Abstract
Coevolution with parasites is thought to maintain genetic diversity in host populations. However, while there are sound theoretical reasons to expect heterozygosity and parasite resistance to be related, this pattern has generally been shown only in inbred laboratory and island populations. This leaves doubt as to whether parasite-mediated selection for genetic diversity is in fact a general process. Here we show that haematozoan parasite load is linked to two complementary measures of microsatellite variability in an outbred population of mountain white-crowned sparrows (Zonotrichia leucophrys oriantha) for which we know that parasites reduce fitness. Moreover, each of the genetic measures predicts a subtly different aspect of parasitism. Microsatellite heterozygosity is related to an individual's risk of parasitism, and mean d2 (a broader, more long-term measure of parental relatedness) to the severity of infection among parasitized individuals.
Keywords: parasite–host interaction, population genetics, genetic diversity, haematozoa, heterozygosity
1. Introduction
Heterozygosity and fitness are related in many taxa, so that relatively heterozygous (‘outbred’) individuals are often fitter than more homozygous (‘inbred’) individuals (Brown 1997; Keller & Waller 2002). Resistance to parasites (Hamilton & Zuk 1982) represents a promising mechanism for this relationship, either because inbreeding depression compromises individuals' ability to control infection, or because outbred individuals have a broader array of pathogen resistance (Coltman et al. 1999). Parasites should impose a selection pressure favouring genetic diversity in their hosts whenever resistance to parasites is superior among genetically diverse individuals (Altizer et al. 2001). Interestingly, however, the predicted relationship between parasites and heterozygosity has generally been found only in inbred captive lines (e.g. Arkush et al. 2002) and island populations (Coltman et al. 1999; Reid et al. 2003), or in opportunistically sampled individuals from unknown populations of origin (Acevedo-Whitehouse et al. 2003). This raises the question of whether parasite-mediated selection for genetic diversity is in fact a general process, or restricted to highly inbred systems.
We investigated the relationship between individual parasite load and two complementary measures of genetic variability (microsatellite heterozygosity and diversity) in a large migratory population of mountain white-crowned sparrows (Zonotrichia leucophrys oriantha). Individuals were screened for the presence and intensity of infection by blood-borne Haemoproteus, the most common haematozoan in this population and one known to negatively affect several aspects of fitness including body condition (Richardson 1997), male mating success (MacDougall-Shackleton et al. 2002) and nesting success (Derryberry et al. 2003). For each individual, we calculated microsatellite heterozygosity and mean d2, a measure of within-individual diversity based on the squared difference in repeat units between alleles (Coulson et al. 1998). These two measures provide slightly different information concerning an individual's degree of inbreeding versus outbreeding. Heterozygosity reflects recent matings between related ancestors, whereas mean d2 measures the relatedness of parental haplotypes via more distant events in the pedigree, such as long-term divergence between subpopulations (Neff 2004a,b).
2. Methods
(a) Study population and subjects
We determined microsatellite genotypes and blood-borne parasite loads for 48 adult male mountain white-crowned sparrows breeding near Tioga Pass, California, during May and June of 1999. These birds are highly mobile throughout their breeding range, and ubiquitous in the subalpine meadows of the Sierra Nevada. The study population has been shown to harbour a high level of genetic variability as assessed by microsatellite markers (MacDougall-Shackleton & MacDougall-Shackleton 2001; MacDougall-Shackleton et al. 2002); the frequent presence of ‘immigrants’ (males singing songs characteristic of other geographical areas) further suggests that subpopulations are connected by occasional migration. Subjects included seven males that, based upon song characteristics, appeared to be immigrants to the study area. These males bred normally within the study population, and did not differ from local singers (n=41) in terms of either heterozygosity (immigrants' mean (s.e.)=0.67 (0.08); locals' mean (s.e.)=0.65 (0.02); t=0.422, p=0.68) or mean d2 (immigrants' mean (s.e.)=14.33 (4.53), locals' mean (s.e.)=13.04 (1.65); t=0.291, p=0.77). Females were not included in this study because immigrant/local status cannot be established for females, who do not sing, and because females are hemizygous at one of the (sex-linked) loci used.
We captured individuals in seed-baited Potter traps in May 1999 before the onset of nesting. A small (<50 μl) blood sample was collected from each bird via brachial venipuncture, which was used for subsequent genetic analyses and to determine parasite load (see § 2b,c below).
(b) Genetic analyses
We determined microsatellite genotypes at the loci Gf 01, 05, and 06 (Petren 1998), Mme 1 and 7 (Jeffrey et al. 2001), and Escμ 1 and 4 (Hanotte et al. 1994). All of these loci were in Hardy–Weinberg equilibrium, and no pairs of loci were found to be in linkage disequilibrium. Heterozygosity was calculated as the proportion of these seven loci at which an individual was heterozygous. For each individual, d2 was calculated at each locus by subtracting the number of repeat units for one allele from the number of repeat units for the other allele, and squaring the result; mean d2 was calculated for each individual as the individual's average d2 value across all seven loci (Coulson et al. 1998).
(c) Parasite analyses
We prepared thin films from blood collected from each male shortly (less than one month) after spring arrival on the breeding grounds, before the onset of nesting. We allowed slides to air-dry before fixing in 100% methanol and subsequent staining. For each slide, we examined 10 fields of view (each containing approximately 1000 erythrocytes) under oil immersion, and noted the number of haematozoa (genus Haemoproteus) present. These parasites are transmitted from infected to uninfected hosts via biting flies (Ceratopogonidae and Hippoboscidae; Atkinson & van Riper 1991). Parasite loads (number of Haemoproteus per 10 000 erythrocytes) were then normalized via square-root transformation.
3. Results
Parasite load was negatively associated with both heterozygosity (generalized linear model F47=8.209, r2=0.133, p=0.006) and mean d2 (F47=5.107, r2=0.080, p=0.029). Interestingly, heterozygosity and mean d2 each appeared to predict different aspects of parasitism. Infected birds (n=12) were less heterozygous than uninfected birds (n=36; t=2.357, p=0.023; figure 1), but did not differ in mean d2 (infected mean (s.e.)=9.182 (1.990); uninfected mean (s.e.)=14.578 (1.904); t=1.539, p=0.131). Conversely, mean d2 but not heterozygosity was negatively related to parasite load among infected birds (generalized linear model: mean d2, F11=10.920, r2=0.522, p=0.008; figure 2; heterozygosity, F11=1.847, r2=0.072, p=0.20). Non-parametric tests (Spearman rank correlations and Mann–Whitney U-test) yielded qualitatively identical results in all cases. Thus, heterozygosity appears to be linked to the risk of Haemoproteus infection, while mean d2 is related to the intensity of infection among parasitized individuals.
Figure 1.
Mountain white-crowned sparrows infected by Haemoproteus (n=12) had lower microsatellite heterozygosity than did uninfected individuals (n=36). Values shown are means+s.e.
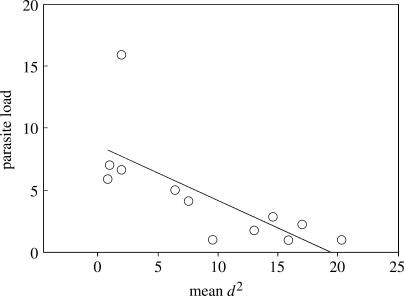
Figure 2.
Parasite load (number of Haemoproteus per 10 000 erythrocytes) of infected mountain white-crowned sparrows decreased with increasing mean d2. Parasite load values have been square-root transformed.
4. Discussion
Our results confirm the existence of parasite-mediated selection favouring genetic variability in an outbred non-island population for which we know parasites reduce reproductive success. Microsatellites are selectively neutral, so the relationship between genetic variability and parasite load is probably owing either to (i) a general effect whereby heterozygosity at these neutral markers is related to an individual's inbreeding coefficient and thus to heterozygosity throughout the genome, including at disease-resistance loci, or (ii) a local effect in that the markers studied are in linkage disequilibrium with disease-resistance loci (Hansson & Westerberg 2002). That is, microsatellite heterozygosity and diversity are presumably associated with heterozygosity and diversity at functional loci that affect an individual's response to parasites and other pathogens.
Parasite load is generally thought to reflect immunocompetence, such that intense infection suggests a poor ability to control parasites (van Riper et al. 1986; Atkinson & van Riper 1991). The correlational nature of our study precludes distinguishing between differences in susceptibility versus differences in exposure to parasites (for example, via behavioural mechanisms) to explain the lower parasite loads of genetically variable individuals. Clearly, experimental immune challenges (Reid et al. 2003) are warranted. Similarly, without knowing whether infections were acquired at the breeding grounds or earlier (over winter or during migration), we cannot rule out the possibility that some individuals may have had latent infections undetectable by blood films. However, this possibility was minimized by collecting blood samples at the beginning of the breeding season, when individuals are likely to be physiologically stressed and latent infections are likely to recur (Scheuerlein & Ricklefs 2004).
Our finding that different genetic measures (heterozygosity and mean d2) each predict a distinct aspect of parasitemia (risk of infection versus severity of infection) should be viewed with some caution. For example, heterozygosity and mean d2 differ in their respective distributions, especially when a limited number of loci are surveyed, which could explain some of the observed differences in predictive power. Still, the observed relationships between heterozygosity and infection risk, and mean d2 and infection intensity, are intriguing and demand further exploration. Heterozygosity may be linked to the likelihood of possessing a parasite-specific immune factor, thus reducing the risk of initial infection. Conversely, high values for mean d2 might be associated with general, highly polygenic measures of health and the ability to minimize parasite load once these infections are acquired.
Whatever the mechanisms, these findings are particularly intriguing in light of the fact that males immigrating to this population suffer higher haematozoan parasite loads and lower mating success than do local males (MacDougall-Shackleton et al. 2002). Thus, heterozygote advantage in terms of parasite load may counteract the high parasitemia of immigrants, who are likely to produce the most heterozygous offspring. A parallel situation has been reported in an island population of song sparrows (Melospiza melodia) in which immigrants are less likely than residents to breed, but the outbred offspring of these immigrants have high survivorship (Marr et al. 2002). Similarly, migration among subpopulations may (through maintaining genetic variability) enhance the ability of mountain white-crowned sparrows to cope with endoparasites and other pathogens. Parasite-mediated maintenance of genetic diversity may thus be both more general and more complex than previously thought.
Acknowledgments
We thank Scott MacDougall-Shackleton, Jeff Markert, Bryan Neff and Trevor Pitcher and two anonymous referees for comments on the manuscript; Shallin Busch, Sharon Lee and Regan Marsh for field assistance; and Marty Morton for field advice. This work was supported by the National Science Foundation (NSF 9801580, 9806765).
References
- Acevedo-Whitehouse K, Gulland F, Greig D, Amos W. Disease susceptibility in California sea lions. Nature. 2003;422:35. doi: 10.1038/422035a. [DOI] [PubMed] [Google Scholar]
- Altizer S, Foufopoulos J, Gager A. Conservation and diseases. In: Levin S.A, editor. Encyclopedia of biodiversity. Vol. 2. Academic Press; San Diego, CA: 2001. pp. 109–126. [Google Scholar]
- Arkush K.D, Giese A.R, Mendenca H.L, McBride A.M, Marty G.D, Hedrick P.W. Resistance to three pathogens in the endangered winter-run Chinook salmon (Oncorhyncus tshawytscha): effects of inbreeding and major histocompatibility complex genotypes. Can. J. Fish. Aquat. Sci. 2002;59:966–975. [Google Scholar]
- Atkinson C.T, van Riper C., III . Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye J.E, Zuk M, editors. Bird–parasite interactions: ecology, evolution and behaviour. Oxford University Press; Oxford: 1991. pp. 19–48. [Google Scholar]
- Brown J.L. A theory of mate choice based on heterozygosity. Behav. Ecol. 1997;8:60–65. [Google Scholar]
- Coltman P.W, Pilkington J.G, Smith J.A, Pemberton J.M. Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. [DOI] [PubMed] [Google Scholar]
- Coulson T.N, Pemberton J.M, Albon S.D, Beaumont M, Marshall T.C, Slate J, Guinness F.E, Clutton-Brock T.H. Microsatellites reveal heterosis in red deer. Proc. R. Soc. B. 1998;265:489–495. doi: 10.1098/rspb.1998.0321. (doi:10.1098/rspb.1998.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry E.P, Foufopoulos J, Dobson A.P, MacDougall-Shackleton E.A, Hahn T.P. An investigation of the effects of two haematozoa on reproductive success in mountain white-crowned sparrows. Int. Comp. Biol. 2003;42:1219. [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Hanotte O, Zanon C, Pugh A, Greig C, Dixon A, Burke T. Isolation and characterization of microsatellite loci in a passerine bird—the reed bunting Emberiza schoeniclus. Mol. Ecol. 1994;3:529–530. doi: 10.1111/j.1365-294x.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002;11:2467–2474. doi: 10.1046/j.1365-294x.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- Jeffrey K.J, Keller L.F, Arcese P, Bruford M.W. The development of microsatellite loci in the song sparrow, Melospiza melodia (Aves) and genotyping errors associated with good quality DNA. Mol. Ecol. Notes. 2001;1:11–13. [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. [Google Scholar]
- MacDougall-Shackleton E.A, MacDougall-Shackleton S.A. Cultural and genetic evolution in mountain white-crowned sparrows: song dialects are associated with population genetic structure. Evolution. 2001;55:2568–2575. doi: 10.1111/j.0014-3820.2001.tb00769.x. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton E.A, Derryberry E.P, Hahn T.P. Non-local male mountain white-crowned sparrows have lower paternity and higher parasite loads than males singing local dialect. Behav. Ecol. 2002;13:682–689. [Google Scholar]
- Marr A.B, Keller L.F, Arcese P. Heterosis and outbreeding depression in descendants of natural immigrants to an inbred population of song sparrows (Melospiza melodia) Evolution. 2002;56:131–142. doi: 10.1111/j.0014-3820.2002.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Neff B.D. Stabilizing selection on genomic divergence in a wild fish population. Proc. Natl Acad. Sci. USA. 2004a;101:2381–2385. doi: 10.1073/pnas.0307522100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff B.D. Mean d2 and divergence time: transformations and standardizations. J. Hered. 2004b;95:165–171. doi: 10.1093/jhered/esh021. [DOI] [PubMed] [Google Scholar]
- Petren K. Microsatellite primers from Geospiza fortis and cross-species amplification in Darwin's finches. Mol. Ecol. 1998;7:1771–1788. doi: 10.1046/j.1365-294x.1998.00518.x. [DOI] [PubMed] [Google Scholar]
- Reid J.M, Arcese P, Keller L.F. Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter-generational effects. Proc. R. Soc. B. 2003;270:2151–2157. doi: 10.1098/rspb.2003.2480. (doi:10.1098/rspb.2003.2480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, M. I. 1997 An investigation of parasite–host interactions between Haemoproteus and Plasmodium, and mountain white crowned sparrows (Zonotrichia leucophrys oriantha). B.Sc. thesis, Princeton University.
- Scheuerlein A, Ricklefs R.E. Prevalence of blood parasites in European passeriform birds. Proc. R. Soc. B. 2004;271:1363–1370. doi: 10.1098/rspb.2004.2726. (doi:10.1098/rspb.2004.2726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riper C, III, van Riper S.G, Goff M.L, Laird M. The epizootiology and epidemiology of malaria in Hawaiian land birds. Ecol. Monogr. 1986;56:327–344. [Google Scholar]