]
- Singh A, Potter A, Newhouse P (2004) Nicotinic acetylcholine receptor system and neuropsychiatric disorders. IDrugs 7: 1096–1103 [PubMed] [Google Scholar]
- Smith MM, Lindstrom J, Merlie JP (1987) Formation of the alpha-bungarotoxin binding site and assembly of the nicotinic acetylcholine receptor subunits occur in the endoplasmic reticulum. J Biol Chem 262: 4367–4376 [PubMed] [Google Scholar]
- Sweileh W, Wenberg K, Xu J, Forsayeth J, Hardy S, Loring RH (2000) Multistep expression and assembly of neuronal nicotinic receptors is both host-cell- and receptor-subtype-dependent. Brain Res Mol Brain Res 75: 293–302 [DOI] [PubMed] [Google Scholar]
- Touroutine DV, Fox RM, Von Stetina SE, Burdina AO, Miller DM III, Richmond JE (2005) ACR-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the C. elegans neuromuscular junction. J Biol Chem 280: 27013–27021 [DOI] [PubMed] [Google Scholar]
- Towers PR, Edwards B, Richmond JE, Sattelle DB (2005) The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor alpha subunit. J Neurochem 93: 1–9 [DOI] [PubMed] [Google Scholar]
- Treusch S, Knuth S, Slaugenhaupt SA, Goldin E, Grant BD, Fares H (2004) Caenorhabditis elegans functional orthologue of human protein h-mucolipin-1 is required for lysosome biogenesis. Proc Natl Acad Sci USA 101: 4483–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol 346: 967–989 [DOI] [PubMed] [Google Scholar]
- Wanamaker CP, Christianson JC, Green WN (2003) Regulation of nicotinic acetylcholine receptor assembly. Ann N Y Acad Sci 998: 66–80 [DOI] [PubMed] [Google Scholar]
- Wang JM, Zhang L, Yao Y, Viroonchatapan N, Rothe E, Wang ZZ (2002) A transmembrane motif governs the surface trafficking of nicotinic acetylcholine receptors. Nat Neurosci 5: 963–970 [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Mathias A, Gautam M, Hall ZW (1999) Metabolic stabilization of muscle nicotinic acetylcholine receptor by rapsyn. J Neurosci 19: 1998–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ (2003) The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci 72: 2055–2061 [DOI] [PubMed] [Google Scholar]
- Zakrzewska E, Perron M, Laroche A, Pallotta D (2003) A role for GEA1 and GEA2 in the organization of the actin cytoskeleton in Saccharomyces cerevisiae. Genetics 165: 985–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Claude A, Chun J, Shields DJ, Presley JF, Melancon P (2006) GBF1, a cis-Golgi and VTCs-localized ARF-GEF, is implicated in ER-to-Golgi protein traffic. J Cell Sci 119: 3743–3753 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data