Abstract
PURPOSE
Recent clinical trials have evaluated treatment strategies for chronic infection with hepatitis C virus (HCV) in patients co-infected with human immunodeficiency virus (HIV). Our objective was to use these data to examine the cost-effectiveness of treating HCV in an urban cohort of co-infected patients.
METHODS
A computer-based model, together with available published data, was used to estimate lifetime costs (2004 US dollars), life expectancy, and incremental cost per year of life saved (YLS) associated with 3 treatment strategies: (1) interferon-alfa and ribavirin; (2) pegylated interferon-alfa; and (3) pegylated interferon-alfa and ribavirin. The target population included treatment-eligible patients, based on an actual urban cohort of HIV-HCV co-infected subjects, with a mean age of 44 years, of whom 66% had genotype 1 HCV, 16% had cirrhosis, and 98% had CD4 cell counts >200 cells/mm3.
RESULTS
Pegylated interferon-alfa and ribavirin was consistently more effective and cost-effective than other treatment strategies, particularly in patients with non-genotype 1 HCV. For patients with CD4 counts between 200 and 500 cells/mm3, survival benefits ranged from 5 to 11 months, and incremental cost-effectiveness ratios were consistently less than $75,000 per YLS for men and women of both genotypes. Due to better treatment efficacy in non-genotype 1 HCV patients, this group experienced greater life expectancy gains and lower incremental cost-effectiveness ratios.
CONCLUSIONS
Combination therapy with pegylated interferon-alfa and ribavirin for HCV in eligible co-infected patients with stable HIV disease provides substantial life-expectancy benefits and appears to be cost-effective. Overcoming barriers to HCV treatment eligibility among urban co-infected patients remains a critical priority.
Keywords: Hepatitis C virus (HCV), Human immunodeficiency virus (HIV), Cost-effectiveness, Peginterferon-alfa and ribavirin, Clinical guidelines, Treatment eligibility
Among the estimated 950,000 persons infected with human immunodeficiency virus (HIV) in the United States, approximately 30% are co-infected with the hepatitis C virus (HCV).1,2 While highly active antiretroviral therapy (HAART) has essentially transformed HIV to a chronic disease, co-infected patients are increasingly vulnerable to complications of chronic liver disease, including cirrhosis and liver failure. Compared with HCV mono-infected patients, they tend to have higher levels of HCV RNA and to progress more rapidly to cirrhosis and end-stage liver disease.3 Mortality attributable to end-stage liver disease has steadily increased since 1996, and in some HIV patient populations it is now the leading cause of death.4 The impact of HCV on HIV progression is more controversial.5-7
CLINICAL SIGNIFICANCE.
In patients infected with both HIV and HCV, therapy with pegylated interferon-alfa and ribavirin for HCV increases life-expectancy and appears to be cost-effective.
In clinical trials among patients with HCV mono-infection, combination therapy with pegylated interferon-alfa and ribavirin has produced sustained virologic response rates ranging from 54% to 63%.8-10 Recently, 4 randomized controlled trials evaluated combination therapy with pegylated interferon-alfa and ribavirin compared with interferon-alfa and ribavirin in patients with HIV-HCV co-infection.11-14 The largest of these trials, the AIDS Pegasys Ribavirin International Co-infection Trial (APRICOT), was conducted at 95 centers in 19 countries with 868 subjects and yielded a sustained virologic response rate of 40%.11 Based on the APRICOT findings, in February 2005 the United States (US) Food and Drug Administration approved pegylated interferon-alfa-2a and ribavirin for the treatment of HCV in patients with HIV.15
To the best of our knowledge, only one cost-effectiveness analysis evaluating the treatment of HCV in HIV-HCV co-infected patients has been published. Kuehne et al demonstrated that combination therapy for histologically moderate HCV in co-infected patients resulted in an increase in quality-adjusted life expectancy while incurring costs comparable with other well-accepted clinical interventions.16 However, this analysis was performed before randomized controlled trials had established approximate treatment efficacy rates in HIV-HCV co-infected patients, and the APRICOT trial rates of sustained virologic response were generally lower than the lower bounds of the sensitivity analysis performed by Kuehne and colleagues. Since this prior cost-effectiveness analysis was conducted, considerable progress has been made in discerning treatment efficacy rates and relative risk estimates for progression of liver disease in HIV-HCV co-infected patients.
Our objective was to use recent prospective data regarding eligibility for interferon-based treatment, the impact of HIV on the progression of HCV-related liver disease, and demonstrated treatment efficacy from clinical trials to consider the potential health benefits, economic costs, and cost-effectiveness of treatment for HCV among an urban cohort of co-infected patients with stable HIV disease.
METHODS
Overview
We modified an existing Markov model of HCV17 to reflect co-infection with HIV and examined the cost-effectiveness of the following strategies for HCV treatment in the treatment-eligible segment of an urban co-infected cohort18: combination therapy with interferon-alfa-2a and ribavirin; monotherapy with pegylated interferon-alfa-2a; and combination therapy with pegylated interferon-alfa-2a and ribavirin.
Population characteristics (mean age, Metavir score distribution, and mean CD4 cell count) for the modeled cohort were derived from a subgroup of the Hepatitis and AIDS Liver Outcomes (HALO) Study cohort that was co-infected with HIV and HCV, and eligible for treatment (Table 1).18
Table 1.
Demographic Characteristics of the Treatment-eligible HIV-HCV Co-infected Subgroup of the HALO Cohort18
| Variable | Treatment-eligible* n = 44 (%) |
|---|---|
| Age (years ± SD) | 44.8 ± 7.3 |
| Sex | |
| Male | 34 (77) |
| Female | 10 (23) |
| CD4 cell count >200 cells/mm3 | 43 (98) |
| HCV genotype | |
| 1 | 29 (66) |
| 2, 3, or 4 | 15 (34) |
| Liver biopsy results (Metavir score)† | |
| F1 | 8 (25) |
| F2 | 14 (44) |
| F3 | 5 (16) |
| F4 | 5 (16) |
Exclusion criteria for treatment eligibility in this cohort included the following: nonadherence (missing >3 clinic appointments), ongoing alcohol or drug use (other than marijuana) in the preceding 6 months, active psychiatric illness (defined as symptomatic psychosis or depression or a suicide attempt within the previous year), active medical illness (defined as ongoing illness that is a contraindication to interferon therapy or is associated with a life expectancy of <3 years), decompensated liver disease (defined as a Child Pugh score of >7), advanced HIV disease (defined as a CD4 cell count of <100 cells/mm3 regardless of HIV viral load, or a count of 100-200 cells/mm3 with a viral load of >10,000 copies per milliliter), neutrophil count of <1.5 × 109 cells per liter, and platelet count of <75 × 109 cells liter. Analyses are stratified on HCV genotype (ie, 1 versus non-1), which affects response to therapy, and sex, which affects rate of fibrosis progression.
Metavir scores were available for 32 subjects. Percentages add to more than 100 due to rounding.
We followed the recommendations of the US Panel on Cost-Effectiveness in Health and Medicine,19 adopting a societal perspective (although we excluded patient time costs) and discounting all costs and clinical consequences at a rate of 3% per year. The comparative efficiencies of alternative treatment strategies were measured by the incremental cost-effectiveness ratio, defined as the additional cost of a specific treatment strategy divided by its additional health benefit, expressed here as years of life saved (YLS). The incremental ratio for a strategy was computed in comparison with the next most effective option after eliminating strategies that were dominated (more costly and less effective than other options) or ruled out by extended (weak) dominance (strategies with higher incremental cost-effectiveness ratios than more effective options). We conducted sensitivity analyses to assess the influence of varying uncertain parameters and adopting alternative assumptions on our results.
Model
A deterministic state-transition Markov model (DATA 4.0; TreeAge Software Inc., Williamstown, Mass) was used to simulate the natural history of HCV infection in patients co-infected with HIV. Early stages of liver disease were classified using the Metavir scoring system, which characterizes the extent of fibrosis that results as damaged liver cells are repaired, including no fibrosis (F0), portal fibrosis without septa (F1), portal fibrosis with few septa (F2), numerous septa without cirrhosis (F3), and cirrhosis (F4). Advanced stages of liver disease were defined clinically as compensated cirrhosis, decompensated cirrhosis (including separate states for ascites, variceal hemorrhage and hepatic encephalopathy), and primary hepatocellular carcinoma (Figure 1). Monthly transition probabilities were derived from the literature and allowed individuals to move through different health states over time.
Figure 1.
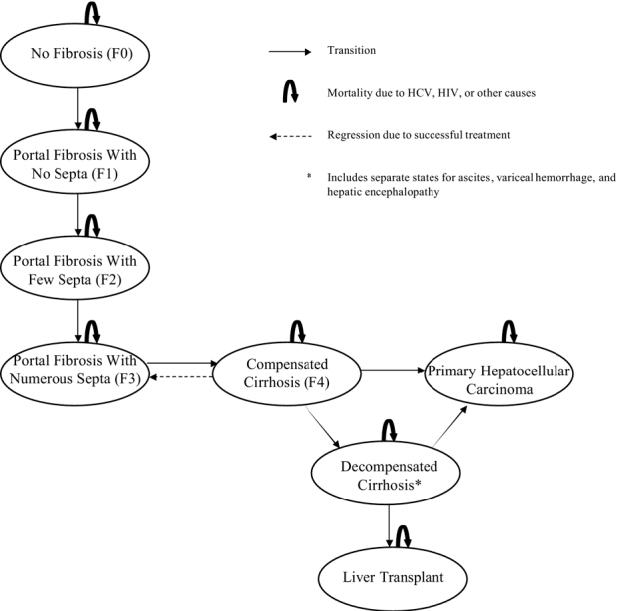
Overview of the Markov model.
We made the following assumptions:
HCV infection may resolve through successful treatment, implying clearance of HCV RNA20;
Patients without a sustained viral response to HCV treatment (as defined by an HCV RNA level of <50 IU per milliliter 24 weeks after completion of therapy) received no clinical benefit and were subject to pretreatment rates of HCV-related liver disease progression11,21;
Patients in early stages of liver disease who experienced a sustained viral response were no longer at risk for HCV-related liver disease22;
Patients with cirrhosis received treatment, and if it was effective they regressed to Metavir stage F3; if treatment was ineffective they were still at risk for decompensated cirrhosis23;
Response to treatment was conditional on genotype11;
The rate of fibrosis progression in the absence of effective treatment was conditional on age and sex, and remained the same for transition to the next higher Metavir stage;
All patients were assumed to have a stable CD4 cell count between 200 and 500 cells/mm3 and to be receiving HAART for HIV; and
Patients with decompensated cirrhosis were eligible for liver transplantation.24,25
Data
Selected parameters used in the model are shown in Table 2. We used baseline age- and sex-specific rates of progression from chronic HCV infection to cirrhosis based on an empirically calibrated model of chronic HCV in mono-infected patients.17,26 We modified these based on data from studies comparing the relative progression in HIV co-infected patients versus mono-infected patients.27-30 We assumed that progression from cirrhosis to decompensated cirrhosis was similar in co-infected patients and mono-infected patients, and used rates derived from a cost-effectiveness study in mono-infected patients.31 Excess mortality due to HIV was based on data from the Multicenter AIDS Cohort Study, from which CD4-specific rates were derived to parameterize a natural history model of HIV/AIDS published by Freedberg et al32; an additive relationship to age- and sex-standardized mortality rates was assumed. We then compared the model’s predictions of cirrhosis prevalence with a published study by Di Martino et al that was not used for natural history parameter estimation.33 Among an HIV-HCV co-infected cohort of former intravenous drug users with an average 10.6-year duration of HCV infection and a mean CD4 count of 482 cells/mm3, 59% of whom had received interferon monotherapy (overall efficacy of 6.4%) and 73% of whom were male, 8.75% had cirrhosis at baseline and 17.5% had cirrhosis at follow-up 4.7 years later.33 Our model predicted that in a cohort of patients with this sex and treatment profile and duration of HCV infection, 16% had cirrhosis over the same follow-up period.
Table 2.
Base Case Values for Model Parameters
| Variable | Base Case |
|---|---|
| HCV natural history parameters*17,26-29,31 | |
| Fibrosis progression in men, age (years)† | |
| 0 | 0.149 |
| 50 | 0.169 |
| 60 | 0.298 |
| ≥70 | 0.406 |
| Fibrosis progression in women, age (years)† | |
| 0 | 0.108 |
| 50 | 0.135 |
| 60 | 0.208 |
| ≥70 | 0.284 |
| Cirrhosis to ascites | 0.025 |
| Cirrhosis to hepatic encephalopathy | 0.004 |
| Cirrhosis to hepatocellular carcinoma | 0.015 |
| Cirrhosis to variceal hemorrhage | 0.011 |
| Liver transplant probability | 0.031 |
| Mortality rate per person | |
| Ascites | 0.110 |
| Hepatic encephalopathy (first year) | 0.680 |
| Hepatic encephalopathy (subsequent years) | 0.400 |
| Hepatocellular carcinoma | 0.433 |
| Transplant (first year) | 0.210 |
| Transplant (subsequent years) | 0.057 |
| Variceal hemorrhage (first year) | 0.400 |
| Variceal hemorrhage (subsequent years) | 0.130 |
| Treatment parameters11 | |
| Treatment response probability | |
| Interferon-alfa + ribavirin | |
| Genotype 1 | 0.07 |
| Non-genotype 1 | 0.18 |
| Pegylated interferon-alfa | |
| Genotype 1 | 0.14 |
| Non-genotype 1 | 0.31 |
| Pegylated interferon-alfa + ribavirin | |
| Genotype 1 | 0.29 |
| Non-genotype 1 | 0.58 |
| Probability of early virologic response (EVR) | |
| Interferon-alfa + ribavirin | 0.38 |
| Pegylated interferon-alfa | 0.55 |
| Pegylated interferon-alfa + ribavirin | 0.71 |
| Treatment mortality probability | 0.002 |
| Costs (2004 US $)31,34-37 | |
| Treatment Protocol 1‡ | |
| Interferon-alfa + ribavirin | $15,568 |
| Pegylated interferon-alfa | $19,305 |
| Pegylated interferon-alfa + ribavirin | $27,880 |
| Treatment Protocol 2§ | |
| Interferon-alfa + ribavirin | $8,769 |
| Pegylated interferon-alfa | $13,109 |
| Pegylated interferon-alfa + ribavirin | $22,022 |
| Costs of annual HCV care | |
| Chronic HCV | $140 |
| Compensated cirrhosis | $1,017 |
| Ascites | $4,280 |
| Variceal hemorrhage, first year | $23,669 |
| Variceal hemorrhage, subsequent years | $4,632 |
| Hepatic encephalopathy, first year | $15,192 |
| Hepatic encephalopathy, subsequent years | $3,519 |
| Hepatocellular carcinoma | $40,828 |
| Liver transplant, first year | $134,458 |
| Liver transplant, subsequent years | $23,481 |
| Costs of annual HIV care | |
| CD4 count 200-500/mm3 | $5,096 |
| Three-drug antiretroviral therapy | $13,752 |
Annual rates per person are presented here except where specified, but these were converted to monthly probabilities in the model.
Fibrosis progression rates are assumed to be linearly interpolated in these ranges. Progression rates were the same for Metavir stages F0 to F4.
All patients receive the full 48-week course of therapy.
Patients without an early virologic response receive only 12 weeks of therapy; protocol assumes nondrug costs by week 12 are two-thirds of nondrug costs for 48 weeks of therapy.
Annual costs of care related to chronic HCV infection and liver disease included detailed estimates of resource utilization, including hospitalizations, outpatient visits, laboratory tests, medications, and interventions.31 Treatment costs were based on average wholesale drug prices34 combined with previously published cost estimates for clinic visits, laboratory tests, and the treatment of adverse events.35 The annual costs of HIV care for patients on HAART with CD4 count between 200 and 500 cells/mm3 were obtained from a recently published model for HIV screening.36,37
In the base case we assumed treatment for 48 weeks,11-13 and dosages resembled those in APRICOT: for interferon and ribavirin, 3 million IU interferon alfa-2a subcutaneously 3 times/week plus 800 mg ribavirin/day; for pegylated interferon, 180 μg of pegylated interferon alfa-2a subcutaneously weekly; and for pegylated interferon and ribavirin, pegylated interferon as described above plus 800 mg ribavirin/day. We made the conservative assumption that all patients completed the full course of medication. In sensitivity analyses, we explored a second treatment protocol and assumed that patients without an early virologic response at week 12 discontinued treatment. The percentage of such patients was drawn from APRICOT and varied by treatment arm. We assessed early treatment withdrawal due to adverse events or abnormal laboratory values in a sensitivity analysis.
RESULTS
Table 3 shows the discounted lifetime costs, life expectancy, and incremental cost per YLS for each treatment strategy, stratified by sex and genotype. For the men in our modeled cohort, the average discounted life expectancy without treatment was 11.6 years and lifetime costs were $240,300. In men with genotype 1, treatment for HCV provided incremental gains ranging from 1.0 to 5.2 months compared with no therapy. Combination therapy with pegylated interferon and ribavirin dominated all other strategies because it was both more effective and had a lower (more attractive) cost-effectiveness ratio. Compared with no therapy, its incremental cost-effectiveness ratio was $73,000 per YLS.
Table 3.
Cost-effectiveness of 4 Strategies by Treatment Protocol, Sex, and Genotype for Patients with CD4 Count 200-500 Cells/mm3
| Strategy* | Cost, 2004 US$ | Life Expectancy, Years | Incremental Cost per YLS |
|---|---|---|---|
| Men | |||
| Genotype 1 | |||
| No treatment | $240,300 | 11.63 | - |
| Interferon + ribavirin | $256,400 | 11.71 | † |
| Pegylated interferon | $261,100 | 11.83 | † |
| Pegylated interferon + ribavirin | $271,700 | 12.06 | $73,000 |
| Non-genotype 1 | |||
| No treatment | $240,300 | 11.63 | - |
| Interferon + ribavirin | $257,900 | 11.88 | † |
| Pegylated interferon | $263,400 | 12.09 | † |
| Pegylated interferon + ribavirin | $275,600 | 12.52 | $39,700 |
| Women | |||
| Genotype 1 | |||
| No treatment | $252,200 | 12.28 | - |
| Interferon + ribavirin | $268,400 | 12.37 | † |
| Pegylated interferon | $273,200 | 12.48 | † |
| Pegylated interferon + ribavirin | $284,000 | 12.73 | $70,700 |
| Non-genotype 1 | |||
| No treatment | $252,200 | 12.28 | - |
| Interferon + ribavirin | $270,000 | 12.55 | † |
| Pegylated interferon | $275,700 | 12.76 | † |
| Pegylated interferon + ribavirin | $288,400 | 13.20 | $39,300 |
Assumes 48 weeks of HCV therapy for all patients.
Dominated strategy.
For men with non-genotype 1 HCV, treatment provided incremental gains ranging from 3.0 to 10.7 months compared with no therapy. Again, combination therapy with pegylated interferon and ribavirin was the dominant strategy. Compared with no therapy, its incremental cost-effectiveness ratio was $39,700 per YLS. Results in women were very similar.
In an exploratory analysis assuming cessation of treatment when no early virologic response occurs, the rank ordering of strategies remained the same. The incremental cost-effectiveness ratio for combination therapy with pegylated interferon and ribavirin became more attractive—lower by 19% in men with genotype 1 ($59,300 per YLS) and by 17% in men with non-genotype 1 ($33,100 per YLS). Again, results in women were similar.
Results were relatively insensitive to varying parameters across plausible ranges for treatment efficacies among patients with cirrhosis and treatment risks. Results were most sensitive to variation in the annual excess death rate due to HIV, fibrosis progression rates and treatment efficacies in noncirrhotic patients. Results were moderately sensitive to drug costs. For men with genotype 1 infection, when the excess mortality due to HIV was reduced by 97%, the incremental cost-effectiveness ratio decreased to $41,400 (base case: $73,000 per YLS). When excess mortality was increased 11-fold to represent death rates in patients with a history of severe opportunistic infections, the incremental cost-effectiveness ratio increased so greatly that treatment was dominated by nontreatment.
We conducted a 2-way sensitivity analysis in which we varied the effectiveness of combination therapy with pegylated interferon and ribavirin, and the relative risk of fibrosis progression due to co-infection with HIV (Figure 2). When treatment efficacy exceeded 50%, cost-effectiveness ratios were consistently less than $50,000 per YLS, regardless of the relative risk of fibrosis progression. When efficacy was >25%, ratios were consistently <$100,000 per YLS across the entire plausible range of relative risk assumptions. However, when efficacy was <25%, the relative risk of progression had slightly more influence on the cost-effectiveness of treatment.
Figure 2.
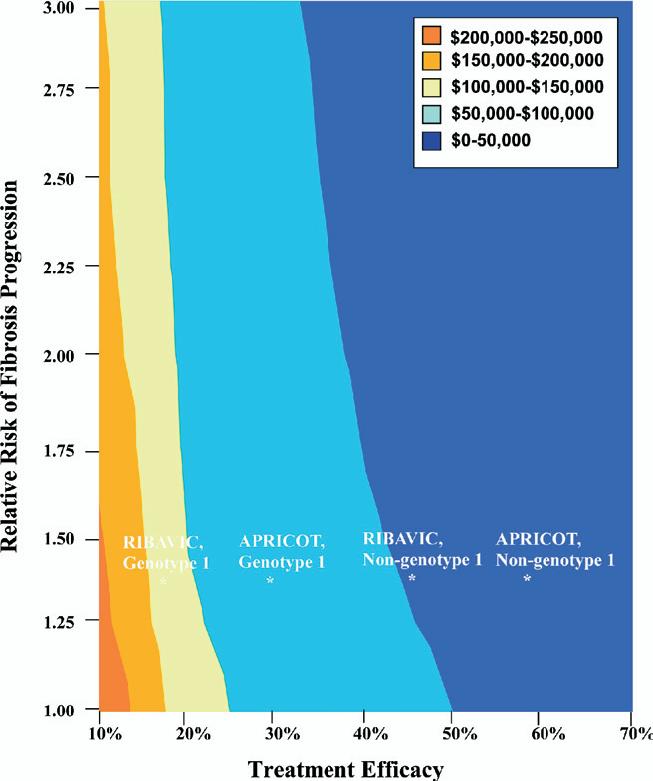
Two-way sensitivity analysis for treatment efficacy and the relative risk of fibrosis progression due to co-infection with HIV. * Indicates percentage of patients with a sustained viral response. The base case value for relative risk of fibrosis progression was 1.35. Efficacy points are merely for reference and do not necessarily represent cost-effectiveness ratios for the particular trial or study they mark due to different drug regimens, protocols, and subject characteristics.
Results were sensitive to the discount rate used. With no discounting, the incremental cost-effectiveness ratio was approximately 60% lower than in the base case, while a discount rate of 5% resulted in a ratio that was 140% higher than the base case. Varying the cost of pegylated interferon and ribavirin between 50% and 150% of the base-case resulted in cost-effectiveness ratios ranging from $56,300 to $88,500 per YLS.
DISCUSSION
We found that treatment with pegylated interferon and ribavirin for chronic HCV infection in an urban cohort coinfected with HIV, with characteristics similar to the treatment-eligible subgroup in the HALO Study, will provide substantial life expectancy gains. These gains are greatest in patients with non-genotype 1 infection. Combination therapy with pegylated interferon and ribavirin was the most effective and cost-effective treatment strategy regardless of genotype or sex.
There were substantial differences in the cost-effectiveness ratios between patients with genotype 1 and nongenotype 1 HCV, mainly attributable to differences in treatment response rates. There were only small differences in the cost-effectiveness of treatment for men and women.
Four trials to date have evaluated the efficacy of HCV treatment in HIV-HCV co-infected patients; we used efficacy results from the largest multicenter trial, APRICOT, for the base case analysis.11-14 Each trial found treatment to be more efficacious in non-genotype 1 patients, but the magnitude of the differences varied by trial, as shown in Figure 2. Because cost-effectiveness ratios are sensitive to treatment efficacy, a wide range of cost-effectiveness ratios is possible. Despite our conservative assumptions, our cost-effectiveness results may be particularly favorable because APRICOT efficacies are high relative to other trials’ results. However, the APRICOT study’s treatment protocol requiring 800 mg of ribavirin daily has become a relatively standard clinical practice. The AIDS Clinical Trials Group (ACTG) trial, on the other hand, administered ribavirin according to a dose-escalation schedule.13 The Agence Nationale de Recherches sur le Sida (ANRS) HCO2-RIBAVIC trial’s exclusion criteria for subjects was less stringent than the other trials, allowing patients with alcohol intake up to 40 grams per day for women or 50 grams per day for men to participate; 21% of this study population had psychiatric disorders, and 40% had bridging fibrosis or cirrhosis.12 These factors may explain the higher efficacies demonstrated in the APRICOT study, but may also indicate that the RIBAVIC trial results might be more applicable to the urban cohort modeled here if treatment eligibility criteria are relaxed.
In contrast to results from a recent cost-effectiveness analysis in HCV mono-infected patients, the present study suggests that monotherapy with pegylated interferon and combination therapy with interferon and ribavirin are dominated strategies in co-infected populations.17 Our results also differ from those of Kuehne et al,16 because this earlier cost-effectiveness analysis of HIV-HCV co-infected patients assumed treatment efficacy and protocols were the same as in mono-infected patients. The APRICOT study not only demonstrated that treatment efficacy is substantially lower in co-infected patients, but that combination therapy with interferon and ribavirin rarely results in a sustained viral response, even in non-genotype 1 patients. While Kuehne et al found this strategy to be cost-effective ($18,500 per YLS relative to no treatment in genotype 1 patients and $63,500 per YLS relative to 24 weeks of combination therapy with interferon and ribavirin in non-genotype 1 patients), our analysis suggests that combination therapy with pegylated interferon and ribavirin dominates other treatment strategies.
Due to limitations in data that would ideally inform a model of co-infection, our goals for this analysis were modest and exploratory in nature, aiming to update a previous analysis with treatment efficacy data and identify influential parameters. Implications of our analysis are restricted to a specific target population of treatment-eligible patients with stable HIV disease and stable CD4 cell counts between 200 and 500 cells/mm3. A more sophisticated model of co-infection that fully represents the natural history of HIV disease will be necessary to explore important questions regarding the optimal timing of treatment for chronic HCV relative to antiretroviral therapy for HIV disease, and potential interactions or additive toxicities between treatments. Other limitations of our study include the uncertainty surrounding many of the model’s parameters. Also, we did not consider health-related quality of life in co-infected patients, and all of our costs were literature-based.
We chose to use population characteristics and treatment eligibility criteria from an urban cohort that has been previously described.18 While the treatment-eligible portion of the HALO Study cohort was in many respects similar to the population studied by APRICOT, efficacy as demonstrated in a randomized controlled trial is not the same as effectiveness in a typical clinical setting. Prospective studies currently underway with the HALO Study and other cohorts will provide insight to treatment effectiveness in a particular population of HIV-HCV patients. It is important to note that only 30% of the co-infected subgroup of the HALO Study cohort was eligible for HCV treatment; the remainder were not eligible for a variety of reasons, including unstable social circumstances, concern about potential adverse effects, and concern about ability to work during the treatment course.38
As treatment-eligible co-infected patients are not currently the norm, further studies are needed to establish the effectiveness of combination HCV therapy in populations with low eligibility for treatment. Overcoming barriers to HCV treatment eligibility and initiation in HIV-HCV coinfected patients remains a priority, now that combination therapy has been demonstrated efficacious in certain populations. For co-infected patients with stable HIV disease, treatment appears to be not only life-prolonging but cost-effective as well.
Acknowledgment
Supported by the National Institute on Alcohol Abuse and Alcoholism R01-AA13216 and the National Institute of Allergy and Infectious Disease K24 AI062476.
References
- 1.U.S. Department of Health and Human Services . HIV/AIDS Statistics, NIAID Facts and Figures. Jul, 2004. [Google Scholar]
- 2.Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S77–S84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 3.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KB, Guest JL, Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA Cohort Study. Clin Infect Dis. 2004;39:1507–1513. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 8.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 9.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 10.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 11.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 12.Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 13.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laguno M, Murillas J, Blanco JL, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18:F27–F36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Food and DrugAdministration Center for Drug Evaluation and Research CDER FastTrack Products Approved Since 1998 Through September 30, 2005. Available at: http://www.fda.gov/cder/rdmt/internetftap.htm. Accessed January 19, 2006.
- 16.Kuehne FC, Bethe U, Freedberg K, Goldie SJ. Treatment for hepatitis C virus in human immunodeficiency virus-infected patients: clinical benefits and cost-effectiveness. Arch Intern Med. 2002;162:2545–2556. doi: 10.1001/archinte.162.22.2545. [DOI] [PubMed] [Google Scholar]
- 17.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228–237. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- 18.Fleming CA, Craven DE, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97–100. doi: 10.1086/344907. [DOI] [PubMed] [Google Scholar]
- 19.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. Oxford University Press; New York, NY: 1996. [Google Scholar]
- 20.Lau DT, Kleiner DE, Ghany MG, Park Y, Schmid P, Hoofnagle JH. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology. 1998;28:1121–1127. doi: 10.1002/hep.510280430. [DOI] [PubMed] [Google Scholar]
- 21.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 22.Marcellin P, Boyer N, Gervais A, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med. 1997;127:875–881. doi: 10.7326/0003-4819-127-10-199711150-00003. [DOI] [PubMed] [Google Scholar]
- 23.Arima M, Terao H, Kashima K, Arita T, Nasu M, Nishizono A. Regression of liver fibrosis in cases of chronic liver disease type C: quantitative evaluation by using computed image analysis. Intern Med. 2004;43:902–910. doi: 10.2169/internalmedicine.43.902. [DOI] [PubMed] [Google Scholar]
- 24.Maida I, Nunez M, Gonzalez-Lahoz J, Soriano V. Liver transplantation in HIV-HCV coinfected candidates: what is the most appropriate time for evaluation? AIDS Res Hum Retroviruses. 2005;21:599–601. doi: 10.1089/aid.2005.21.599. [DOI] [PubMed] [Google Scholar]
- 25.Moreno S, Fortun J, Quereda C, et al. Liver transplantation in HIV-infected recipients. Liver Transpl. 2005;11:76–81. doi: 10.1002/lt.20318. [DOI] [PubMed] [Google Scholar]
- 26.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–773. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Sierra C, Arizcorreta A, Diaz F, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491–498. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 28.Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52:1035–1040. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 30.Marine-Barjoan E, Saint-Paul MC, Pradier C, et al. Impact of antiretroviral treatment on progression of hepatic fibrosis in HIV/hepatitis C virus co-infected patients. AIDS. 2004;18:2163–2170. doi: 10.1097/00002030-200411050-00008. [DOI] [PubMed] [Google Scholar]
- 31.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 32.Freedberg KA, Scharfstein JA, Seage GR, 3rd, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–136. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 33.Di Martino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology. 2001;34:1193–1199. doi: 10.1053/jhep.2001.29201. [DOI] [PubMed] [Google Scholar]
- 34.Red Book 2004: Pharmacy’s Fundamental Reference. Thomson Healthcare; Stamford, CT: 2004. [Google Scholar]
- 35.Wong JB, Poynard T, Ling MH, Albrecht JK, Pauker SG. Cost-effectiveness of 24 or 48 weeks of interferon alpha-2b alone or with ribavirin as initial treatment of chronic hepatitis C. International Hepatitis Interventional Therapy Group. Am J Gastroenterol. 2000;95:1524–1530. doi: 10.1111/j.1572-0241.2000.2089_1.x. [DOI] [PubMed] [Google Scholar]
- 36.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 37.Bozzette SA, Joyce G, McCaffrey DF, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001;344:817–823. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 38.Fleming CA, Tumilty S, Murray JE, Nunes D. Challenges in the treatment of patients coinfected with HIV and hepatitis C virus: need for team care. Clin Infect Dis. 2005;40(Suppl 5):S349–S354. doi: 10.1086/427452. [DOI] [PubMed] [Google Scholar]


