Abstract
Tuberoinfundibular (TI) dopamine (DA) neurons are spared in Parkinson’s disease (PD), a disorder that causes degeneration of midbrain nigrostriatal (NS) and mesolimbic (ML) DA neurons. This pattern of susceptibility has been demonstrated in acute complex I inhibitor-induced models of PD, and extrinsic factors such as toxin distribution, bioactivation, entry into the cell and sequestration into vesicles are postulated to underlie the resistance of TIDA neurons. In the present experiments, direct exposure to rotenone or 1-methyl-4-phenylpyridinium (MPP+) had no effect on mediobasal hypothalamic TIDA neurons, but significantly increased the percentage of apoptag immunoreactive neurons in midbrain primary NSDA and MLDA cultures. In vivo MPTP exposure caused an initial decrease (by 4hr) in DA in brain regions containing axon terminals of TIDA (median eminence [ME]), NSDA (striatum [ST]) and MLDA (nucleus accumbens [NA]) neurons. By 16hr after MPTP treatment, DA concentrations in ME returned to control levels, while ST and NA DA levels remained low up to 32hr after treatment with MPTP. When mice and rats were chronically treated with MPTP and rotenone, respectively, the same pattern of susceptibility emerged. TIDA neurons were unaffected while NSDA neurons suffered loss of cell bodies and axon terminal DA. These experiments demonstrate that the resistance of hypothalamic TIDA neurons is not likely to be due to extrinsic factors, and that further examination of the intrinsic properties of these neurons may elucidate mechanisms that can be translated into neuroprotective strategies in PD.
Keywords: MPTP, Rotenone, Hypothalamus, Susceptibility, Neurodegeneration, Tyrosine Hydroxylase
Tuberoinfundibular (TI) dopamine (DA) neurons are part of a heterogeneous group of DA neurons in the brain that originate in the arcuate nucleus (ARC) of the mediobasal hypothalamus (MBH) and project ventrally to the median eminence (ME) to terminate adjacent to hypophysial portal vessels. DA released into the portal circulation is transported to the anterior pituitary where it tonically inhibits prolactin secretion. Reuptake of DA is mediated by high volume low affinity transporters and low volume high affinity DA transporters (DAT) (Lookingland and Moore, 2005). Unlike other DA neurons in the brain, TIDA neurons are not affected in Parkinson’s disease (PD) (Langston and Forno, 1978, Braak and Braak, 2000) and the mechanisms underlying this selective resistance to neurodegeneration are not fully understood. As such, characterization of the properties of TIDA neurons compared to neurons that degenerate in PD may elucidate mechanisms that impart protection.
Nigrostriatal (NS) DA neurons, emanating from substantia nigra pars compacta (SNpc) and projecting to the striatum (ST), are key components of the extrapyramidal motor system and their degeneration underlies the motor deficits of PD (Dauer and Przedborski, 2003). The mesolimbic (ML) DA neurons originate in the midbrain ventral tegmental area (VTA) and project to the forebrain nucleus accumbens (NA) and frontal cortex. Although both midbrain neuronal populations are affected in PD, neurons in the SNpc undergo more extensive (80-90% loss) degeneration compared to the adjacent VTA neurons (30-40% loss) (German et al., 1989, Fearnley and Lees, 1991). Midbrain DA neurons have long projecting axons, form classic synapses with target neurons, use DAT for reuptake of DA and their activity is regulated, in part, by DA autoreceptor-mediated mechanisms (Lookingland and Moore, 2005).
Neurotoxin-based experimental models of PD have been used to demonstrate a differential susceptibility of DA neuronal subpopulations following acute neurotoxin administration. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is metabolized by monoamine oxidase (MAO) primarily in astrocytes to form 1-methyl-4-phenylpyridinium (MPP+). At modest doses, the complex I inhibitor MPP+ causes toxicity to DA neurons by gaining access to the cell via DAT and depleting the cell’s ATP stores as well as producing reactive oxygen species (Chan et al., 1991, Zang and Misra, 1993). MPP+ can also serve as a substrate for MAO inside these DA neurons and result in inhibition of the enzymatic breakdown of DA into DOPAC (Takamidoh et al., 1987). Furthermore, MPP+ can be packaged into vesicles by the efficient vesicular monoamine transporter, thereby displacing vesicular DA into the cytoplasm (Reinhard et al., 1987, Liu et al., 1992). Rotenone is another complex I inhibitor that has been used to model PD in vitro and in vivo (Betarbet et al., 2002). Rotenone is a lipophillic molecule and unlike MPTP, it does not require bioactivation. Rotenone diffuses into all cells but is not compartmentalized into vesicles (Betarbet et al., 2000).
TIDA neurons are resistant to modest acute doses of MPTP or MPP+ when administered either systemically or centrally, while NSDA neurons are severely damaged (Melamed et al., 1985, Sundstrom et al., 1987, Willis and Donnan, 1987, Mogi et al., 1988). MLDA neurons also suffer from MPTP toxicity, but to a lesser degree than NSDA neurons (Sundstrom et al., 1990, Hung et al., 1995). While several intrinsic factors have been proposed to explain partial resistance of MLDA neurons to complex I inhibition toxicity (Greene et al., 2005), complete resistance of TIDA neurons to MPTP toxicity is postulated to be due exclusively to extrinsic factors. It is generally believed that decreased MPP+ uptake into these neurons, due to lower DAT expression, may underlie the resistance of TIDA neurons. Others have suggested that differential distribution, reduced bio-activation by astrocytes, or sequestration of MPP+ into vesicles may be responsible for the resistance of TIDA neurons (Hirsch, 1992, Hirsch et al., 1997, Uhl, 1998). Chronic administration of rotenone, another complex I inhibitor that readily crosses membranes, has also been used to selectively damage NSDA neurons, while MLDA neurons are relatively spared (Greene et al., 2005). The effects of rotenone on TIDA neurons, however, have not been documented.
The purpose of these experiments was to test current hypotheses regarding the mechanisms underlying differential susceptibility of TIDA, NSDA and MLDA neurons to acute administration of complex I inhibitors and to determine whether this pattern of susceptibility also exists in chronic neurotoxin models that may more closely model PD.
Experimental Procedures
In Vitro studies
Drugs
1-methyl-4-phenylpyridinium (MPP+)-iodide was dissolved in cell culture media (basal media eagle, Fischer scientific, Pittsburgh, PA) and rotenone was dissolved in ethanol prior to addition to media. The final concentration of ethanol (200 μM) was held constant and media containing 200 μM ethanol was used for control samples in the rotenone cell culture experiments. Toxin-containing media was filter-sterilized. All drugs were obtained from Sigma (St. Louis, MO).
Primary cell cultures
Postnatal 2-4 day old mice were used to prepare primary neuronal cultures from MBH and midbrain as previously described (Smeyne and Smeyne, 2002). After decapitation, brains were rapidly removed, placed on a chilled sterile dissection stage, and the midbrain and MBH were dissected under microscopic visualization. Dissected tissue was placed in a neutral buffered dissociation media, incubated in papain/DNAse and dissociated by gentle trituration (Worthington Biochemical Corp, Lakewood, NJ). Cells were re-suspended in plating media with 2% of rat serum and viability was tested with 0.4% trypan blue. Cell density was adjusted to 1.2×106 cells/ml and plated on Lab-Tek 4-well Permanox™ chamber slides coated with poly-D-lysine (Fisher Scientific, Pittsburgh, PA). Cells were kept in a 5% CO2 incubator at 37°C and replaced every 3 days with feeding media containing heat-inactivated 10% horse and 5 % fetal bovine serum. Mitotic inhibitors (cytosine-1-B-D-arabinofuranoside, 5 μm) were added after 48h to inhibit glial proliferation.
Toxicity assays for primary neurons
After five days of culture, cells were exposed to toxin- or vehicle-containing media for 24h and then fixed with Zamboni’s solution. Cells were incubated in rabbit anti-TH polyclonal antibody (Chemicon, Temecula, CA) and subsequently in secondary antibody (Rhodamine-conjugated goat anti-rabbit, Jackson Immunoresearch, West Grove, PA). Apoptotic nuclei were detected with a modification of the terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) method using an Apoptag Plus fluorescein in situ apoptosis detection kit (Chemicon, Temecula, CA). Cells were analyzed using laser-scanning microscopy on a Zeiss LSM5 (PASCAL) equipped with three lasers for detecting protein co-localization and image analysis software. In the resulting images green represented Apoptag staining and red signified TH-IR. An observer blind to treatment group determined the number of TH-IR cells and percentage of TH-IR cells with apoptotic nuclei in each chamber. A total of 4 chambers were used for each treatment group. An additional two chambers of cells without treatment were incubated in primary antibody solution without anti-TH antibody or without anti-digoxigenin antibody to control for nonspecific cross-reactivity of the secondary antibodies.
In Vivo studies
Animals
Neurotoxin studies were conducted on male mice (8-10 weeks of age, C57/Bl Jackson Labs, Bar Harbor, ME) or rats (Lewis 250-300 g, Charles River laboratories, Wilmington, MA). Animals were housed two to four per cage, maintained in a temperature (22 ± 1 °C) and light controlled (12L:12D) room, and provided with food and tap water ad libitum. Use of animals was with prior approval by the All University Committee on Animal Use and Care.
Drugs
MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) hydrochloride was dissolved in saline. Probenecid (p-[dipropylsulfamoyl]benzoic acid) was dissolved in 0.1N NaOH and adjusted to pH 7.2 with 0.1M Tris HCL buffer. Rotenone was dissolved in dimethylsulfoxide (DMSO): polyethylene glycol (PEG) (1:1). All compounds are commercially available (Sigma, St. Louis, MO).
Time Course of Acute MPTP administration
Mice (n=8/group) were treated with MPTP (20 mg/kg; s.c.) or vehicle and sacrificed 4, 8, 16 or 32h later. All vehicle-treated animals were sacrificed 4 hours after injection.
Chronic MPTP administration
Mice (n=8/group) were treated with MPTP (20 mg/kg; s.c.) using a previously described chronic administration regimen (Petroske et al., 2001, Drolet et al., 2004). All animals received probenecid (250 mg/kg; i.p.) 30min prior to MPTP/vehicle administration in order to increase the half-life of MPP+ (Lau et al., 1990). Probenecid alone does not produce DA neuronal toxicity (Lau et al., 1990). A total of 10 treatments were given over 35 days (once every 3.5 days) and animals were sacrificed 21 days following the last treatment.
Chronic rotenone administration
One Alzet osmotic minipump (Alza, Palo Alto, CA) filled with rotenone (Sigma, St. Louis, MO) or vehicle was implanted under the skin of each rat. The pumps were incubated in sterile 0.9% saline at 37 °C overnight. Isoflurane (Abbott Laboratories, North Chicago, IL) was used as the anesthetic agent. The control rats (n=8) received dimethylsulfoxide (DMSO): polyethylene glycol (PEG) (1:1) only. The treated rats (n=20) received 3.0 mg/kg/day rotenone in DMSO:PEG (1:1) (calculated based on weight at the time of surgery). All rats were sacrificed 28 days after pump implantation. Only animals that demonstrated behavioral motor deficits were selected for neurochemical analysis based on a recommendation by Dr. T. Sherer (Todd Sherer, Research programs, Michael J. Fox Foundation for Parkinson’s Disease Research, personal communication).
Tissue preparation
Following appropriate treatments, animals were decapitated and brains were quickly removed and placed on an ice-cooled glass stage. The ME was microdissected from the ventral surface of the brain and placed in tissue buffer (0.1 M phosphate-citric buffer containing 20% methanol [pH 2.5]). For the acute studies, the entire brain was immediately frozen following the microdissection of ME. For the chronic studies, the brain was quickly bisected in the mid-saggital plane. One half of the brain was immersion fixed in 4% paraformaldehyde and stored at 4°C for histological processing. The remaining half brain was rapidly frozen over dry ice. Frontal sections (500 μm) were prepared from the frozen brains in a cryostat (-10° C), and the striatum (ST) and nucleus accumbens (NA) were dissected from appropriate sections according to the method of Palkovits (Palkovits, 1973). These samples were placed in 65 (NA) and 100μl (ST) of tissue buffer and stored at -20° C until neurochemical analysis.
Neurochemistry
All samples were thawed on the day of analysis and sonicated with 3 one-second bursts (Sonicator Cell Disruptor, Heat Systems-Ultrasonic, Plainview, NY, USA) and centrifuged at 12,000 rpm for 1min. The supernatants were removed and analyzed for DA and 3,4-dihyroxyphenylacetic acid (DOPAC) content using high performance liquid chromatography coupled with electrochemical detection (HPLC-EC) (Lindley et al., 1990). The amounts of these compounds in the tissue samples were determined by comparing peak height values (as determined by a Hewlett Packard Integrator, Model 3395) with those obtained from external standards run on the same day. Tissue pellets were dissolved in 1 N NaOH and assayed for protein (Lowry et al., 1951).
Immunohistochemistry
Frontal sections (60 μm) through the hypothalamus and midbrain (bregma −2.5 to −3.7mm; (Franklin and Paxinos, 1996)) were prepared from the fixed and cryoprotected half brains with a cryostat (-19°C) using Multiblock™ processing (Neuroscience Associates, Knoxville, TN). Immunohistochemistry was performed on free floating sections using a primary rabbit anti-TH antibody (Chemicon, Temecula, CA), followed by a biotin-conjugated, goat anti-rabbit secondary antibody (Jackson Immunoresearch, West Grove, PA). Bound peroxidase was visualized with 0.05% 3-3’-diaminobenzidine tetrahydrochloride with 0.01% hydrogen peroxide using an ABC Elite kit (Vector Laboratories, Burlingame, VT). Alternate sections were stained with Cresyl Violet to determine if decreases in TH-immunoreactive (IR) cell counts correspond with loss of neurons.
Unbiased stereological cell counting
Using the StereoInvestigator software (version 4.03, MicroBrightfield, Inc. 2000), sections were viewed on the screen at low magnification (4X), ARC, SNpc and VTA were delineated from the rostral through the caudal ends. The first plane was a randomly chosen section within 180 μm of the most rostral plane of the ARC, SNpc or VTA as determined by a mouse atlas (Franklin and Paxinos, 1996). The delineated sections were evenly spaced apart (240 μm).
Nissl or TH-immunoreactive (IR) neurons were counted using the Optical Fractionator method, an unbiased stereological technique that employs a systematic, random strategy in which cells in a defined region are sampled. This method results in a quantifiable estimate of the total population of cells within a given region (West et al., 1991, Schmitz, 1997, Schmitz and Hof, 2000, 2005). The 3-dimentional probe used to sample from the entire ARC, SNpc or VTA yields counts that are independent of cell shape and size or conformational changes in the tissue. A counting frame (60×60μm) was utilized and a fraction of the delineated cells were sampled. Cell counts were made through 78% (14μm) of the total depth of the tissue (18μm) allowing for a 2μm guard zone at the top and bottom of each section. TH-IR and Nissl stained cells were identified with a consistent distribution over the depth of the counting frame, thus confirming sufficient reagent penetration throughout the thickness of the tissue slice.
Nissl or TH-IR counts were performed using a 60X objective and cells were counted only if the top of the nucleus came into focus within the virtual counting frame and within the delineated region of interest. Neurons were counted only if they showed Nissl or TH-IR within the cell body. For Nissl stained sections, cells that were larger in size (>15 μm in diameter) and had a defined nucleus were counted as neurons. The smaller cells (<15 μm in diameter) that were amorphous in shape and did not have a well-defined nucleus were considered glia and were not counted. For estimates of number of neurons per half brain, the section periodicity was 4 as every fourth section was selected for analysis (West et al., 1991, Schmitz, 1997). The coefficient of error for each estimate was calculated and was less than 0.1 (Gundersen, m=1) (Gundersen and Jensen, 1987).
Statistical Analysis
Sigma Stat software version 2.03 was used to make statistical comparisons among groups using one or two-way analysis of variance. If a significant interaction was detected, post-hoc analysis was followed by between group comparisons using Tukey’s test. Differences with a probability of error of less than 5% were considered statistically significant.
Results
To investigate the hypothesis that differential distribution or drug bioactivation accounts for the lack of susceptibility of TIDA neurons to MPTP, primary neuronal cultures from mediobasal hypothalamus containing TIDA neurons and the midbrain containing NSDA and MLDA neurons were exposed directly to MPP+. As demonstrated in Figure 1, exposure to 10 μM MPP+ had no effect on mediobasal hypothalamic cultures but it significantly increased the percentage of DA neurons expressing apoptag in the midbrain.
Figure 1. The effects of MPP+ on primary mediobasal hypothalamic (MBH) and midbrain DA neurons.
Primary neurons were treated with vehicle-containing media (white bars), 5 μM MPP+ (gray bars) or 10 μM MPP+ (black bars) for 24h. Cells were fixed and co-labeled with antibodies for TH and Apoptag to identify DA neurons and cells undergoing apoptosis. The percentages of TH-immunoreactive (IR) neurons expressing Apoptag were determined. Data points represent means at each concentration and error bars represent +1.0 SEM. (*) Values that are significantly different from vehicle-treated group (P≤0.05).
In order to control for differential uptake and sequestration into vesicles, primary neuronal cultures from mediobasal hypothalamus containing TIDA neurons and the midbrain containing NSDA and MLDA neurons were exposed to rotenone, as the neuronal uptake of this neurotoxin is transporter independent. Figure 2 demonstrates that exposure to 30nM rotenone did not affect apoptag-IR DA neurons in the hypothalamic cultures but significantly increased the percentage of apoptag-IR DA neurons in midbrain cultures.
Figure 2. The effects of rotenone on primary mediobasal hypothalamic (MBH) and midbrain DA neurons.
Primary neurons were treated with vehicle (white bars), 3 nM rotenone (gray bars) or 30 nM rotenone (black bars) for 24h. Cells were fixed and co-labeled with antibodies for TH and Apoptag to identify DA neurons and cells undergoing apoptosis. The percentages of TH-immunoreactive (IR) neurons expressing Apoptag were determined. Data points represent means at each concentration and error bars represent +1.0 SEM. (*) Values that are significantly different from vehicle-containing media treated group (P<0.05).
Figure 3 demonstrates the time-course of the response of TIDA, NSDA and MLDA neurons to a single systemic injection of MPTP in vivo. ME, ST and NA DA levels significantly decreased by 4 hours after MPTP treatment. ME DA levels increased at 8 hours, and were similar to controls at 16, and 32 hours post-injection. In contrast, DA levels in ST decreased further and remained low (~25% of controls). NA maintained low levels of DA (~70% of control) at 8, 16, and 32 hours post-MPTP injection.
Figure 3. Time course of the effects of one injection of MPTP on TIDA, NSDA and MLDA nerve terminals.
Mice were treated with vehicle or MPTP (20mg/kg; s.c.) and killed 4, 8, 16 and 32 hours after injection. Vehicle treated animals are considered time 0. DA levels in ME, ST and NA were calculated as a percentage of control animals. Lines represent means of groups (n = 8/group) of NSDA (circle), MLDA (square) and TIDA (triangle) neurons and vertical bars indicate +1.0 SEM. Filled symbols represent values from MPTP-treated groups that are significantly different from vehicle treated controls (P≤0.05). Actual concentrations of DA in control animals are as follows (mean ng DA per mg protein ± 1.0 SEM): ME, 175±12; ST, 172±7; NA, 71±3.
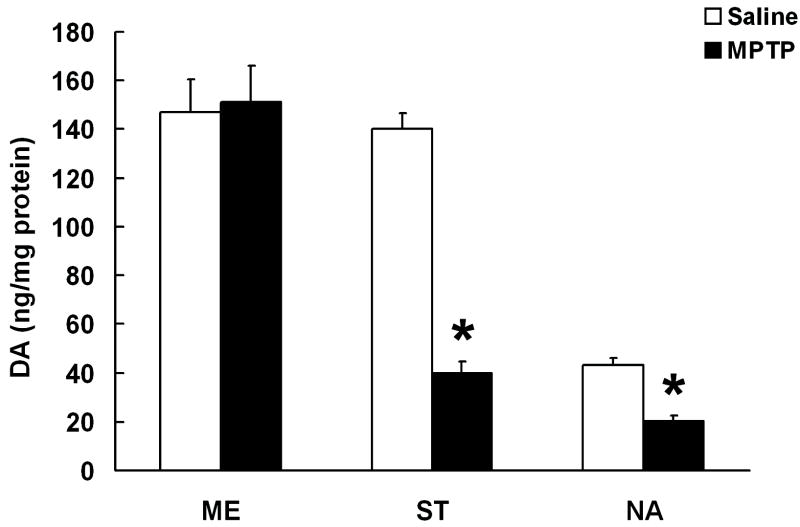
Figure 4 demonstrates that after repeated exposure to MPTP, DA concentrations are similar to controls in ME, while they are significantly reduced in ST (25% of controls) and NA (50% of controls).
Figure 4. The effects of chronic MPTP treatment on TIDA, NSDA and MLDA nerve terminals.
DA concentrations in the ST, NA and ME of mice following chronic administration of saline (white bars, 1ml/kg) or MPTP (black bars, 20 mg/kg, s.c., every 3.5 days for 10 doses). All animals received probenecid (250 mg/kg, i.p.) 30 min prior to treatments to prolong the brain half-life of MPP+. Columns represent means of groups (n = 6-8/group) and vertical bars indicate +1.0 SEM. (*) Values for MPTP treated groups that are significantly different from vehicle treated controls (P≤0.05).
As demonstrated in Figure 5, no cell loss was observed in ARC or VTA of animals chronically exposed to MPTP, but there was a significant loss of TH-IR and Nissl cell bodies in SNpc.
Figure 5. The effects of chronic MPTP administration on TIDA, NSDA and MLDA cell bodies.
TH (A) and Nissl (B) cell counts as determined by unbiased stereological cell counts in ARC, SNpc and VTA of mice treated with chronic saline (white bars) or 20 mg/kg MPTP (black bars). Columns represent means of cell counts per hemisphere and vertical lines indicate +1.0 SEM (n = 4-6/group). (*) Values for MPTP-treated groups that are significantly different from vehicle treated controls (P≤0.05).
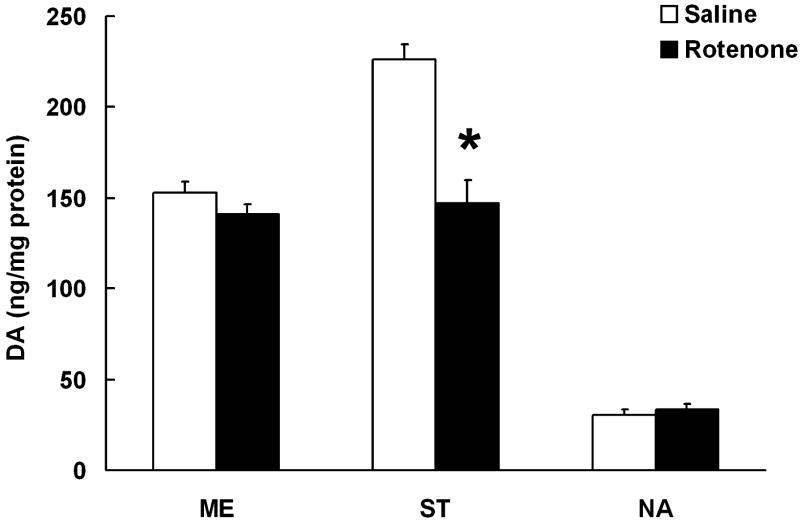
Chronic rotenone treatment resulted in no change in DA concentrations in ME and NA, while ST DA levels were significantly reduced.
Discussion
These experiments characterize unique mechanisms underlying the response of TIDA neurons to neurotoxin exposure. A novel temporal profile of neurotoxin-induced neurochemical changes is observed in TIDA versus NSDA neurons. Specifically, TIDA neurons respond to low-dose MPTP exposure with a substantial DA loss from their nerve terminals 4 hours after a single injection. The initial loss of DA in TIDA neurons is followed by a rapid recovery that continues to up to 16 hours after MPTP treatment. The timeline of DA loss and recovery in TIDA neurons is consistent with the short half life of MPP+ in the brain (Cosi and Marien, 1999, Saporito et al., 2000). Taken together, the time-course of toxin-induced neurochemical changes in TIDA neurons suggests that, even though DAT expression is low in TIDA terminals, MPP+ entry into these cells is sufficient to disrupt neurotransmitter storage.
NSDA and MLDA terminals also lose DA 4 hours after MPTP treatment, but unlike TIDA neurons, they do not recover from MPTP toxicity for up to 32 hours after acute toxin exposure. In fact, NSDA neurons appear to have ongoing DA loss between 4 and 8 hours post MPTP-injection, during which time TIDA neurons are rapidly recovering. Differences in TIDA and NSDA neuronal regulation of DA synthesis, packaging, re-uptake and metabolism may impart protection from toxicity. In NSDA neurons, excess DA leakage out of axon terminals binds to D2 autoreceptors and thereby inhibits further DA synthesis and release. Inhibition of DA release in these neurons (coupled with decreased vesicular monoamine transporter and MAO activity) would lead to excess levels of cytoplasmic DA that cannot be enzymatically metabolized. Cytosolic DA and its metabolites are likely involved in degeneration of NSDA neurons via formation of reactive oxygen species and highly reactive quinone molecules (Lotharius and O’Malley, 2000). MPTP causes increased levels of quinone-modified proteins in NSDA terminals in vivo (Teismann et al., 2003), and the same pattern of increased quinone-modified proteins can be seen in vitro after treatment with MPP+ (Lotharius and O’Malley, 2000). TIDA neurons lack this autoreceptor-mediated regulatory feedback and continue to release DA, which is subsequently cleared by the hypophysial portal blood. As such, cytoplasmic levels of DA in TIDA terminals would not experience the increase seen in NSDA neurons following neurotoxic insult.
These experiments also confirm that MLDA neurons are partially resistant to the toxic effects of complex I inhibition. The initial response of MLDA neurons after 4 hours is very similar to that of NSDA neurons, but NSDA terminals continue to lose DA between 4 and 8 hours, whereas MLDA terminals maintain these DA levels. MLDA neurons, however, do not show the rapid recovery that TIDA neurons experience at 8 and 16 hours after MPTP injection. Furthermore, DA concentrations in MLDA nerve terminals are similar after one injection and repeated injections of MPTP. These results suggest that MLDA neurons may be composed of a heterogeneous group of neurons; some that display resistance and some that are vulnerable to neurotoxic insult and do not recover. Although some phenotypic markers have been suggested (German et al., 1992), the features that separate the resistant from the susceptible neurons in the VTA are largely unknown, making it difficult to study the neuroprotective properties of the resistant subpopulation of DA neurons within this region.
The pattern of susceptibility of DA neuronal subpopulations, as estimated by the magnitude of nerve terminal DA loss is very similar after single-acute and repeated-chronic injections of MPTP. Chronic administration of rotenone also specifically damages NSDA neurons, while MLDA and TIDA neurons are spared. Systemic exposure to rotenone results in a milder neurotoxic insult to NSDA neurons as compared with MPTP. Intra-animal variations in rotenone-induced neurotoxicity have been reported and non-responders could have diluted the overall magnitude of response. To address this possibility, a subset of rotenone-treated animals that demonstrated motor behavioral deficits were assessed in the current study as a strategy to reduce confounding animal-animal variation in toxin responses. The difference in the magnitude of DA loss between MPTP and rotenone-treated animals could also reflect a dose–dependent phenomenon. Since higher doses of chronically administered rotenone are not well tolerated and result in high animal mortality, we could not directly explore the effects of higher doses.
In addition to characterizing the unique temporal profile of neurotoxin-induced neurochemical responses amongst TIDA, NSDA and MLDA neurons, three commonly proposed explanations for the relative resistance of TIDA neurons to neurotoxic insult were tested. Direct exposure to MPP+ or rotenone did not affect primary DA neurons in the mediobasal hypothalamus, yet the same concentrations of the complex I inhibitors markedly increased apoptosis of midbrain primary DA neurons. This suggests that the differential sensitivity of TIDA and NSDA neurons to these toxins is not due to drug distribution to the targeted neurons. Moreover, the lack of a direct effect of MPP+ on TIDA neurons suggests that the resistance to MPTP is not due to bio-activation of MPTP to MPP+ by astrocytes in the mediobasal hypothalamus. The observation that TIDA neurons do have an initial response to MPTP supports the conclusion that toxin distribution and bio-activation are not important factors in determining the ultimate fate of these neurons following neurotoxic insult. Finally, the resistance of TIDA neurons to rotenone toxicity in vitro and in vivo demonstrates that the differential toxin uptake via DAT or sequestration into vesicles is not responsible for the differential susceptibility of these neuronal populations since rotenone is a lipophillic molecule that does not require DAT to enter neurons and is not compartmentalized into vesicles (Le et al., 1999, Betarbet et al., 2000, Masliah et al., 2000, Bywood and Johnson, 2003). Taken together this data suggests that factors extrinsic to TIDA neurons, specifically toxin distribution, bio-activation, entry into the cell and sequestration into vesicles, are not responsible for their resistance to complex I inhibition. A unique phenotypic response to neurotoxin exposure is most likely due to unique intrinsic to hypothalamic TIDA neurons since these neurons appear resistant to both MPTP (a dopamine specific toxicant) and rotenone (a toxicant not specific for dopamine neurons) when compared to mesencephalic dopamine neurons. The possibility that paracrine factors secreted by other non-dopaminergic hypothalamic cells may mediate the selective resistance of TIDA neurons has not been excluded by the current experiments. In the context of PD, however, it appears that the hypothalamic DA neurons are the uniquely resistant cell type while other non-dopaminergic cell groups in the hypothalamus appear to be affected by the disease process (Braak and Braak, 2000).
TIDA neurons are likely resistant to neurotoxin-induced damage due to an intrinsic neuronal phenotype that is distinct amongst DA neuronal subpopulations. Further examination of intrinsic cellular events that underlie initial response, the rapid recovery and the long-term effects of neurotoxin exposure in TIDA neurons may uncover beneficial adaptive neuronal responses that could be exploited as targets for neuroprotective therapies. For example, gene expression differences in key cellular pathways may account for the differential susceptibility of TIDA, NSDA and MLDA neurons to cell death. A recent study providing an initial analysis of gene expression profile comparisons of NSDA and MLDA neurons suggested several molecular pathways that may be involved in neuroprotection (Greene et al., 2005). These pathways include energy metabolism, neuropeptide neurotransmission, and aberrant kinase or phosphatase signaling pathways amongst others. Studying gene expression profiles of TIDA neurons may identify novel neuroprotective properties that are distinct from those observed in MLDA neurons since we have now demonstrated that the response to neurotoxin exposure amongst TIDA, MLDA and NSDA neurons are distinct. Identification of protective and deleterious properties in DA neuronal populations with divergent responses to neurotoxins can provide a method to discover targets that can subsequently be translated into neuroprotective treatments for patients with PD.
Figure 6. The effects of chronic rotenone administration on TIDA, NSDA and MLDA nerve terminals.
DA concentrations in the ST, NA and ME in rats following chronic, systemic administration of vehicle (DMSO:PEG 1:1; 0.25 μl/day; white bars) or rotenone (4 mg/kg/day; black bars) for 28 days using a s.c. osmotic minipump. Columns represent means and vertical bars indicate +1.0 SEM. (*) Values for rotenone treated groups that are significantly different from vehicle treated controls (P<0.05).
Acknowledgments
This work was supported by the Michigan State University Foundation and National Institute of Health grant NS04905701. The authors would like to thank Ms. Kendra Atkinson and Mr. Troy Frayer for technical assistance, Dr. Joseph Nunez for assistance with stereology techniques, and Dr. Juli Wade for providing the stereology equipment.
Abbreviations
- TI
tuberoinfundibular
- DA
dopamine
- PD
Parkinson’s disease
- NS
nigrostriatal
- ML
mesolimbic
- MPP+
1-methyl-4-phenylpyrindium
- ME
median eminence
- ST
striatum
- NA
nucleus accumbens
- ARC
arcuate nucleus
- SNpc
substantia nigra pars compacta
- VTA
ventral tegmental area
- DAT
dopamine transporter
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- TH-IR
tyrosine hydroxylase immunoreactive
- MBH
mediobasal hypothalamus
- MAO
monoamine oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson’s disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247(Suppl 2):II3–10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- Bywood PT, Johnson SM. Mitochondrial complex inhibitors preferentially damage substantia nigra dopamine neurons in rat brain slices. Exp Neurol. 2003;179:47–59. doi: 10.1006/exnr.2002.8044. [DOI] [PubMed] [Google Scholar]
- Chan P, DeLanney LE, Irwin I, Langston JW, Di Monte D. Rapid ATP loss caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse brain. J Neurochem. 1991;57:348–351. doi: 10.1111/j.1471-4159.1991.tb02134.x. [DOI] [PubMed] [Google Scholar]
- Cosi C, Marien M. Implication of poly (ADP-ribose) polymerase (PARP) in neurodegeneration and brain energy metabolism. Decreases in mouse brain NAD+ and ATP caused by MPTP are prevented by the PARP inhibitor benzamide. Ann N Y Acad Sci. 1999;890:227–239. doi: 10.1111/j.1749-6632.1999.tb07998.x. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; 1996. [Google Scholar]
- German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol. 1989;26:507–514. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- Greene JG, Dingledine R, Greenamyre JT. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis. 2005;18:19–31. doi: 10.1016/j.nbd.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hirsch EC. Why are nigral catecholaminergic neurons more vulnerable than other cells in Parkinson’s disease? Ann Neurol. 1992;32(Suppl):S88–93. doi: 10.1002/ana.410320715. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y. Neuronal vulnerability in Parkinson’s disease. J Neural Transm Suppl. 1997;50:79–88. doi: 10.1007/978-3-7091-6842-4_9. [DOI] [PubMed] [Google Scholar]
- Hung HC, Tao PL, Lee EH. 1-Methyl-4-phenyl-pyridinium (MPP+) uptake does not explain the differential toxicity of MPP+ in the nigrostriatal and mesolimbic dopaminergic pathways. Neurosci Lett. 1995;196:93–96. doi: 10.1016/0304-3940(95)11856-r. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS. The hypothalamus in Parkinson disease. Ann Neurol. 1978;3:129–133. doi: 10.1002/ana.410030207. [DOI] [PubMed] [Google Scholar]
- Lau YS, Trobough KL, Crampton JM, Wilson JA. Effects of probenecid on striatal dopamine depletion in acute and long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Gen Pharmacol. 1990;21:181–187. doi: 10.1016/0306-3623(90)90898-v. [DOI] [PubMed] [Google Scholar]
- Le W, Conneely OM, Zou L, He Y, Saucedo-Cardenas O, Jankovic J, Mosier DR, Appel SH. Selective agenesis of mesencephalic dopaminergic neurons in Nurr1-deficient mice. Exp Neurol. 1999;159:451–458. doi: 10.1006/exnr.1999.7191. [DOI] [PubMed] [Google Scholar]
- Lindley SE, Gunnet JW, Lookingland KJ, Moore KE. 3,4-Dihydroxyphenylacetic acid concentrations in the intermediate lobe and neural lobe of the posterior pituitary gland as an index of tuberohypophysial dopaminergic neuronal activity. Brain Res. 1990;506:133–138. doi: 10.1016/0006-8993(90)91209-y. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- Lookingland KJ, Moore KE. Functional neuroanatomy of hypothalamic dopaminergic neuroendocrine systems. In: Dunnett SB, editor. Handbook of Chemical Neuroanatomy. Vol. 21. Amsterdam: Elsevier; 2005. pp. 433–521. [Google Scholar]
- Lotharius J, O’Malley KL. The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem. 2000;275:38581–38588. doi: 10.1074/jbc.M005385200. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Melamed E, Rosenthal J, Globus M, Cohen O, Frucht Y, Uzzan A. Mesolimbic dopaminergic neurons are not spared by MPTP neurotoxicity in mice. Eur J Pharmacol. 1985;114:97–100. doi: 10.1016/0014-2999(85)90528-x. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kojima K, Kiuchi K, Nagatsu T. Effects of systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine to mice on tyrosine hydroxylase, L-3,4-dihydroxyphenylalanine decarboxylase, dopamine beta-hydroxylase, and monoamine oxidase activities in the striatum and hypothalamus. J Neurochem. 1988;50:1053–1056. doi: 10.1111/j.1471-4159.1988.tb10572.x. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Reinhard JF, Jr, Diliberto EJ, Jr, Viveros OH, Daniels AJ. Subcellular compartmentalization of 1-methyl-4-phenylpyridinium with catecholamines in adrenal medullary chromaffin vesicles may explain the lack of toxicity to adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1987;84:8160–8164. doi: 10.1073/pnas.84.22.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito MS, Thomas BA, Scott RW. MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J Neurochem. 2000;75:1200–1208. doi: 10.1046/j.1471-4159.2000.0751200.x. [DOI] [PubMed] [Google Scholar]
- Schmitz C. Towards more readily comprehensible procedures in disector stereology. J Neurocytol. 1997;26:707–710. doi: 10.1023/a:1018506113256. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Smeyne RJ. Method for culturing postnatal substantia nigra as an in vitro model of experimental Parkinson’s disease. Brain Res Brain Res Protoc. 2002;9:105–111. doi: 10.1016/s1385-299x(02)00117-4. [DOI] [PubMed] [Google Scholar]
- Sundstrom E, Fredriksson A, Archer T. Chronic neurochemical and behavioral changes in MPTP-lesioned C57BL/6 mice: a model for Parkinson’s disease. Brain Res. 1990;528:181–188. doi: 10.1016/0006-8993(90)91656-2. [DOI] [PubMed] [Google Scholar]
- Sundstrom E, Stromberg I, Tsutsumi T, Olson L, Jonsson G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res. 1987;405:26–38. doi: 10.1016/0006-8993(87)90986-3. [DOI] [PubMed] [Google Scholar]
- Takamidoh H, Naoi M, Nagatsu T. Inhibition of type A monoamine oxidase by 1-methyl-4-phenylpyridine. Neurosci Lett. 1987;73:293–297. doi: 10.1016/0304-3940(87)90261-8. [DOI] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR. Hypothesis: the role of dopaminergic transporters in selective vulnerability of cells in Parkinson’s disease. Ann Neurol. 1998;43:555–560. doi: 10.1002/ana.410430503. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Willis GL, Donnan GA. Histochemical, biochemical and behavioural consequences of MPTP treatment in C-57 black mice. Brain Res. 1987;402:269–274. doi: 10.1016/0006-8993(87)90033-3. [DOI] [PubMed] [Google Scholar]
- Zang LY, Misra HP. Generation of reactive oxygen species during the monoamine oxidase-catalyzed oxidation of the neurotoxicant, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Biol Chem. 1993;268:16504–16512. [PubMed] [Google Scholar]