Abstract
Ambient temperature exerts a prominent influence on sleep. In rats and humans, low ambient temperatures generally impair sleep, whereas higher temperatures tend to promote sleep. The purpose of the current study was to evaluate sleep patterns and core body temperatures of C57BL/6J mice at ambient temperatures of 22°C, 26°C and 30°C under baseline conditions, after sleep deprivation (SD), and after infection with influenza virus. C57BL/6J mice were surgically implanted with electrodes for recording electroencephalogram (EEG) and electromyogram (EMG) and with intraperitoneal transmitters for recording core body temperature (Tc) and locomotor activity. The data indicate that higher ambient temperatures (26°C and 30°C) promote spontaneous slow wave sleep (SWS) in association with reduced delta wave amplitude during SWS in C57BL/6J mice. Furthermore, higher ambient temperatures also promote recuperative sleep after SD. Thus, in mice, higher ambient temperatures reduced sleep depth under normal conditions, but augmented the recuperative response to sleep loss. Mice infected with influenza virus while maintained at 22 or 26°C developed more SWS, less rapid eye movement sleep, lower locomotor activity and greater hypothermia than did mice maintained at 30°C during infection. In addition, despite equivalent viral titers, mice infected with influenza virus at 30°C showed less leucopenia and lower cytokine induction as compared with 22 and 26°C, respectively, suggesting that less inflammation develops at the higher ambient temperature.
Keywords: sleep, ambient temperature, sleep deprivation, influenza infection
Introduction1
Evironmental temperature exerts complex effects on mammalian sleep and wakefulness (Alfoldi et al., 1990; Parmeggiani and Rabini, 1970; Ray et al., 2004; Rosenthal and Vogel, 1993; Roussel et al., 1984; Schmidek et al., 1972; Thomas and Kumar, 2002). In general, shifting the ambient temperature (Ta) toward the thermoneutral zone increases the time spent in slow wave sleep (SWS) and rapid eye movement sleep REMS (Roussel et al., 1984). Maintaining mice at a warm ambient temperature (Ta) (30°C) increases time in both SWS and REMS, whereas exposure to cold temperatures (10 or 18°C) reduces sleep (Parmeggiani and Rabini, 1970; Roussel et al., 1984; Szymusiak and Satinoff, 1984). Thus, assessment of sleep in mice, as well as in other species, requires information on environmental temperature and the thermoneutral zones of species under investigation (Glotzbach and Heller, 1976).
Like ambient temperature, infectious or inflammatory challenge (e.g., infection with or administration of bacterial, viral or fungal organisms or components) can alter sleep patterns and core body temperature (Tc) (Conrad et al., 1997; Gourmelon et al., 1986; Kent et al., 1988; Kimura-Takeuchi et al., 1992a; Kimura-Takeuchi et al., 1992b; Kozak et al., 1995 ; Shoham and Krueger, 1988; Szekely and Szolcsanyi, 1978; Toth and Krueger, 1989). Furthermore, ambient temperature can influence the response to inflammatory challenge (Conrad et al., 1997; Rudaya et al., 2005; Szekely and Szolcsanyi, 1978). For example, in rats, treatment with endotoxin causes fever and changes in SWS that appear to be independent of Ta but REMS is reduced in rats that are maintained on either side of the thermoneutral zone (Conrad et al., 1997).
Mice inoculated with influenza virus typically develop hypothermia rather than fever (Fang et al., 1995; Kimura-Takeuchi et al., 1992a; Kimura-Takeuchi et al., 1992b; Klein et al., 1992; Toth and Williams, 1999; Toth and Hughes, 2004), and also show strain-dependent variation in sleep (Toth et al., 1995; Toth and Williams, 1999). The pathogenesis and host immune response elicited in response to influenza infection has been well characterized in mice. When administered via the intranasal route in anesthetized mice, influenza virus infects cells of the upper and lower respiratory mucosa (Murphy et al., 1990; Renegar et al., 2000). Signs of clinical illness (hypothermia, reduced locomotor activity, and reduced food and water intakes) develop within 1–2 days after inoculation (Conn et al., 1995; Klein et al., 1992; Toth et al., 1995), suggesting that viral components and/or cell death secondary to viral replication contribute to the production of illness. Increased concentrations of sleep-modulatory cytokines, including interleukin (IL)-1β, tumor necrosis factor (TNF)-α and interferon (IFN)- αβ, develop in bronchoalveolar lavage fluid within 36 h after inoculation (Conn et al., 1995; Hennet et al., 1992; Raut et al., 1975), and increased cytokine gene expression occurs in mediastinal lymph nodes within 3 days (Carding et al., 1993). Neutrophils are important in the primary response to influenza infection (Fujisawa et al., 1987) and their numbers increase as the viral titer increases in infected lung (Wyde et al., 1982), contributing to the immunologic and pathologic manifestations of infection. Macrophages comprise a large proportion of the inflammatory cells in the lungs of influenza-infected mice (Allan et al., 1990; Wyde et al., 1978). Phagocytic cells also probably release sleep-inducing microbial components and cytokines during infectious disease, (Fincher et al., 1996; Johannsen et al., 1991} and neuraminidase from influenza virus induces the secretion of the sleep-promoting cytokines, TNF and IL1 by murine macrophages in vivo and in vitro (Houde and Arora, 1990). Depletion of phagocytes prior to a sublethal infection with influenza virus results in greater virus growth and mortality in mice, as well as decreased expression of cytokines and chemokines (Tumpey et al., 2005). Collectively these data indicate that phagocytic cells control the replication and spread of virus after intranasal infection of mice with influenza.
Given the significance of Ta in determining sleep changes and the effect of viral infections in altering sleep and core temperature, variation in Ta could alter effects of influenza infection on both sleep and core temperature in mice. To assess the effects of ambient temperature on sleep and immune responses in mice, we evaluated the impact of three environmental temperatures (22, 26, and 30°C) on C57BL/6J mice under normal conditions, after sleep deprivation (SD), and during influenza infection. Because ambient temperature markedly altered the sleep and temperature responses to influenza infection, we also evaluated the impact of environmental temperature on selected measures of the innate immune response after influenza infection.
Methods
Animal surgery
Adult male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used in these experiments. The Laboratory Animal Care and Use Committee of the Southern Illinois University School of Medicine approved all animal procedures used in this study. Mice were housed in individual cages in sound-attenuated, temperature-controlled (22°C, 26°C, or 30°C) chambers under a 12:12 h light:dark cycle beginning 1 week prior to surgery. Mice were surgically implanted with instrumentation for monitoring of the electroencephalogram (EEG), electromyogram (EMG), locomotor activity and Tc. Mice were anesthetized with a mixture of ketamine (50 mg/kg, subcutaneous) and xylazine (50 mg/kg, subcutaneous) and were supplemented with additional anesthetic during surgery if needed. Standard aseptic techniques were used. In brief, four insulated stainless steel wires (Plastics One, Roanoke, VA) were visually positioned parallel to and under the skull in bilateral frontal (1 mm anterior to bregma and 2 mm to the left and right of midline) and parietotemporal (3–4 mm posterior to bregma and 2 mm to the left and right of midline) positions to serve as EEG electrodes. All electrodes were inserted into a pedestal that was secured to the skull with dental acrylic. One of the electrodes was made continuous with cable shielding and served as a ground; this electrode was not used for data acquisition. Two of the other three electrodes were referenced against each other in the combination that provided the best visual differentiation of three vigilance states (wakefulness, SWS and REMS). EMG electrodes (Plastics One, Roanoke, VA) were placed subcutaneously overlying nuchal muscles of the left and right sides of the body and were referenced against each other. During the same surgery, mice were also implanted with intra-abdominal transmitters (Data Sciences International, St.Paul, MN) to allow telemetric recording of locomotor activity and Tc. The analgesic ibuprofen (1.0 mg/ml) was provided in the drinking water from one day before through 5 days after surgery. Animals were allowed 14 days for recovery from surgery and habituation to the chamber environment.
Experimental protocols
To permit collection of EEG and EMG data, mice were tethered to a six-channel electrical commutator with a lightweight flexible cable (Plastics One, Roanoke, VA) and were acclimated to the tether for at least three days. Past work has shown that this acclimation period is adequate for obtaining stable recordings of sleep (Toth et al., 1995). Throughout all recording sessions, the mice could move freely in their cages and had continuous access to food and water. After a total temperature acclimation period of 21 days (1 week prior to surgery and 14 days after surgery), monitoring of sleep, Tc, and locomotor activity was initiated at light onset (lights on for 12 h beginning at 9:00 A.M) in the absence of any experimental manipulation and continued for 24 h (experimental day 1).
For assessment of the homeostatic response to sleep loss, mice were subjected to 6 h of enforced wakefulness beginning immediately after light onset on the next day (experimental day 2). Wakefulness was maintained by gently stimulating the mice whenever they assumed a sleep-like posture or developed high delta-wave amplitude (DWA) on the EEG. After the SD period, recording continued for the remaining 18 h of day 2 without further disturbing the mice. The mice were given another 24 h period (day 3) of recuperation from the effects of SD.
The same mice were used the next day (day 4) to assess the impact of influenza infection on sleep at different ambient temperatures. A total of 24 mice were studied, in cohorts of 2 to 4 at each temperature. Basal patterns of sleep were recorded for 24 h without treatment (day 4). Sleep patterns obtained on day 4 did not differ significantly from those obtained on day 1. Immediately after light onset on the next day (day 5), mice were anesthetized lightly with methoxyflurane and inoculated intranasally with 25 µl of allantoic fluid that contained approximately 1,000 hemagglutinating units (HAU) of strain A/HKx1 influenza virus (H3N2) (Toth et al., 1995). This is a mouse-adapted, laboratory generated recombinant of A/PR8/34 (H1N1) and A/Aichi (H3N2). Viral stocks were prepared by inoculation of embryonated chicken eggs in the laboratory of Dr. Robert Webster of the Department of Virology at St. Jude. Virus-infected allantoic fluid was harvested and titered, and maintained in frozen aliquots at −80°C. The dose of ∼ 1000 HAU approximates the LD10 for 12-weekold male C57BL/6J mice. Recording continued for 72 h after inoculation. Mice were then euthanized by exsanguination under isoflurane anesthesia. Lungs were collected and homogenized immediately. Aliquots of the lung homogenates were frozen for subsequent culture to verify the presence of pulmonary infection and for measurement of pulmonary cytokines and chemokines. The sleep patterns that developed after influenza inoculation were compared to those obtained during the baseline period for each animal. Previous work has shown that intranasal inoculation with uninfected allantoic fluid does not influence the normal sleep-wake patterns of C57BL/6J mice (Toth et al., 1995).
Data acquisition and analysis
EEG and EMG signals were processed through an 8-channel Grass polygraph. The amplified EEG signals were electronically band pass filtered in delta (1–4 Hz) and theta (4–8 Hz) frequency ranges (Quality Software, Springfield, IL), and the filtered signals were digitally sampled (Quality Software) at a rate of 16 Hz. The sampled data were rectified and averaged in 10-sec epochs. A computer-assisted scoring method employing custom software (Quality Software) was used to assign vigilance states to each 10-sec epoch of the recording period. To accomplish this, EEG tracings for each animal were examined visually to determine a threshold DWA associated with SWS and a threshold theta: delta ratio associated with REMS. EMG tracings were examined to determine threshold amplitudes associated with movement. A computer algorithm used these thresholds to assign vigilance states to each 10-sec epochs over the entire recording period. Three vigilance states were defined as follows: 1) SWS (DWA values above threshold in the absence of movement for any two consecutive 10-sec intervals); 2) REM sleep (low DWA, and high theta: delta amplitude in the absence of movement); and 3) wakefulness (all time intervals that did not conform to requirements for SWS or REMS). Two scorers visually reviewed the data to assign thresholds, and one scorer reviewed all computer-scored data to verify the accuracy of the computerized scorings.
The percentage of time spent in SWS and REMS were calculated for twelve 2-h intervals during each 24-h recording period. Differences were assessed using analysis of variance (ANOVA) to allow between- and within-group comparisons for effects of strain, phase (light vs. dark), condition (baseline, post sleep deprivation, or post influenza infection), and interactions. The length (in min) and number of bouts of SWS and REMS and mean DWA during SWS (expressed as a percentage of the average value measured on the baseline day) were also calculated. All values are expressed as mean ± standard error of mean (SEM).
The Data Sciences International software uses vendor-supplied calibration factors to convert the frequency signals emitted by the abdominal transmitters into temperature values that are expressed in °C. Temperature values were measured and stored every 10 min over the entire recording period. Locomotor activity is detected based on movement of the animal (i.e., the abdominal transmitter) across the DSI receiver positioned under the cage. Activity counts, which reflect transmitter movement, were summed and stored across each 10 min interval for the entire recording period.
Blood and pulmonary measurements
Blood was collected by intracardiac puncture of anesthetized mice at 72 h after influenza infection. Mice were then euthanized by cervical dislocation without recovery from anesthesia. Lungs were collected and homogenized immediately. Aliquots of the lung homogenates were frozen for subsequent measurement of viral titers and a panel of cytokines. Whole blood was assessed for total white blood cell (WBC) count and differential analysis using a Hemavet hematology instrument (CDC Technologies, Oxford, CT).
Pulmonary virus titers were measured using the tissue culture infectivity dose50 (TCID50) method. Lungs were rapidly thawed at 37°C, homogenized in 1mL of sterile phosphate buffered saline (PBS), and centrifuged at 1000 X g. Two aliquots were then frozen at −80°C until analysis. For viral titers, half-log dilutions of the supernatant were added in triplicate to Madin-Darby canine kidney cell cultures in 96-well microtiter plates. Cells were incubated for 4 days at 37°C/5% CO2. Supernatants were then assayed for hemagglutination of chicken red blood cells. Viral titers are expressed as the dilution infecting 50% of the tissue culture wells (TCID50). The remainder of the lung homogenate was processed for cytokine analysis as described below.
Cytokine/chemokine protein levels in lung
Lung homogenates prepared for viral titers above were further diluted 1:5 in PBS containing Complete Protease Inhibitor Cocktail (Roche, Camarillo, CA). The tissue homogenate was then centrifuged at 13,700 × g at 4°C for 5 min, the supernatant removed, and filtered through 0.8 µm filter. Aliquots were then frozen at −80°C until assayed. IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, granulocyte colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), keratinocyte derived chemokine (KC), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), TNF-α, and IFN-γ protein levels were measured in lung supernatant using the LINCOPlex system based on Luminex® X-Map technology (LINCO Research, St. Charles, MO). X-Map technology allows simultaneous determination of all 13 cytokines/chemokines in a single 50µL sample. Samples were assayed in duplicate and are reported as pg/lung.
Statistics
Sleep, activity and temperature were analyzed using a three factor analysis of variance (ANOVA), with strain treated as a between-subjects variable, and days and phase (nested in days) treated as within-subjects variables. If a significant interaction was detected between treatment and phase, a stepdown analysis was used to isolate the treatment(s) that generated the interaction. Further analysis of simple effects (separately for light and dark phases) was conducted on the individual treatment data to determine how they differed from the other treatments, using Student’s t-test with Bonferroni correction (SPSS, Chicago, IL).
White blood cell parameters and individual cytokine and chemokine levels in lung were analyzed separately using a one-way ANOVA with Tukey post hoc comparisons. If the cytokine values for individual samples were below the level of detection, the minimum measurable value for that cytokine was used to allow conservative assessment of significant differences between groups. For viral titers, the raw data was first rank transformed. Mean values were then compared using one-way ANOVA with Tukey post hoc assessment. All values presented are means ± SEM for indicated sample sizes. An α level of p ≤ 0.05 was considered to indicate a statistically significant effect. Error bars and asterisks shown in text and figures represent standard errors and significance levels, respectively.
Results
Spontaneous patterns of sleep, core body temperature and activity in C57BL/6J mice at 3 different ambient temperatures
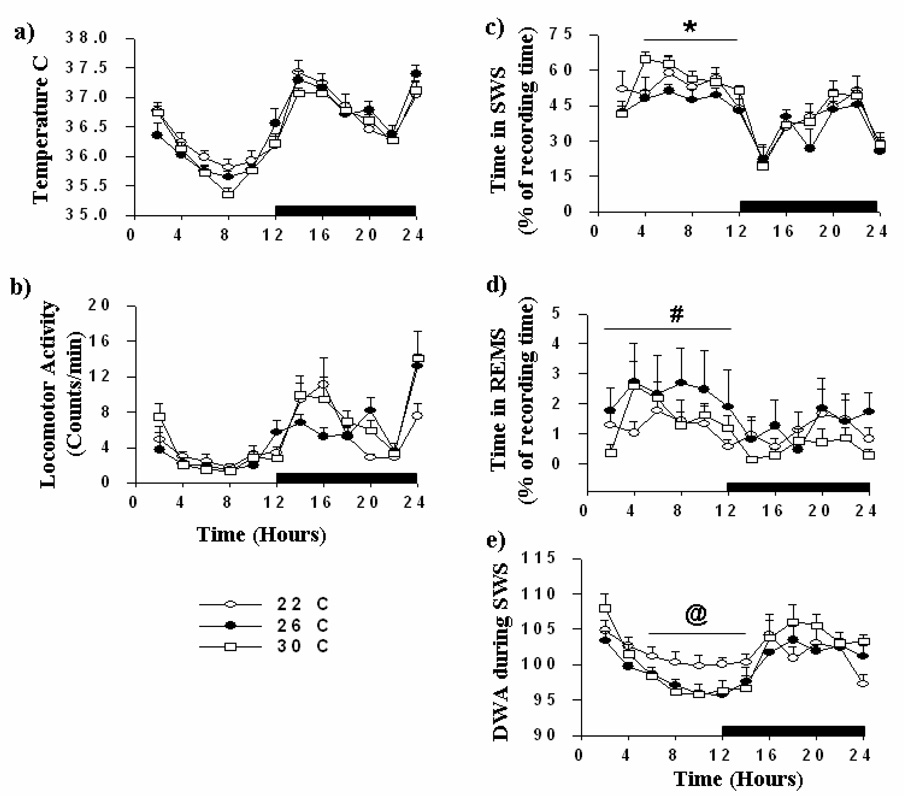
All mice showed clear diurnal variation in sleep at each of the three Ta, spending more time in sleep during the light phase of the 24-h cycle (Fig. 1). Some statistically significant changes in sleep occurred as a function of Ta, although the effect sizes associated with these changes were modest. As compared with mice housed at 26°C (n = 7), mice at 30°C (n = 10) spent significantly more time in SWS during the light phase (Fig. 1c; p< 0.01). During the light phase, the SWS bout length was significantly longer in mice at 30°C as compared to mice at 26°C, but the number of SWS bouts did not differ significantly at the two Ta (data not shown). Diurnal variation in DWA during SWS was significantly less in mice housed at 22°C (n = 7) as compared with mice at 26 and 30°C (Fig. 1e; p< 0.01). During the light phase, the time spent in REMS was significantly higher at 26°C as compared with mice housed at 22°C (Fig. 1d; p<0.05). Locomotor activity and Tc were not significantly influenced by Ta (Fig. 1a,b).
Fig.1. Spontaneous slow wave sleep (SWS), rapid eye movement sleep (REMS), delta wave amplitude (DWA) during SWS, core body temperature, and locomotor activity of C57BL/6J mice housed at three ambient temperatures.
After acclimation to the respective ambient temperature for 21 days, C57BL/6J mice (n=7 for 22 and 26°C, n=10 for 30°C) were monitored for 24 h without experimental manipulation. Black bars represent the dark phase of the 24 h light:dark cycle (lights off at 9:00 PM and on at 9:00 AM). Open circles, closed circles, and open squares indicate mice housed at 22°C, 26°C, and 30°C, respectively. Horizontal bars with symbols denote significant differences (p<0.05) as follows: *, 26 vs. 30°C; #, 22 vs. 26°C; @, 22°C vs. both 26 and 30°C. Data points are the means ± SEM.
Sleep patterns after 6 h of sleep deprivation
Mice (n = 7 per group) were subjected to 6 h of SD, beginning at light onset. During the recovery period, time spent in SWS was 31% and 37% greater in mice at 22 and 26°C (p<0.05) as compared with the corresponding baseline day. In addition mice, housed at 22 and 26°C showed greater increases in DWA during SWS (6% and 9%, respectively) (p<0.05) during the initial 2-h after the SD period (Fig.2 a, d, g).
Fig.2. Sleep patterns after 6 h of sleep deprivation (SD) in C57BL/6J mice housed at 22, 26, and 30°C.
Sleep was monitored for 24 h without experimental treatment (baseline, closed circles) [slow wave sleep (SWS) (top panels: a, d, g), rapid eye movement sleep (REMS) (middle panels: b, e, h), delta wave amplitude (DWA) (lower panels: c, f, i)]. Mice were then subjected to 6 h of SD (open circles), beginning at light onset (9:00 AM), and were then permitted to engage in spontaneous sleep. Black bars on the abscissa denote the dark phase of the 24 h light:dark cycle. Asterisks indicate significant differences between baseline and SD days (p<0.05; n=7 in each group).
Mice housed at 30°C spent 29% more time in SWS (p<0.05) during the initial 2 h of the recovery period after SD as compared to the baseline day. These mice also showed a second phase of rebound sleep during the initial 4 h after dark onset as compared with the baseline day (p<0.05). In addition, during the initial 2 h after SD, DWA during SWS was 19% greater (p<0.05, Fig. 2c, f, i ) and bout lengths were longer (p<0.05) as compared with the baseline period. At 30°C, increases in SWS and DWA during SWS persisted during the second 2 h segment of the recovery period.
At all three Ta, increases in DWA during SWS were followed by reduced DWA during the dark phase (Fig.2c, f, i; p< 0.05). The magnitude of these reductions was progressively greater with higher temperatures. Thus, although mice housed at warmer Ta (30°C) show a comparable recovery of SWS time as compared to mice housed at lower temperatures, their sleep is deeper (as reflected by increased DWA during SWS) and more consolidated (as reflected by longer bout lengths).
Time spent in REMS after SD was not significantly influenced by Ta (Fig 2b, e, h). However, relative to the baseline period, REMS increased during hours 10–12 after SD at all ambient temperatures (Fig. 2b, e, h; p<0.05 for all temperatures). Sleep parameters measured during the 24 h period after SD did not differ significantly from values obtained on the baseline day (data not shown).
Sleep patterns, core temperature, locomotor activity and cytokine concentrations after influenza infection
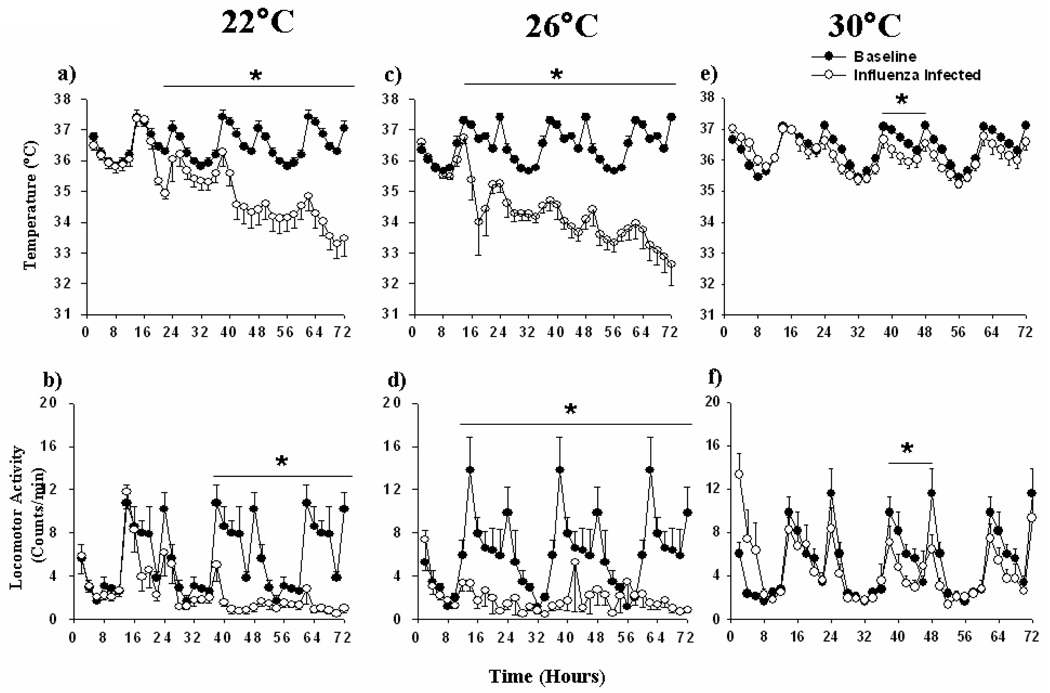
As compared with the baseline period, mice housed at 22 and 26°C developed hypothermia and reduced locomotor activity after inoculation with influenza virus (p<0.01; Fig.3a–d). As compared with control values collected at each temperature, the hypothermia and reduced locomotor activity began earlier and was greater in magnitude at 26°C as compared with 22°C and persisted for the duration of the recording period (p<0.01). At 30°C, mice showed mild hypothermia and reduced locomotion between 36 to 48 h after inoculation (dark phase) (Fig.3e, f; p<0.01).
Fig. 3. Core body temperature and locomotor activity after influenza inoculation at three ambient temperatures.
Core body temperature (a, c, e) and locomotor activity (b, d, f) was monitored for 24 h before (black circles) and 72 h after (open circles) inoculation of C57BL/6J mice with influenza virus at 22, 26, and 30°C (n=7 at 22 and 26°C and n=10 at 30°C). The X-axis indicates the time after inoculation in hours. 24 h baselines were repeat-plotted over the 72 h of post-inoculation values to facilitate visual comparison. ANOVA revealed significant differences (‘*’, p<0.01) in core body temperature and locomotor activity between pre- and post-influenza inoculation days in mice housed at all three ambient temperatures (p<0.01). Individual data points represent mean ± SEM. Intervals 12–24, 36–48, and 54–72 are the dark phases of the 24 h light:dark cycles.
After infection, the mice housed at 22 and 26°C spent significantly more time in SWS (p<0.05 for both Ta) during the dark phases after inoculation (Fig.4a, d) as compared with the baseline day. This increase in SWS was due to a greater number of SWS bouts (p<0.05, Table 1). However, the length of individual bouts of SWS was reduced (p<0.05) indicating fragmented sleep (Table 1). Time spent in REMS was significantly reduced in mice housed at 22 and 26°C, as compared with the respective baseline periods (Fig.4b, e; p<0.05). The reduction in REMS began at about 18–24 h after inoculation and persisted for the duration of the recording period. The decrease in REMS was due to reductions in both the duration and number of REMS episodes after influenza infection (p<0.01; Table 1).
Fig.4. Sleep patterns of C57BL/6J mice before and after influenza inoculation.
Sleep patterns were monitored for 24 h without experimental treatment. Mice were then inoculated with ~1000 haemagglutinating units of influenza virus immediately after light onset (“time 0”). Panels show (top to bottom) percentage of time spent in SWS (a, d, g), time spent in REMS (b, e, h), and DWA during SWS at ambient temperatures of (c, f, i) 22, 26, and 30°C (n=7 at 22 and 26°C and n=10 at 30°C). Individual data points represent mean ± SEM. Asterisks indicate significant differences (p<0.05) between the baseline day (closed circles) and days after influenza infection (open circles). Intervals 12–24, 36–48, and 54–72 are the dark phases of the 24 h light:dark cycles. Values obtained on the baseline day are repeat-plotted over the 72 h of post-inoculation values to facilitate visual comparison.
Table 1.
SWS and REMS architecture after influenza infection at different ambient temperatures
| Group & Measure | Baseline | Day 1 | Day 2 | Day 3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| LP | DP | LP | DP | LP | DP | LP | DP | ||
| 22° C (n = 7) | |||||||||
| SWS bouts | Number | 37.0±1.8 | 29.2±2.7 | 41.1±2.3 | 40.2±3.5* | 59.7±2.4* | 47.4±3.3 | 52.5±2.4 | 44.4±2.4* |
| Duration | 1.9±0.2 | 1.6±0.2 | 1.7±0.1 | 1.3±0.1 | 1.1±0.1 | 0.9±0.1 | 1.0±0.1 | 1.1±0.1 | |
| REMS bouts | Number | 3.0±0.3 | 3.6±0.7 | 5.0±0.9 | 3.0±0.8 | 2.7±0.5 | 1.5±0.5* | 1.5±0.3* | 1.1±0.3* |
| Duration | 0.5±0.1 | 0.3±0.1 | 0.3±0.0 | 0.2±0.0 | 0.2±0.0* | 0.2±0.0 | 0.1±0.0* | 0.1±0.0* | |
| 26 °C (n = 6) | |||||||||
| SWS bouts | Number | 41.0±2.2 | 25.7±2.2 | 45.7±2.4 | 46.6±3.7* | 65.6±1.4* | 65.2±2.3* | 63.3±2.3* | 58.5±2.5* |
| Duration | 1.5±0.1 | 1.6±0.1 | 1.3±0.1 | 0.9±0.1* | 0.9±0.0* | 0.8±0.0* | 0.9±0.1* | 0.8±0.0* | |
| REMS bouts | Number | 5.0±0.9 | 3.6±0.8 | 4.1±0.9 | 3.3±0.7 | 3.1±0.6* | 2.4±0.4* | 2.0±0.3* | 1.6±0.3* |
| Duration | 0.4±0.1 | 0.2±0.1 | 0.3±0.04 | 0.2±0.02 | 0.3±0.0 | 0.2±0.0 | 0.3±0.1 | 0.2±0.0 | |
| 30° C (n = 10) | |||||||||
| SWS bouts | Number | 36.0±1.6 | 25.3±1.8 | 35.1±2.0 | 37.4±2.1 | 46.1±1.6* | 35.6±1.7* | 40.4±1.7 | 36.4±1.9* |
| Duration | 2.2±0.1 | 2.7±0.2 | 2.1±0.1 | 1.8±0.1 | 1.8±0.1 | 1.9±0.1 | 2.1±0.1 | 2.1±0.2 | |
| REMS bouts | Number | 6.0±0.6 | 2.1±0.3 | 6.5±0.7 | 3.8±0.5 | 6.7±0.6 | 3.4±0.4 | 5.6±0.5 | 3.3±0.4 |
| Duration | 0.4±0.0 | 0.3±0.0 | 0.3±0.0 | 0.2±0.0 | 0.3±0.0 | 0.3±0.0 | 0.3±0.0 | 0.3±0.0 | |
Data are expressed as averages of values measured during the 12 h light (LP; 9:00 A.M to 9:00 P.M) and dark phases (DP; 9:00 P.M to 9:00 A.M) of the diurnal cycle. Values are expressed as mean ± SEM.
denotes p < 0.05 as compared with the corresponding measurement on the baseline day.
Mice housed at 30°C showed progressive increases in time spent in SWS during the dark phase on day 2 and 3 after inoculation ( Fig.4g; p<0.05). Time spent in REMS was not significantly altered by influenza infection at 30°C (Fig. 4h).
Influenza infection did not change DWA during SWS in mice housed at 22°C (Fig. 4g). However, DWA during SWS was reduced at 26°C, predominantly during the dark phase, on all 3 days after infection (Fig. 4h; p<0.01). This finding suggests that although C57BL/6J mice spent more time in SWS, their plane of sleep was relatively lighter. In contrast, in mice housed at 30°C, DWA during SWS was increased at 50 h after inoculation (p<0.05); this effect persisted for about 12 h (Fig. 4i).
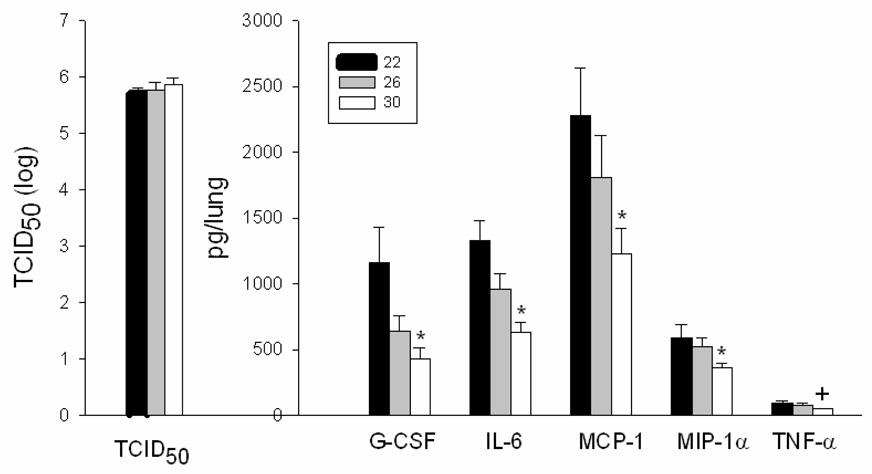
Mice were euthanized at 72 h after infection, and lungs were collected for measurement of pulmonary viral titers and a panel of cytokines. Viral titers were equivalent at all ambient temperatures (p>0.05; 22°C: 105.4 TCID50/ml, n = 9; 26°C: 105.1 TCID50/ml, n = 11; 30°C: 105.2 TCID50/ml, n=12) (Fig. 5). When compared to normal values for uninfected C57BL/6J mice, infected mice at all ambient temperatures had lower total white blood cell counts (WBC) with a greater percentage of neutrophils and lower percentage of lymphocytes (Table 2). Total white blood cell counts and numbers of neutrophils, lymphocytes, and monocytes in circulation were higher in mice housed at 30°C as compared with 22°C, indicating significantly less leukopenia at 30 °C (Table 2; p<0.05 for all parameters). WBC differential analysis demonstrated that mice housed at 26°C and 30°C had a significantly higher percentage of neutrophils as compared with mice housed at 22°C (Table 2; p<0.05).
Fig. 5. Viral titers and cytokine and chemokine concentrations in lung homogenates of influenza infected C57BL/6J mice.
Viral titers (TCID50) and concentrations of granulocyte-colony stimulating factor (G-CSF), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and tumor necrosis factor-α (TNF-α) were measured in lung homogenates of C57BL/6J mice at 72 h after influenza infection. Values represent mean ± SEM (n = 9 at 22°C; n = 11 at 26°C; n = 12 at 30°C). *, p<0.05 at 30°C as compared with 22°C; +, p = 0.08 at 30°C as compared with 22°C.
Table 2.
White blood cell differential analyses at 72 h after influenza infection of mice maintained at three ambient temperatures
| Group (N) | WBC | Neutrophils | Lymphocytes | Monocytes | N:L | |||
|---|---|---|---|---|---|---|---|---|
| K/uL | K/uL | % | K/uL | % | K/uL | % | (ratio) | |
| Jax norm (uninfected) | 10.8 | 0.86 | 8 | 9.40 | 87 | 0.11 | 1 | 0.09 |
| 22°C (9) | 2.46 ± 0.26 | 0.36 ± 0.09 | 15 | 1.90 ± 0.18 | 77 | 0.18 ± 0.03 | 7 | 0.19 |
| 26°C (10) | 3.69 ± 0.50 | 1.18 ± 0.29 * | 32 | 2.24 ± 0.25 | 61 | 0.22 ± 0.03 | 6 | 0.53* |
| 30°C (12) | 5.48 ± 0.68 * | 1.65 ± 0.29 * | 30 | 3.41 ± 0.41* | 62 | 0.36 ± 0.06 * | 7 | 0.48* |
WBC, white blood cell; N:L, neutrophil:lymphocyte. WBC and differential counts are presented as thousand per microliter and as a percentage of total WBC. Values are mean ± SEM. Normal values for C57BL/6J mice were obtained from the Jackson Laboratory.
p<0.05 compared with values at 22°C.
Analyses of a panel of cytokines in lung homogenates revealed significant differences as a function of ambient temperature. Concentrations of G-CSF, IL-6, MCP-1, and MIP-1α, were significantly lower at 30°C as compared with 22°C (Fig. 5; p<0.05). Concentrations of TNF-α (p = 0.08; Fig. 5) and IL-1α, IL-1β, IL-2, IL-10, and KC (p>0.05; data not shown) were not significantly influenced by ambient temperature at 72 h post infection. GM-CSF, IL-4 and IFN-γ were below limits of detection at all ambient temperatures.
Discussion
The data reported here show a significant influence of ambient temperature on time spent asleep in C57BL/6J mice, particularly after SD and influenza infection. Under baseline conditions, C57BL/6J mice spend more time in SWS at 30°C compared with 26°C, have lower DWA during SWS (indicating reduced depth of sleep) at ambient temperatures of 26 and 30 vs. 22°C, and spend more time in REMS at 26°C compared with 22°C. After SD, C57BL/6J mice show a rebound in SWS time and DWA during SWS at all three ambient temperatures, but the increase in DWA is progressively greater at higher temperatures. Influenza infection causes hypothermia, reduced locomotor activity, and increased SWS at 22°C; these effects are greater at 26°C, but are attenuated at 30°C. In contrast, during influenza infection, DWA during SWS is progressively reduced at higher ambient temperatures. Pulmonary viral titers were equivalent at all ambient temperatures. However, plasma WBC counts were higher at 30°C as compared with 22 and 26°C. Pulmonary concentrations of G-CSF, IL-6, MIP-1 α, and MCP-1 at 72 h after infection were lower at 30°C as compared with lower ambient temperatures. Taken together, these data indicate that higher ambient temperatures promote greater depth of sleep after sleep deprivation. During influenza infection, the intermediate ambient temperature (26°C) promotes sleep, yet the highest (30°C) attenuates sleep. Hypothermia is also reduced at the highest ambient temperature. Immune indices suggest less inflammation at 30°C despite equivalent viral titers.
Two key implications of our study are as follows. First, various laboratories that study sleep in mice report using ambient temperatures ranging from 22°C to 29°C (Gourine et al., 2002; Huang et al., 2006; Kozak and Kozak, 2003; Li et al., 2003; Morrow and Opp, 2005; Obal et al., 2005; Oka et al., 2003; Toth et al., 1995; Toth and Opp, 2001; Vyazovskiy et al., 2002). Data collected from mice housed under different temperatures may therefore vary depending on an interaction between the ambient temperature and the condition of the animal. In some rodent species, even small fluctuations in ambient temperature can change the physiology or behavior. For example, the thermoregulatory responses to LPS are strongly influenced by ambient temperature in mice (Conrad et al., 1997; Rudaya et al., 2005; Shoham and Krueger, 1988). Furthermore, the range and extent of the so-called thermoneutral zone, which is defined as the temperature range in which thermal stress is minimal, is not a constant interval and can vary even within a single species depending on the environmental and experimental conditions (Romanovsky et al., 2002). These factors, together with the current data, reinforce the importance of considering environmental temperature when evaluating the sleep data.
Second, the ambient temperature of 30°C appears to diminish the physiologic signs of influenza infection (i.e., a minimal increase in SWS without a concomitant change in core temperature) and to modify processes of host defense (i.e., reduced induction of pro-inflammatory cytokines and chemokines and relatively less leucopenia at 30°C). Concentrations of IL-6, G-CSF, MIP1-α, and MCP-1 in influenza-infected lungs were lower and leukopenia and lymphocytopenia were attenuated at 30°C, despite equivalent viral titers at all three ambient temperatures. Collectively, these data suggest that pro-inflammatory processes are blunted and anti-inflammatory processes are augmented at 30°C, thereby changing the inflammatory milieu and subsequently reducing its impact on sleep and core temperature. IL-6 and MIP1-α have been associated with altered sleep after infectious or inflammatory challenge in mice, and exogenous administration of G-CSF reduces sleep in humans (Bauer et al., 1994; Opp and Toth, 2003; Schuld et al., 1999). Although MCP-1 lacks a documented role in sleep regulation, it could indirectly contribute to sleep modulation by recruiting mononuclear cells to the site of inflammation and causing downstream induction of other sleep-modulatory substances. In addition, mice are relatively poikilothermic as compared to larger mammals, and variation in environmental temperature may differentially affect enzyme activity and other regulatory processes.
In our study, environmental temperature altered sleep in normal mice more prominently during the light phase of the diurnal cycle, as evident in the increased SWS time at 30°C. Others have similarly reported that effects of temperature on sleep are more prominent during the light phase (Alfoldi et al., 1990; Glotzbach and Heller, 1976; Obal et al., 1983; Oka et al., 2003; Ray et al., 2004; Schmidek et al., 1972; Thomas et al., 2000). DWA during SWS was lower at 26 and 30°C as compared with 22°C, suggesting a light plane of sleep at higher ambient temperatures. Whether mice would prefer temperatures that promote longer duration or greater depth of sleep is currently unknown. The poikilothermic nature of mice may contribute to the absence of consistent or graded trends in the relationship of ambient temperature to the other variables measured.
In response to SD, mice did not show significant changes in the total amount of SWS as a function of ambient temperature, but they did exhibit a greater elevation of DWA during SWS and longer duration of SWS bouts after SD at higher ambient temperatures. These findings suggest that higher temperatures promote the depth and consolidation of sleep after sleep loss, but not its duration. Thus, the effects of temperature on DWA under normal conditions and after sleep loss differ. Environmental warmth and extended periods of wakefulness are both associated with elevated brain temperatures (Franken et al., 1992; Gao et al., 1995; Glotzbach and Heller, 1976; Thomas and Kumar, 2002; Wehr, 1992) and may influence sleep via common mechanisms (Gao et al., 1995). However, changes in brain temperature are not associated with changes in DWA, suggesting that independent mechanisms regulate brain temperature and sleep intensity (Franken et al., 1992). In several species, recovery of SWS after sleep loss occurs at the expense of REMS (Borbely and Neuhaus, 1979; Friedman et al., 1979; Takahashi et al., 1997), and REMS rebound occurs relatively later during the course of sleep recovery. We similarly observed a REMS rebound during the dark phase of the diurnal cycle and a reduction in the magnitude of the REMS rebound with progressively higher ambient temperatures.
Although influenza infection causes fever in humans and ferrets (Husseini et al., 1982), mice infected with influenza virus develop hypothermia (Fang et al., 1995; Kimura-Takeuchi et al., 1992a; Kimura-Takeuchi et al., 1992b; Klein et al., 1992; Toth and Williams, 1999). When given access to a thermal gradient, influenza-infected mice move to a warmer environment, implying that they prefer warmth or feel cold as a result of viral-induced hypothermia (Kimura-Takeuchi et al., 1992b). However, hypothermia appears to promote survival in mice treated with endotoxin (Lagerspetz and Väätäinen, 1987) or inoculated with influenza virus (Klein et al., 1992), perhaps by reducing energy needs during infection-induced anorexia. Energy conservation may be particularly important in mice due to their high basal metabolic rate. Maintaining a fever would create an additional metabolic demand. However, just as rats maintained in a cold environment do not become febrile (Conrad et al., 1997, Rudaya et al., 2005; Szelenyi and Szekely, 1979), hypothermia did not develop in infected mice housed at 30°C. This finding could indicate either that the elevated ambient temperature maintains normothermia in infected mice, or, alternatively, that the internal signals that generate hypothermia do not develop at the warmer temperature. The latter possibility is suggested by the altered immune parameters measured in the mice maintained at 30°C.
Changes in SWS, REMS and thermoregulation can occur simultaneously yet without interdependence (Morrow and Opp, 2005; Parmeggiani and Rabini, 1970; Parmeggiani, 2003). The present study and previous work by us and others demonstrates concurrent changes in SWS, REMS, and core temperature during influenza infection in mice (Fang et al., 1995; Kimura-Takeuchi et al., 1992a; Kimura-Takeuchi et al., 1992b; Toth and Williams, 1999). In our data, the hypothermia persisted for the duration of the recording period at the lower ambient temperature, whereas the increase in SWS was restricted to the dark phase of the diurnal cycle on the first two days after infection, suggesting that distinct mechanisms regulate sleep and temperature.
Current models suggest that during systemic immune or inflammatory challenges, a peripheral signal (e.g., cytokine production elaborated in the lungs in response to influenza infection) eventually impacts the brain and triggers local induction of cytokines that then modulate sleep (Long et al., 1992; Marshall and Born, 2005; Opp and Toth, 2003). Thus, the thermoregulatory and sleep alterations associated with influenza infection may reflect altered cytokine production in the periphery and/or the brain. Altered sleep is typically viewed as a sickness behavior. With regard to influenza, our data confirm that the infection changes sleep, and further indicate that both sleep and facets of the inflammatory response are modified by changes in ambient temperature. We and others have also demonstrated that different ambient temperature alone can influence sleep even in normal healthy mice (Alfoldi et al., 1990; Parmeggiani and Rabini, 1970; Ray et al., 2004; Rosenthal and Vogel, 1993; Roussel et al., 1984; Schmidek et al., 1972; Thomas and Kumar, 2002).
In conclusion, data reported here indicate that ambient temperature influences sleep not only under normal conditions but also after SD and influenza infection. Exposure to warm ambient temperature has been proposed as a non-pharmacological method of increasing sleep (Rosenthal and Vogel, 1993). Modulating ambient temperatures may be a potential approach to promoting recuperation from sleep loss and mitigating the adverse effects of viral infection.
Acknowledgements
The authors thank Lisa Cox, Joel Reichensperger, and Chris Bosgraaf for their excellent technical assistance, Dr. Tom Gardiner for developing the sleep scoring software, and Drs. Larry Hughes and Steve Verhulst for guidance with statistics. This work was supported by NIH grants RR017543 and HL070522.
Footnotes
ANOVA, analysis of variance; DWA, delta-wave amplitude; EEG, electroencephalogram; EMG, electromyogram; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte macrophage-colony stimulating factor; HAU, hemagglutinating units; IFN-γ, interferon–γ; IL, interleukin; KC, keratinocyte derived chemokine; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; PBS, phosphate-buffered saline; REMS, rapid-eye-movement sleep; SD, sleep deprivation; SEM, standard error of the mean; SWS, slow-wave sleep; Ta, ambient temperature; TcTc, core temperature; TCID50, tissue culture infectivity dose50; TNF-α, tumor necrosis factor-α; TNZ, thermoneutral zone.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alfoldi P, Rubicsek G, Cserni G, Obal F., Jr Brain and core temperatures and peripheral vasomotion during sleep and wakefulness at various ambient temperatures in the rat. Pflugers. Arch. 1990;417(3):336–341. doi: 10.1007/BF00371001. [DOI] [PubMed] [Google Scholar]
- Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza: Consequences of depleting CD4+ T cells. J Immunol. 1990;144(10):3980–3986. [PubMed] [Google Scholar]
- Bauer J, Hohagen F, Ebert T, Timmer J, Ganter U, Krieger S, Lis S, Postler E, Voderholzer U, Berger M. Interleukin-6 serum levels in healthy persons correspond to the sleep-wake cycle. Clin. Investig. 1994;72(4):315. doi: 10.1007/BF00180048. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Neuhaus HU. Sleep-deprivation: effects on sleep and EEG in the rat. J. Comp. Physiol. 1979;133:71–87. [Google Scholar]
- Carding SR, Allan W, McMickle A, Doherty PC. Activation of cytokine genes in T cells during primary and secondary murine influenza pneumonia. J Exp Med. 1993;177(2):475–482. doi: 10.1084/jem.177.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn CA, McClellan JL, Maassab HF, Smitka CW, Majde JA, Kluger MJ. Cytokines and the acute phase response to influenza virus in mice. Am. J. Physiol. 1995;268(1 Pt 2):R78–R84. doi: 10.1152/ajpregu.1995.268.1.R78. [DOI] [PubMed] [Google Scholar]
- Conrad A, Bull DF, King MG, Husband AJ. The effects of lipopolysaccharide on the fever response in rats at different ambient temperatures. Physiol. Behav. 1997;62:1197–1201. doi: 10.1016/s0031-9384(97)00166-2. [DOI] [PubMed] [Google Scholar]
- Fang J, Sanborn CK, Renegar KB, Majde JA, Krueger JM. Influenza viral infections enhance sleep in mice. Proc. Soc. Exp. Biol. Med. 1995;210:242–252. doi: 10.3181/00379727-210-43945. [DOI] [PubMed] [Google Scholar]
- Fincher EF, 4th, Johannsen L, Kapas L, Takahashi S, Krueger JM. Microglia digest Staphylococcus aureus into low molecular weight biologically active compounds. Am J Physiol. 1996;271(1 Pt 2):R149–R156. doi: 10.1152/ajpregu.1996.271.1.R149. [DOI] [PubMed] [Google Scholar]
- Franken P, Tobler I, Borbely AA. Cortical temperature and EEG slow-wave activity in the rat: analysis of vigilance state related changes. Pflugers. Arch. 1992;420(5–6):500–507. doi: 10.1007/BF00374625. [DOI] [PubMed] [Google Scholar]
- Friedman L, Bergmann BM, Rechtschaffen A. Effects of sleep deprivation on sleepiness, sleep intensity, and subsequent sleep in the rat. Sleep. 1979;1:369–391. doi: 10.1093/sleep/1.4.369. [DOI] [PubMed] [Google Scholar]
- Fujisawa H, Tsuru S, Taniguchi M, Zinnaka Y, Nomoto K. Protective mechanisms against pulmonary infection with influenza virus. I. Relative contribution of polymorphonuclear leukocytes and of alveolar macrophages to protection during the early phase of intranasal infection. J Gen Virol. 1987;68(Pt 2):425–432. doi: 10.1099/0022-1317-68-2-425. [DOI] [PubMed] [Google Scholar]
- Gao B, Franken P, Tobler I, Borbély AA. Effect of elevated ambient temperature on sleep, EEG spectra, and brain temperature in the rat. Am. J. Physiol. 1995;268:R1365–R1373. doi: 10.1152/ajpregu.1995.268.6.R1365. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC. Central nervous regulation of body temperature during sleep. Science. 1976;194:537–539. doi: 10.1126/science.973138. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Gourine VN, Tesfaigzi Y, Caluwaerts N, Van Leuven F, Kluger MJ. Role of α2-macroglobulin in fever and cytokine responses induced by lipopolysaccharide in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R218–R226. doi: 10.1152/ajpregu.00746.2001. [DOI] [PubMed] [Google Scholar]
- Gourmelon P, Briet D, Court L, Tsiang H. Electrophysiological and sleep alterations in experimental mouse rabies. Brain. Res. 1986;398:128–140. doi: 10.1016/0006-8993(86)91258-8. [DOI] [PubMed] [Google Scholar]
- Hennet T, Ziltener HJ, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149(3):932–939. [PubMed] [Google Scholar]
- Houde M, Arora DJ. Stimulation of tumor necrosis factor secretion by purified influenza virus neuraminidase. Cell Immunol. 1990;129(1):104–111. doi: 10.1016/0008-8749(90)90190-3. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Mochizuki T, Qu WM, Hong ZY, Watanabe T, Urade Y, Hayaishi O. Altered sleep-wake characteristics and lack of arousal response to H3receptor antagonist in histamine H1receptor knockout mice. Proc. Natl. Acad. Sci. U. S. A. 21. 2006;103(12):4687–4692. doi: 10.1073/pnas.0600451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseini RH, Sweet C, Collie MH, Smith H. Elevation of nasal viral levels by suppression of fever in ferrets infected with influenza viruses of differing virulence. J. Infect. Dis. 1982;145:520–524. doi: 10.1093/infdis/145.4.520. [DOI] [PubMed] [Google Scholar]
- Johannsen L, Wecke J, Obal F, Jr, Krueger JM. Macrophages produce somnogenic and pyrogenic muramyl peptides during digestion of staphylococci. Am J Physiol. 1991;260(1 Pt 2):R126–R133. doi: 10.1152/ajpregu.1991.260.1.R126. [DOI] [PubMed] [Google Scholar]
- Kent S, Price M, Satinoff E. Fever alters characteristics of sleep in rats. Physiol. Behav. 1988;44:709–715. doi: 10.1016/0031-9384(88)90051-0. [DOI] [PubMed] [Google Scholar]
- Kimura-Takeuchi M, Majde JA, Toth LA, Krueger JM. The role of double-stranded RNA in induction of the acute-phase response in an abortive influenza virus infection model. J. Infect. Dis. 1992a;166(6):1266–1275. doi: 10.1093/infdis/166.6.1266. [DOI] [PubMed] [Google Scholar]
- Kimura-Takeuchi M, Majde JA, Toth LA, Krueger JM. Influenza virus-induced changes in rabbit sleep and acute phase responses. Am. J. Physiol. 1992b;263:R1115–R1121. doi: 10.1152/ajpregu.1992.263.5.R1115. [DOI] [PubMed] [Google Scholar]
- Klein MS, Conn CA, Kluger MJ. Behavioral thermoregulation in mice inoculated with influenza virus. Physiol. Behav. 1992;52:1133–1139. doi: 10.1016/0031-9384(92)90472-e. [DOI] [PubMed] [Google Scholar]
- Kozak W, Zheng H, Conn CA, Soszynski D, Van der Ploeg LHT, Kluger MJ. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1β-deficient mice. Am. J. Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- Kozak W, Kozak A. Differential rols of nitric oxide synthase isoforms in fever of different etiologies: studies using NOS gene-deficient mice. J. Appl. Physiol. 2003;94:2534–2544. doi: 10.1152/japplphysiol.01042.2002. [DOI] [PubMed] [Google Scholar]
- Lagerspetz KYH, Väätäinen T. Bacterial endotoxin and infection cause behavioural hypothermia in infant mice. Comp. Biochem. Physiol. 1987;88A:519–521. doi: 10.1016/0300-9629(87)90074-0. [DOI] [PubMed] [Google Scholar]
- Li S, Goorha S, Ballou LR, Blatteis CM. Intracerebroventricular, interleukin-6, macrophage inflammatory protein-1β and IL-18: pyrogenic and PGE2-mediated? Brain Res. 2003;922:76–84. doi: 10.1016/j.brainres.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Long NC, Morimoto A, Nakamori T, Murakami N. Systemic injection of TNF- α attenuates fever due to IL-1 β and LPS in rats. Am. J. Physiol. 1992;263:R987–R991. doi: 10.1152/ajpregu.1992.263.5.R987. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. Brain-immune interactions in sleep. Int. Rev. Neurobiol. 2005;52:93–131. doi: 10.1016/s0074-7742(02)52007-9. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav. Immun. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8(5):497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Tobler I, Borbely AA. Effect of ambient temperature on the 24-hour sleep-wake cycle in normal and capsaicin-treated rats. Physiol. Behav. 1983;30(3):425–430. doi: 10.1016/0031-9384(83)90148-8. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Garcia-Garcia F, Kacsoh B, Taishi P, Bohnet S, Horseman ND, Krueger JM. Rapid eye movement sleep is reduced in prolactin-deficient mice. J. Neurosci. 2005;25(44):10282–10289. doi: 10.1523/JNEUROSCI.2572-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J. Physiol. 2003;551:945–654. doi: 10.1113/jphysiol.2003.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp MR, Toth LA. Neural-immune interactions in the regulation of sleep. Front. Biosci. 2003;8:d768–d779. doi: 10.2741/1061. [DOI] [PubMed] [Google Scholar]
- Parmeggiani PL, Rabini C. Sleep and environmental temperature. Arch. Ital. Biol. 1970;108:369–387. [PubMed] [Google Scholar]
- Parmeggiani PL. Thermoregulation and sleep. Front. Biosci. 2003;8:s557–s567. doi: 10.2741/1054. [DOI] [PubMed] [Google Scholar]
- Raut S, Hurd J, Blandford G, Heath RB, Cureton RJ. The pathogenesis of infections of the mouse caused by virulent and avirulent variants of an influenza virus. J Med Microbiol. 1975 Feb;8(1):127–136. doi: 10.1099/00222615-8-1-127. [DOI] [PubMed] [Google Scholar]
- Ray B, Mallick HN, Kumar VM. Changes in thermal preference, sleep-wakefulness, body temperature and locomotor activity of rats during continuous recording for 24 hours. Behav. Brain. Res. 2004;154(2):519–526. doi: 10.1016/j.bbr.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Renegar KB, Crouse D, Floyd RA, Krueger J. Progression of influenza viral infection through the murine respiratory tract: the protective role of sleep deprivation. Sleep. 2000;23(7):859–863. [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Rosenthal AM, Vogel GW. The effect of a 3-day increase of ambient temperature toward the thermoneutral zone on rapid eye movement sleep in the rat. Sleep. 1993;16(8):702–705. doi: 10.1093/sleep/16.8.702. [DOI] [PubMed] [Google Scholar]
- Roussel B, Turrillot P, Kitahama K. Effect of ambient temperature on the sleep-waking cycle in two strains of mice. Brain. Res. 1984;294(1):67–73. doi: 10.1016/0006-8993(84)91310-6. [DOI] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am. J. Physiol. Integr. Comp. Physiol. 2005;289:R1244–R1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- Schmidek WR, Hoshino K, Schmidek M, Timo-Iaria C. Influence of environmental temperature on the sleep-wakefulness cycle in the rat. Physiol. Behav. 1972;8(2):363–371. doi: 10.1016/0031-9384(72)90384-8. [DOI] [PubMed] [Google Scholar]
- Schuld A, Mullington J, Hermann D, Hinze-Selch D, Fenzel T, Holsboer F, Pollmächer T. Effects of granulocyte colony-stimulating factor on night sleep in humans. Am. J. Physiol. 1999;276:R1149–R1155. doi: 10.1152/ajpregu.1999.276.4.R1149. [DOI] [PubMed] [Google Scholar]
- Shoham S, Krueger JM. Muramyl dipeptide-induced sleep and fever: effects of ambient temperature and time of injections. Am. J. Physiol. 1988;255(1 Pt 2):R157–R165. doi: 10.1152/ajpregu.1988.255.1.R157. [DOI] [PubMed] [Google Scholar]
- Szekely M, Szolcsanyi J. Endotoxin and prostaglandin fever of newborn guinea pigs at different ambient temperatures. Acta. Physiol. Acad. Sci. Hung. 1978;51(3):293–298. [PubMed] [Google Scholar]
- Szelenyi Z, Szekely M. Comparison of the effector mechanisms during endotoxin fever in the adult rabbit. Acta. Physiol. Acad. Sci. Hung. 1979;54(1):33–41. [PubMed] [Google Scholar]
- Szymusiak R, Satinoff E. Ambient temperature-dependence of sleep disturbances produced by basal forebrain damage in rats. Brain. Res. Bull. 1984;12(3):295–305. doi: 10.1016/0361-9230(84)90057-1. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Fang J, Kapás L, Wang Y, Krueger J. Inhibition of brain interleukin-1 attenuated sleep rebound after sleep deprivation in rabbits. Am. J. Physiol. 1997;273:R677–R682. doi: 10.1152/ajpregu.1997.273.2.R677. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Erokwu BO, Yamamoto BK, Ernsberger P, Bishara O, Strohl KP. Alterations in respiratory behavior, brain neurochemistry and receptor density induced by pharmacologic suppression of sleep in the neonatal period. Developmental Brain Research. 2000;120:181–189. doi: 10.1016/s0165-3806(00)00008-0. [DOI] [PubMed] [Google Scholar]
- Thomas TC, Kumar VM. Effect of ambient temperature on brain temperature and sleep-wakefulness in medial preoptic area lesioned rats. Indian J. Physiol. Pharmacol. 2002;46(3):287–297. [PubMed] [Google Scholar]
- Toth LA, Krueger JM. Effects of microbial challenge on sleep in rabbits. F.A.S.E.B. J. 1989;3:2062–2066. doi: 10.1096/fasebj.3.9.2663582. [DOI] [PubMed] [Google Scholar]
- Toth LA, Rehg JE, Webster RG. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J. Neuroimmunol. 1995;58:89–99. doi: 10.1016/0165-5728(94)00193-r. [DOI] [PubMed] [Google Scholar]
- Toth LA, Williams RW. A quantitative genetic analysis of slow-wave sleep in influenzainfected CXB recombinant inbred mice. Behav. Genet. 1999;29(5):339–348. doi: 10.1023/a:1021661901196. [DOI] [PubMed] [Google Scholar]
- Toth LA, Hughes LF. Macrophage participation in influenza-induced sleep enhancement in C57BL/6J mice. Brain. Behav. Immun. 2004;18:375–389. doi: 10.1016/j.bbi.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Toth LA, Opp MR. Cytokine- and microbially-induced sleep responses of interleukin-10 deficient mice. Am. J. Physiol. 2001;280:R1806–R1814. doi: 10.1152/ajpregu.2001.280.6.R1806. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79(23):14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Borbely AA, Tobler I. Interhemispheric sleep EEG asymmetry in the rat is enhanced by sleep deprivation. J. Neurophysiol. 2002;88(5):2280–2286. doi: 10.1152/jn.00304.2002. [DOI] [PubMed] [Google Scholar]
- Wehr TA. A brain-warming function for REM sleep. Neurosci. Biobehav. Rev. 1992;16(3):379–397. doi: 10.1016/s0149-7634(05)80208-8. [DOI] [PubMed] [Google Scholar]
- Wyde PR, Peavy DL, Cate TR. Morphological and cytochemical characterization of cells infiltrating mouse lungs after influenza infection. Infect Immun. 1978;21(1):140–146. doi: 10.1128/iai.21.1.140-146.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde PR, Wilson MR, Cate TR. Interferon production by leukocytes infiltrating the lungs of mice during primary influenza virus infection. Infect Immun. 1982;38(3):1249–1255. doi: 10.1128/iai.38.3.1249-1255.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]