Abstract
Peroxynitrite, a powerful mutagenic oxidant and nitrating species, is formed by the near diffusion-limited reaction of ·NO and O2⨪ during activation of phagocytes. Chronic inflammation induced by phagocytes is a major contributor to cancer and other degenerative diseases. We examined how γ-tocopherol (γT), the principal form of vitamin E in the United States diet, and α-tocopherol (αT), the major form in supplements, protect against peroxynitrite-induced lipid oxidation. Lipid hydroperoxide formation in liposomes (but not isolated low-density lipoprotein) exposed to peroxynitrite or the ·NO and O2⨪ generator SIN-1 (3-morpholinosydnonimine) was inhibited more effectively by γT than αT. More importantly, nitration of γT at the nucleophilic 5-position, which proceeded in both liposomes and human low density lipoprotein at yields of ≈50% and ≈75%, respectively, was not affected by the presence of αT. These results suggest that despite αT’s action as an antioxidant γT is required to effectively remove the peroxynitrite-derived nitrating species. We postulate that γT acts in vivo as a trap for membrane-soluble electrophilic nitrogen oxides and other electrophilic mutagens, forming stable carbon-centered adducts through the nucleophilic 5-position, which is blocked in αT. Because large doses of dietary αT displace γT in plasma and other tissues, the current wisdom of vitamin E supplementation with primarily αT should be reconsidered.
Keywords: antimutagen, lipid peroxidation, low-density lipoprotein, inflammation
Chronic inflammation induced by activation of phagocytes is a major contributor to the development of cancer and neurodegenerative and cardiovascular diseases in humans (reviewed in ref. 1). Reactive nitrogen oxide species (NOx) appear to contribute to this damage because their stable end-products, nitrite and nitrate, are readily detected in both animals and humans with ongoing inflammation (1). Peroxynitrite is a powerful oxidant and nitrating species formed from the near diffusion-limited reaction of nitric oxide (·NO) with the superoxide radical anion (O2⨪), two radical species generated simultaneously and in close proximity to one another by activated phagocytes (2). Peroxynitrite has been shown to readily react with most biological molecules (lipids, amino acids, and DNA), and as a consequence cause extensive cell damage (1, 3). An important etiological role for oxidants such as peroxynitrite in the above diseases is suggested by the observed inverse correlation between dietary antioxidant consumption and incidence of disease (4, 5), and the increased formation of products such as 3-nitrotyrosine (6) derived from their reaction with biomolecular targets and detected in inflammation.
The main membrane antioxidant vitamin E consists of two major forms, α-tocopherol (αT) and γ-tocopherol (γT), that differ structurally only by a methyl group substitution at the 5-position (see Fig. 4). Whereas αT has the higher vitamin E activity (7), is the more potent antioxidant in vitro (8), and is the primary form in supplements, γT is the principal form of vitamin E in the United States diet (9). That γT has not received much attention may stem from the fact that αT levels in blood plasma and most other tissues are ≈5-fold higher than γT (10), considered to be due to preferential reincorporation of αT into nascent very low-density lipoproteins (11). This ratio further increases (>20-fold) in people that consume αT supplements. However, some of the discrepancy between dietary intake and plasma levels may be caused by more rapid tissue uptake and increased cellular turnover of γT compared to αT (12, 13).
Figure 4.
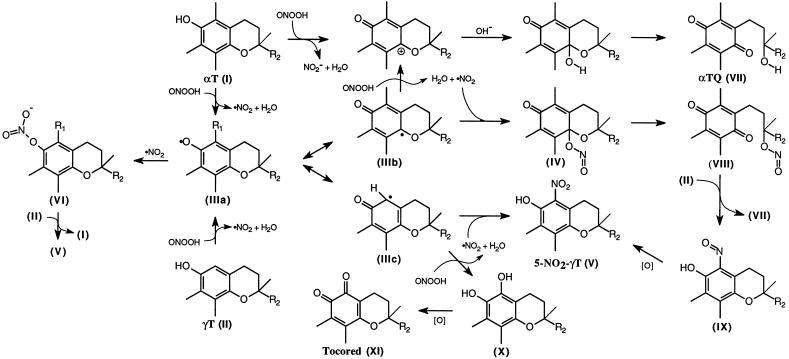
Postulated reactions of NOx with αT and γT during peroxynitrite-induced lipid peroxidation. The reaction mechanism takes into account that αT (I) is principally oxidized to αTQ (VII, compare with ref. 26), whereas γT (II) is mainly nitrated to 5-NO2-γT (V) or oxidized to its ortho-quinone (XI). ·NO2 formed as a result of one-electron oxidation of tocopherol or unsaturated lipids by peroxynitrite is likely to react with one of the resonance forms of the tocopheroxyl radical (IIIa, b, and c) yielding either IV, V, or VI. Although αT inhibits nitrosation reactions (46), it forms reactive nitrite esters with ·NO2 such as VI and VIII (see refs. 27 and 41), that can further nitrosate nucleophilic targets (20). Transnitrosation of γT by IV or VIII to IX may explain why the consumption of γT and the extent of 5-NO2-γT formation are not affected by the presence of αT (see Fig. 2). R1 = CH3 or H, R2 = C16H33. (Reactions are not balanced).
Low plasma levels of αT are a strong predictor for incidence of many of the above diseases (4, 5), prompting several intervention studies using oral supplementation with αT. However, many studies failed to demonstrate a protective effect (5, 14, 15). Furthermore, high doses of αT can increase tumor formation in animals (16–18) and displace γT in plasma and other tissues (19). Together these data indicate a need to evaluate the potential complementarity of the two tocopherols.
Here we investigated the efficacy by which αT and γT inhibit peroxynitrite-induced lipid peroxidation and found that the two tocopherols exhibit fundamentally different reactivities. The results support the recent findings by Cooney et al. that nitrogen dioxide (·NO2)-mediated nitrosation of morpholine is inhibited effectively only by γT and not by αT (20), and that γT is readily nitrated by NOx such as ·NO2 (21). Evidence is now emerging that γT may be as important as αT in the prevention of degenerative diseases (20, 22).
MATERIALS AND METHODS
Chemicals.
Peroxynitrite was synthesized as described (23). 3-morpholinosydnonimine (SIN-1) was a gift from Cassella (Frankfurt). All oxidation and nitration experiments were carried out in 0.1 M sodium phosphate buffer (pH 7.4) treated with Chelex resin (Bio-Rad). Phosphatidylcholine (PC) from soybean was from Fluka and used without further purification (lipid hydroperoxide content <0.1%). Synthetic dimyristoyl and dipalmitoyl PC were from Sigma. d-αT and d-γT (purity >99.7%) were from Fluka, and α-tocopherylquinone (αTQ) from ICN. γ-tocopherol-5,6-quinone (tocored) was synthesized as described (24) and purified by C18 reversed-phase HPLC. Concentrations of tocopherol standard solutions were determined spectrophotometrically (25–27). Solvents suitable for HPLC and organic extraction were from Fisher Scientific or Sigma-Aldrich.
Synthesis and Identification of 5-Nitro-γ-tocopherol (5-NO2-γT).
Nitrous acid-induced nitration of γT was carried out as described by Marcinkiewicz (28). Briefly, 4 ml of glacial acetic acid was added to 100 mg of d-γT (Acros Chemicals, Springfield, NJ, purity >95%) dissolved in ethanol (100 ml), followed by 60 ml of a 2% aqueous solution of sodium nitrite. After 2 min, 40 ml of 20% KOH was added to the orange reaction mixture, followed by 200 ml of water. The product was extracted into hexane (100 ml), washed several times with water, and reduced to an oil by solvent evaporation. The crude product was obtained in 90–95% yield and was purified further by C18 reversed-phase HPLC using 100% methanol as the eluent. As expected (28), exposure of γT to nitrous acid resulted in formation of the 5-nitro, and not the 5-nitroso adduct (29): UV in EtOH 260, 304, and 413 nm. 1H NMR (CDCl3) δ: 0.87 (m, 12H, 4 × CH3), 1.24 (s, 3H, 2C—CH3), 1.0–1.45 (broad m, 21H, 9 × CH2 + 3 × CH), 1.74 (m, 2H, 3—CH2), 2.19 (8C—CH3), 2.23 (7C—CH3), 3.01 (t, 2H, 4—CH2, J = 6.8 Hz), 10.74 (s, 1H, OH). 13C NMR (CDCl3) δ: 12.11 (q), 13.36 (q), 19.69 (q), 19.79 (q), 20.97 (t), 21.78 (t), 22.67 (q), 22.76 (q), 23.65 (q), 24.49 (t), 24.86 (t), 28.03 (d), 30.97 (t), 32.70 (d), 32.84 (d), 37.33 (t), 37.43 (3 × t), 39.41 (t), 39.64 (t), 75.51 (s), 113.50 (s), 125.33 (s), 132.00 (s), 137.12 (s), 145.28 (s), 148.42 (s). The experimentally determined mass for the molecular ion (M•+) of the product was 461.3502 (compare with 461.3505 calculated for C26H47O4N). High-resolution mass spectra were obtained in electron ionization mode on an Autospec-Q mass spectrometer (Micromas, Manchester, United Kingdom) using perfluorokerosene as calibrant. UV spectra were obtained on a Shimadzu PC160 spectrophotometer. 1H and 13C NMR spectra were obtained on a Bruker ACF300 (300 MHz) spectrometer using trimethylsilane as internal standard.
Preparation and Oxidation of Liposomes.
Multilamellar PC liposomes were prepared as reported (30). Reactions were initiated by either rapid mixing (5 sec) of liposomes with a small volume of peroxynitrite at room temperature, or by adding a small volume of SIN-1 to the liposomes and subsequent incubation at 37°C in the dark. Reaction mixtures were analyzed for phosphatidylcholine hydroperoxide (PC-OOH) and tocopherols by direct injection onto the HPLC column without previous extraction and within 30 sec of sampling.
Supplementation, Isolation, and Exposure of Human Low Density Lipoprotein (LDL) to SIN-1.
LDL was isolated from human plasma obtained from two healthy male normolipidemic donors (33 and 55 years old) after fasting (≥12 h) and purified as described (31). LDL was diluted (≈2 mg protein/ml) so that the concentration of free cholesterol (≈1 mM) and tocopherol was roughly equal to that in the corresponding plasma. Extractions for lipid and antioxidant analyses were carried out as described (31), except that acetic acid was omitted to prevent unwanted nitration of γT by residual nitrite. For some experiments, the tocopherol content of LDL was altered by oral supplementation with either 300 mg of αT or γT (in vegetable oil) 12 h before isolation of plasma (with appropriate campus approval and written consent).
Biochemical Analyses.
·NO formation during decomposition of SIN-1 (1 mM) in buffer only was determined using oxyhemoglobin (32) and was 15.1 μM/min (initial rate, n = 2). This method underestimates ·NO release due to reaction with O2⨪ (33, 34) and because of light scattering can not be used when liposomes are present. Therefore, SIN-1 decomposition was monitored at 260 nm using the phospholipid HPLC conditions (see below) to estimate release of ·NO. SIN-1 decomposition was 40.5 ± 2.9 μM/min (initial rate, n = 3), which compared favorably with the formation of total nitrite + nitrate (41.4 ± 3.3 μM/min, n = 3). Nitrate accounted for 68 ± 3.3% of the total product formation, indicating that >70% of the ·NO released from SIN-1 was likely converted to peroxynitrite, and that a large proportion of this peroxynitrite decomposed directly to nitrate without reacting with lipid.
Lipid hydroperoxides were measured by HPLC with chemiluminescence detection (S-3400, Soma Optics, Japan) (31, 35). The borate concentration of the isoluminol buffer was reduced to 20 mM to avoid precipitation. Phospholipids were separated on a weak anion-exchange column (Hewlett–Packard, 20 × 0.46 cm APS-Hypersil, 5 μm particle size) eluted with 1 ml/min 95:5 (vol/vol) methanol/40 mM sodium dihydrogen phosphate. Neutral lipids and cholesteryl ester hydroperoxides (CE-OOH) were separated as described (31). The amount of PC-OOH injected was standardized against PC; in addition, LDL lipids and antioxidants were adjusted to (unesterified) cholesterol (36). Unoxidized lipids were monitored by concurrent UV detection at 210 nm. Purified linoleic acid hydroperoxide was prepared by oxidation of linoleic acid with an azo-initiator or purchased (Cayman Chemicals, Ann Arbor, MI) and used as standard (35).
Lipid-soluble antioxidants and major tocopherol products (including 5-NO2-γT) were measured by HPLC with electrochemical detection (LC-4B, Bioanalytical Systems, West Lafayette, IN) (31). Separation was performed on an LC-18 column (Supelco, 25 × 0.46 cm, 5-μm particle size) with an LC-8 guard column. β-tocopherol does not separate from γT under these conditions, but in human plasma contributes to less than 10% of the two tocopherols combined (19). Because nitration at the 5-position increases γT’s oxidation potential, it was raised from +0.6 to +0.9 V (electrode 2). αTQ and tocored were detected at −0.55 V (electrode 1). Because of interferences with the oxidative mode, tocored values are only approximations. Peak identity of 5-NO2-γT [ɛ410 in EtOH = 1,976 M−1·cm−1, ɛ410 in hexane = 2,367 M−1·cm−1, (37)] was verified by coelution with and comparison of the hydrodynamic voltammogram to synthetic standard.
Data Analysis.
The initial rate and maximum extent of SIN-1-induced formation of PC-OOH were calculated from first-order curve fits of the experimental data using Kaleidagraph 3.0 (Synergy Software, Reading, PA). Statistical difference (student t test, one-sided, unpaired) was accepted at the P < 0.05 level unless otherwise indicated.
RESULTS
Based on elemental analysis and spectral comparison with known nitrophenols, Marcinkiewicz (28) proposed that the major reaction product of γT with nitrous acid is the nitro-adduct, rather than the nitroso-adduct, as originally reported (29). To substantiate this, we analyzed the major product by UV, 1H and 13C NMR, and mass spectrometry and found that all analytical data (see Materials and Methods) matched those obtained for the yellow product formed during the reaction of γT with ·NO2 (21): 5-NO2-γT (see Fig. 4). Nitration rather than nitrosation was supported by the high-resolution mass spectrum of the major reaction product isolated, which best fit the elemental composition C28H47O4N. Additionally, the loss of the C5 proton, large downfield shift of the C6-OH proton, and ≈25 ppm downfield shift of the C5 carbon relative to γT are consistent with attachment of a nitro-group to the C5 position. Together the data unambiguously identify the major reaction product as 5-NO2-γT. The rapid reaction (<2 min for completion) and high yield (>90%) of γT nitration indicates that the 5-position of γT is highly nucleophilic and reactive toward electrophilic NOx such as nitrosonium (NO+), nitronium (NO2+) ions, or their equivalents, consistent with the many reports of nitrous acid-mediated nitration of ortho- or para-unsubstituted phenols (38).
Inhibition of Peroxynitrite-Induced Lipid Peroxidation.
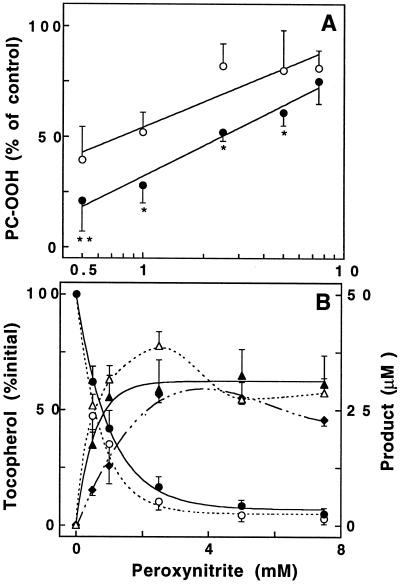
Peroxynitrite caused concentration-dependent and rapid accumulation (<30 sec) of PC-OOH in soybean PC liposomes (not shown), which was inhibited both by αT and by γT (Fig. 1A). Significantly, up to 5 mM peroxynitrite inhibition was greater for γT than αT. Peroxynitrite also caused dose-dependent liposomal depletion of the two tocopherols (Fig. 1B). The loss of αT was accompanied by the formation of its two-electron oxidation product αTQ (dashed lines); γT was converted to both 5-NO2-γT (solid lines) and its ortho-quinone tocored. In contrast, the para-quinone of γT (i.e., the equivalent of αTQ) could not be detected. At low peroxynitrite concentrations, αTQ and 5-NO2-γT each accounted for ≈40–50% of the respective loss of tocopherol and tocored formation represented another ≈25–35% of γT lost; i.e., the total accountable loss of γT was ≈75% (Fig. 1B). A similar product profile is observed when γT is exposed to ·NO2 (21), suggesting the formation of ·NO2 during peroxynitrite-induced lipid peroxidation.
Figure 1.
Inhibition of peroxynitrite-induced PC-OOH formation by αT and γT (A) and fate of tocopherols (B). Soybean PC liposomes (20 mM) containing either 0.1 mM αT (○) or γT (•) were exposed to various concentrations of peroxynitrite and subsequently analyzed for the different reactants and products by HPLC as described in Materials and Methods. (A) PC-OOH formation in the presence of tocopherol is shown as a percentage of that formed in its absence (control = 100%). Accumulation of PC-OOH reached a maximum at ≈5 mM peroxynitrite. Asterisks represent significant difference between αT- and γT-containing liposomes; **P ≥ 0.01 and *P ≥ 0.05. Data are presented on a log scale. (B) Loss of either αT (○) or γT (•), and the corresponding increase in αTQ (▵), or 5-NO2-γT (▴) and tocored (♦), respectively. Decomposed peroxynitrite caused neither oxidation of tocopherols nor of lipids. Data shown are mean values ± SEM of four complete sets of independent experiments. Paired t test was used.
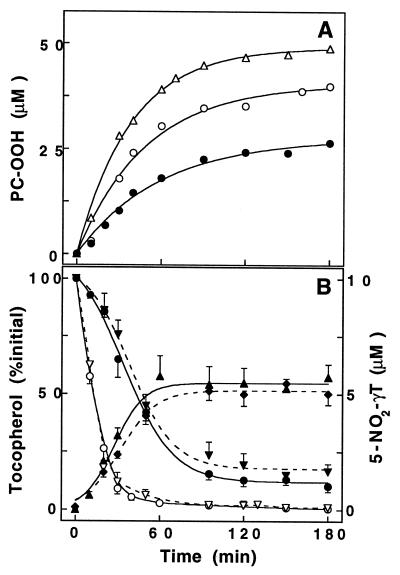
To mimic more physiological conditions, the ·NO and O2⨪ -generator SIN-1 was used to produce peroxynitrite continuously and at low concentrations. Consistent with previous findings (34, 39), incubation of soybean PC liposomes with SIN-1 resulted in time-dependent formation of PC-OOH (Fig. 2A). As expected from the ≈20 min half-life of SIN-1 under our conditions, accumulation of PC-OOH did not proceed in a linear fashion and was completed within ≈2 h postincubation. Similar to the situation with peroxynitrite, addition of either αT or γT inhibited SIN-1-induced PC-OOH accumulation (Fig. 2A). A summary of the data using a range of tocopherol concentrations is shown in Table 1. At 20 μM, the initial rate of PC-OOH formation was inhibited significantly more by γT than αT. Again, incubation with SIN-1 caused time-dependent loss of either αT or γT (Fig. 2B, solid lines). However, the loss of γT was significantly slower than that of αT, revealing the difference in reactivity between the two. Again nitration accounted for ≈50% of total γT lost independent of the initial tocopherol concentration (e.g., 46.3 ± 5.7%, n = 5, at 20 μM). More importantly, the presence of αT did not significantly affect γT nitration, since the extent of 5-NO2-γT formation in liposomes with extra αT was similar to that in liposomes that contained γT alone (Fig. 2B, dashed lines). The remainder of the γT loss was accounted for by the formation of tocored, whereas αT was converted near-quantitatively (>90%) to αTQ (not shown). Also, αT did not significantly spare γT or further increase the γT-mediated inhibition of SIN-1-induced PC-OOH formation (Table 1).
Figure 2.
Time-dependent formation of PC-OOH (A) and nitration of γT (B) during exposure of PC liposomes to SIN-1. Soybean PC liposomes (20 mM) without (▵), or with either αT (○) or γT (•) (20 μM) were incubated with SIN-1 (1 mM) at 37°C and periodically analyzed for PC-OOH (A), and tocopherols and its products (B). (A) One experiment typical of at least four independent experiments is shown. Percentage inhibition values of SIN-1-induced PC-OOH formation by tocopherols at various concentrations are given in Table 1. (B) Fate of αT (○, ▿) and γT (•, ▾) during SIN-1-induced liposomal oxidation when each is present individually (solid lines) or combined (broken lines). 5-NO2-γT formation in the presence of αT (♦) was not significantly different from that in its absence (▴). Data shown are mean values ± SEM of four independent experiments. αTQ and tocored values are reported in the text.
Table 1.
Inhibition of SIN-1-induced accumulation of PC-OOH
| Conc., μM | Tocopherol | Percentage inhibition†
|
n | |
|---|---|---|---|---|
| Initial rate | Maximum extent | |||
| 5 | α | 34.7 ± 3.3 | 18.8 ± 11.4 | 3 |
| γ | 31.1 ± 17.8 | 33.4 ± 6.9 | 3 | |
| 10 | α | — | — | — |
| γ | 41.2 ± 17.3 | 53.1 ± 12.8 | 3 | |
| 20 | α | 45.2 ± 8.0 | 36.5 ± 6.6 | 4 |
| γ | 64.7 ± 5.0* | 46.6 ± 4.5 | 5 | |
| ‡α and γ | 71.5 ± 2.5** | 51.6 ± 5.8 | 3 | |
Calculated from the extent of PC-OOH accumulation measured in the presence and absence of tocopherol (control = 100%). Values are mean ± SEM of n measurements. ∗, Significantly different from the corresponding αT value (P = 0.03). ∗∗, Significantly different from αT (P = 0.02) but not γT.
Total concentration = 40 μM.
To investigate the role of lipid peroxidation in γT nitration, formation of 5-NO2-γT was measured in liposomes resistant to oxidation (i.e., made from dimyristoyl or dipalmitoyl PC). Under various conditions, γT nitration induced by either peroxynitrite or SIN-1 (initial rates) only ranged from 3% to 11%, despite somewhat faster consumption of γT than in soybean PC liposomes. In contrast, nitration induced by SIN-1 proceeded more rapidly in liposomes with a high initial content of PC-OOH (not shown). These results suggest that nitration of γT in lipid membranes proceeds in large part via reactions that involve the formation of secondary reactive intermediates.
LDL Experiments.
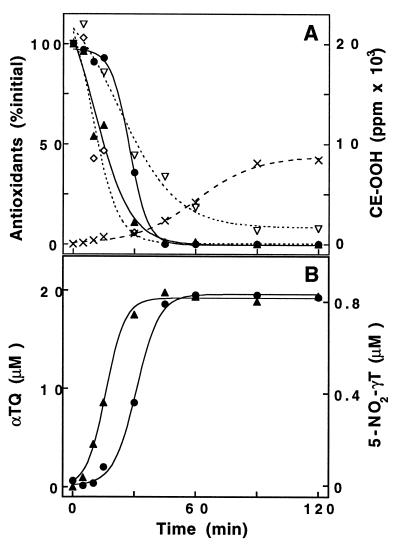
Incubation of human LDL with SIN-1 resulted in time-dependent accumulation of small amounts of CE-OOH even in the presence of endogenous antioxidants. CE-OOH accumulation increased during the rapid and time-dependent SIN-1-induced loss of LDL-associated antioxidants (Fig. 3A). Consistent with previous findings (39), ubiquinol-10 was consumed first (within the first 15 to 20 min) and quantitatively converted to ubiquinone-10 (not shown). Similar to the liposome experiments, consumption of αT preceded that of γT. While αT was quantitatively converted to αTQ, the formation of 5-NO2-γT accounted for ≈75% of total γT lost (Fig. 3B). In this case, however, less than ≈10–15% of lost γT was accounted for by formation of tocored (not shown). As previously observed (39), β-carotene was the least rapidly consumed of the lipid-soluble antioxidants. Lycopene disappeared much more rapidly, comparable to αT, suggesting a much greater reactivity of this carotenoid toward peroxynitrite than β-carotene. In an attempt to delineate the corresponding role of the two tocopherols in SIN-1-induced LDL oxidation, their relative ratio in LDL was altered by short-term oral supplementation with either of the two tocopherols. As expected (11, 19), supplementation with αT increased the mean αT in LDL level from 19.0 to 28.0 μM and reduced that of γT from 1.3 to 0.6 μM. γT supplementation caused a transient increase in γT to 6.2 μM and a decrease in αT to 10.4 μM (mean levels). In contrast to the liposome experiments, supplementation of LDL with γT did not inhibit SIN-1-induced lipid peroxidation (accumulation of either CE-OOH or PC-OOH) more effectively than αT (not shown). In fact, αT appeared to be more protective. However, the yield of 5-NO2-γT formation was always ≈75%, independent of the initial concentration of αT, and despite the fact that nitration consistently lagged behind the conversion of αT to αTQ (compare with Fig. 3). Similar qualitative results were obtained with peroxynitrite (not shown).
Figure 3.
Time-dependent loss of antioxidants and oxidation of lipids (A) and formation of tocopherol reaction products (B) during exposure of human LDL to SIN-1. LDL was incubated with SIN-1 (0.2 mM) at 37°C and analyzed for lipid-soluble antioxidants and CE-OOH (A), and αTQ (▴) and 5-NO2-γT (•) (B). (A) The levels of ubiquinol-10 (initial concentration, 0.93 μM; not shown), lycopene (1.03 μM, ⋄), β-carotene (1.04 μM, ▿), αT (21.0 μM, ▴), and γT (0.98 μM, •) are given as a percentage of initial concentration. CE-OOH are given as ppm of polyunsaturated fatty acids esterified to cholesterol (×). Phospholipid oxidation (measured as PC-OOH) was consistently ≈3-fold lower than that of cholesteryl esters, indicating that SIN-1-induced oxidation of LDL may be tocopherol-mediated (40). One experiment typical of three for unsupplemented LDL is shown. Several experiments in which LDL αT (n = 2) and γT (n = 3) levels were altered by oral supplementation gave similar qualitative and quantitative results (see text for details). γT nitration ranged from 71.9% to 75.4% for LDL supplemented with αT and γT, respectively.
DISCUSSION
Increasing evidence shows that peroxynitrite is involved in pathological processes. Here, we tested the efficacy by which the two major forms of vitamin E inhibit peroxynitrite-induced lipid peroxidation. γT was more effective than αT in inhibiting both peroxynitrite or SIN-1-induced oxidation of PC liposomes (see Fig. 1 and Table 1) but not of LDL. To gain mechanistic insights into how γT and αT modulate peroxynitrite-induced lipid oxidation, the major tocopherol products formed during this process were analyzed. The main product formed from αT was αTQ (VII) (see e.g., Fig. 4). In the case of SIN-1 oxidations, αTQ was formed stoichiometrically, consistent with previous findings (26), though whether this reaction is concerted or sequential has not been definitively established. In contrast, the para-quinone of γT could not be detected in any of our experiments. Instead, γT was nitrated in high yield to 5-NO2-γT (V) or oxidized to the ortho-quinone, tocored (XI). This product pattern is reminiscent of the reaction of γT with ·NO2 (21), a major constituent of cigarette smoke (3) and polluted air, and indicates that the reactions between the two tocopherols and NOx species such as those derived from peroxynitrite are fundamentally different. The fact that exposure of unsaturated lipids to peroxynitrite resulted in lipid hydroperoxide formation indicates bisallylic hydrogen abstraction and suggests a one-electron oxidation reaction by peroxynitrite with concomitant formation of ·NO2. This could be responsible for part of the 5-NO2-γT formed in the present study and is supported by the increased yields of γT nitration when lipid peroxidation is permitted (see Results).
γT as a Defense Against NOx.
·NO2 is reactive toward many biological molecules, including lipid and DNA, and is consequently a mutagen (42). ·NO2 also readily nitrates phenolic residues such as tyrosine (43). γT therefore may trap the membrane-soluble ·NO2 or detoxify the reactive nitrite esters formed (Fig. 4) before it can damage important target molecules. This argument is supported by the finding that γT (but not αT) is a potent inhibitor of NO2·-induced DNA strand breaks in V79 cells (44). While the relative reactivity of NOx toward γT and other targets is beyond the scope of this paper, we found that SIN-1-induced nitration of γT in LDL was far more extensive than that of tyrosine residues (6) in LDL’s apoB protein (unpublished data). Also, the ease with which γT is nitrated by acidified nitrite indicates that γT is highly reactive toward electrophilic NOx. This nucleophilic character of γT may also extend to other membrane-soluble electrophilic mutagens, as demonstrated by rapid chlorination of γT by hypochlorous acid (unpublished data), another product of phagocytes. Furthermore, γT is more efficient than αT in inhibiting interleukin-1β-induced toxicity in rat insulinoma cells (21) and neoplastic transformation of C3H/10T½ fibroblasts (20); model systems in which the target endpoints are proposed to be dependent on NOx formation. Other dietary antioxidants such as carotenoids may also be important NOx scavengers. Interestingly, the consumption of lycopene in LDL incubated with SIN-1 preceded that of β-carotene (see Fig. 3), in line with their relative reactivity toward ·NO2 (ref. 45; C. Rice-Evans, personal communication), and may point to an important role for lycopene in the defense against NOx.
It is likely that the reason for the superior activity of γT in the above experiments is related to its ability to trap NOx as an unreactive carbon-centered adduct. This ability is not shared by αT, which is likely to trap NOx, but remains chemically reactive (Fig. 4, ref. 20). This may explain the dual role of αT in inhibiting and catalyzing nitrosation reactions (46, 47). It remains to be determined if the superior activity of γT in our experiments was due to more efficient trapping of oxidants formed during the peroxidation process (such as NO2·), or if the products formed from γT themselves possess antioxidant activity (47). The less rapid loss of γT compared to αT when the tocopherols were tested individually points to formation of antioxidant active products such as the ortho-hydroquinone of γT (X), which is likely to compete with its consumption (compare with Fig. 2).
αT and γT in Smoking and Inflammation.
The results of the present study suggest that γT complements αT in biological systems as illustrated by the LDL experiments in which the levels of αT have no effect on the degree of γT nitration (see Fig. 3). Thus, the presence of both tocopherols may be required in vivo for optimal protection against NOx. There is indirect support for this argument. For example, plasma levels of γT (but not αT) rapidly increase when long-term smokers (who are exposed to increased amounts of inhaled and phagocyte-derived NOx) cease to smoke (48). Chronic alcohol feeding in rats, a treatment that can cause activation of hepatic macrophages (Kupffer cells), can also affect tocopherol levels. In one of these studies the decrease in plasma and liver γT was significantly greater than that of αT (49), a finding mirrored in human epidemiological studies (50). We also found that zymosan-induced peritonitis in rats, a model for inflammation, leads to a drop in plasma γT levels that outpaces that of αT (unpublished data). While we are still investigating the mechanisms responsible for this effect, increased consumption of γT is a plausible explanation.
αT and γT in Heart Disease.
Although there is a strong inverse correlation between plasma vitamin E levels (which are almost exclusively measured as αT) and incidence of coronary heart disease (4), many αT supplementation studies have failed to demonstrate a protective effect (5, 14, 15). Interestingly, in one of the studies (15), vitamin E appeared to offer protection only when taken up from the diet and not when taken in supplements, which consist primarily of αT. The strongest correlation was shown with the consumption of margarine, nuts, and seeds, all of which are excellent sources of γT (15). Interestingly, a recent clinical evaluation of individuals suffering from coronary heart disease showed decreased serum levels of γT, but not αT (22). Together these findings may point to a critical role for γT in the prevention of cardiovascular diseases.
Concluding Remarks.
We argue that γT may function in vivo as a nucleophilic trap for membrane-soluble NOx and possibly other electrophilic mutagens through its ability to form stable carbon-centered adducts. This capacity of γT to trap electrophilic species like NOx clearly extends to other dietary phenolic “antioxidants” (e.g., flavonoids, tea, and wine polyphenolics). It may therefore be important to test antioxidants not only for their ability to inhibit oxidation reactions but also their ability to trap electrophiles (46). For example, the addition of sesamin to a diet rich in tocopherols markedly increases γT levels in plasma and other tissues but has no effect on αT levels. This may indicate that the phenolic metabolites derived from sesamin spare γT from being consumed (51). Because αT supplementation suppresses γT levels (19), a combination of the two tocopherols that better reflects the ratios found in our diet may be more useful as a supplement than the formulations of vitamin E currently available. Furthermore, 5-NO2-γT and other adducts to γT may be useful biomarkers for the physiological presence of lipid-soluble electrophiles.
Acknowledgments
We thank J. Tsai, D. Cheung, and H. Chang for experimental assistance, T. Klopotowski for translating ref. 28, M. G. Traber, J. Lykkesfeldt, R. V. Cooney, and R. Stocker for helpful discussions, and Henkel Corporation for the gift of γT for human consumption. This work was supported by a Fellowship from the Swiss Foundation for Medical-Biological Stipends (S.C.), Outstanding Investigator Grant from the National Cancer Institute CA39910 (B.N.A.), and National Institute of Environmental Health Sciences Center Grant ESO1896 (B.N.A.).
ABBREVIATIONS
- CE-OOH
cholesteryl ester hydroperoxides
- LDL
low density lipoprotein
- 5-NO2-γT
5-nitro-γ-tocopherol (2,7,8-trimethyl-2-[4′,8′,12′-trimethyltridecyl]-5-nitro-6-chromanol)
- NOx
reactive nitrogen oxide species
- PC
phosphatidylcholine
- PC-OOH
phosphatidylcholine hydroperoxide
- SIN-1
3-morpholinosydnonimine
- αT
α-tocopherol (2,5,7,8-tetramethyl-2-[4′,8′,12′-trimethyltridecyl]-6-chromanol)
- γT
γ-tocopherol (2,7,8-trimethyl-2-[4′,8′,12′-trimethyltridecyl]-6-chromanol)
- tocored
γ-tocopherol-5,6-quinone (2,7,8-trimethyl-2-[4′,8′,12′-trimethyltridecyl]-chroman-5,6-dione)
- αTQ
α-tocopherylquinone (2-[3′-hydroxy-3′,7′,11′,15′-tetramethylhexadecyl]-3,5,6-trimethyl-1,4-benzoquinone)
References
- 1.Christen S, Hagen T M, Shigenaga M K, Ames B N. In: Chronic Infection and Inflammation Lead to Mutation and Cancer. Parsonnet J, Horning S, editors. Oxford: Oxford Univ. Press; 1997. in press. [Google Scholar]
- 2.Bose M, Farnia P. Immunol Lett. 1995;48:59–64. doi: 10.1016/0165-2478(95)02444-1. [DOI] [PubMed] [Google Scholar]
- 3.Pryor W A, Squadrito G L. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 4.Gey K F, Puska P, Jordan P, Moser U K. Am J Clin Nutr. 1991;53:326S–334S. doi: 10.1093/ajcn/53.1.326S. [DOI] [PubMed] [Google Scholar]
- 5.Jha P, Flather M, Lonn E, Farkouh M, Yusuf S. Ann Intern Med. 1995;123:860–872. doi: 10.7326/0003-4819-123-11-199512010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Shigenaga M K, Lee H H, Blount B C, Christen S, Shigeno E T, Yip H, Ames B N. Proc Natl Acad Sci USA. 1997;94:3211–3216. doi: 10.1073/pnas.94.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieri J G, Evarts R P. J Nutr. 1974;104:850–857. doi: 10.1093/jn/104.7.850. [DOI] [PubMed] [Google Scholar]
- 8.Burton G W, Ingold K U. J Am Chem Soc. 1981;103:6472–6477. [Google Scholar]
- 9.Lehmann J, Martin H L, Lashley E L, Marshall M W, Judd J T. J Am Diet Assoc. 1986;86:1208–1216. [PubMed] [Google Scholar]
- 10.Vatassery G T, Johnson G J, Krezowski A M. J Am Coll Nutr. 1983;2:369–375. doi: 10.1080/07315724.1983.10719934. [DOI] [PubMed] [Google Scholar]
- 11.Traber M G, Burton G W, Hughes L, Ingold K U, Hidaka H, Malloy M, Kane J, Hyams J, Kayden H J. J Lipid Res. 1992;33:1171–1182. [PubMed] [Google Scholar]
- 12.Meydani M, Cohn J S, Macauley J B, McNamara J R, Blumberg J B, Schaefer E J. J Nutr. 1989;119:1252–1258. doi: 10.1093/jn/119.9.1252. [DOI] [PubMed] [Google Scholar]
- 13.Tran K, Chan A C. Lipids. 1992;27:38–41. doi: 10.1007/BF02537056. [DOI] [PubMed] [Google Scholar]
- 14.The Alpha-Tocopherol, Beta-Carotene Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 15.Kushi L H, Folsom A R, Prineas R J, Mink P J, Wu Y, Bostick R M. N Engl J Med. 1996;334:1156–1162. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 16.Toth B, Patil K. J Natl Cancer Inst. 1983;70:1107–1111. [PubMed] [Google Scholar]
- 17.McIntosh G H. Nutr Cancer. 1992;17:47–55. doi: 10.1080/01635589209514172. [DOI] [PubMed] [Google Scholar]
- 18.Mitchel R E, McCann R. Carcinogenesis. 1993;14:659–662. doi: 10.1093/carcin/14.4.659. [DOI] [PubMed] [Google Scholar]
- 19.Handelman G J, Machlin L J, Fitch K, Weiter J J, Dratz E A. J Nutr. 1985;115:807–813. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- 20.Cooney R V, Franke A A, Harwood P J, Hatch-Pigott V, Custer L J, Mordan L J. Proc Natl Acad Sci USA. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooney R V, Harwood P J, Franke A A, Narala K, Sundström A-K, Berggren P-O, Mordan L J. Free Radical Biol Med. 1995;19:259–269. doi: 10.1016/0891-5849(95)00019-t. [DOI] [PubMed] [Google Scholar]
- 22.Öhrvall M, Sundlof G, Vessby B. J Intern Med. 1996;239:111–117. doi: 10.1046/j.1365-2796.1996.410753000.x. [DOI] [PubMed] [Google Scholar]
- 23.Pryor W A, Cueto R, Jin X, Koppenol W H, Ngu-Schwemlein M, Squadrito G L, Uppu P L, Uppu R M. Free Radical Biol Med. 1995;18:75–83. doi: 10.1016/0891-5849(94)00105-s. [DOI] [PubMed] [Google Scholar]
- 24.Frampton V L, Skinner W A, Jr, Bailey P S. J Am Chem Soc. 1954;76:282–284. [Google Scholar]
- 25.Miller K W, Lorr N A, Yang C S. Anal Biochem. 1984;138:340–345. doi: 10.1016/0003-2697(84)90819-4. [DOI] [PubMed] [Google Scholar]
- 26.Hogg N, Joseph J, Kalyanaraman B. Arch Biochem Biophys. 1994;314:153–158. doi: 10.1006/abbi.1994.1423. [DOI] [PubMed] [Google Scholar]
- 27.Bourgeois C. Determination of Vitamin E: Tocopherols and Tocotrienols. London: Elsevier; 1992. [Google Scholar]
- 28.Marcinkiewicz S. Acta Pol Pharm. 1967;24:375–378. [PubMed] [Google Scholar]
- 29.Quaife M L. J Biol Chem. 1948;175:605–617. [PubMed] [Google Scholar]
- 30.Stocker R, McDonagh A F, Glazer A N, Ames B N. Methods Enzymol. 1990;186:301–309. doi: 10.1016/0076-6879(90)86123-d. [DOI] [PubMed] [Google Scholar]
- 31.Sattler W, Mohr D, Stocker R. Methods Enzymol. 1993;233:469–489. doi: 10.1016/s0076-6879(94)33053-0. [DOI] [PubMed] [Google Scholar]
- 32.Murphy M E, Noack E. Methods Enzymol. 1994;233:240–250. doi: 10.1016/s0076-6879(94)33027-1. [DOI] [PubMed] [Google Scholar]
- 33.Hogg N, Darley-Usmar V M, Wilson M T, Moncada S. Biochem J. 1992;281:419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darley-Usmar V M, Hogg N, O’Leary V J, Wilson M T, Moncada S. Free Radical Res Comms. 1992;17:9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Brodsky M H, Baker J C, Ames B N. Anal Biochem. 1987;160:7–13. doi: 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]
- 36.Bowry V W, Stanley K K, Stocker R. Proc Natl Acad Sci USA. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcinkiewicz S. Chem Anal (Warsaw) 1972;17:13–20. [Google Scholar]
- 38.Olah G A, Malhotra R, Narang S C. Nitration: Methods and Mechanisms. New York: VCH; 1989. [Google Scholar]
- 39.Jessup W, Mohr D, Gieseg S P, Dean R T, Stocker R. Biochim Biophys Acta. 1992;1180:73–82. doi: 10.1016/0925-4439(92)90029-m. [DOI] [PubMed] [Google Scholar]
- 40.Bowry V W, Stocker R. J Am Chem Soc. 1993;115:6029–6044. [Google Scholar]
- 41.Selander H, Nilsson J L. Acta Pharm Suec. 1972;9:125–128. [PubMed] [Google Scholar]
- 42.Christen S, Gee P, Ames B N. Methods Enzymol. 1996;269:267–278. doi: 10.1016/s0076-6879(96)69027-2. [DOI] [PubMed] [Google Scholar]
- 43.van der Vliet A, Eiserich J P, O’Neill C A, Halliwell B, Cross C E. Arch Biochem Biophys. 1995;319:341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]
- 44.Bittrich H, Matzig A K, Kraker I, Appel K E. Chem Biol Interact. 1993;86:199–211. doi: 10.1016/0009-2797(93)90098-j. [DOI] [PubMed] [Google Scholar]
- 45.Everett S A, Dennis M F, Patel K B, Maddix S, Kundu S C, Willson R L. J Biol Chem. 1996;271:3988–3994. doi: 10.1074/jbc.271.8.3988. [DOI] [PubMed] [Google Scholar]
- 46.Newmark H L, Mergens W J. In: α-Tocopherol (Vitamin E) and its Relationship to Tumor Induction and Development. Zedeck M S, Lipkin M, editors. New York: Plenum; 1981. pp. 127–168. [Google Scholar]
- 47.Kamal-Eldin A, Appelqvist L-Å. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 48.Brown A J. J Nutr Biochem. 1996;7:29–39. [Google Scholar]
- 49.Sadrzadeh S M, Nanji A A, Meydani M. Biochem Pharmacol. 1994;47:2005–2010. doi: 10.1016/0006-2952(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 50.Sinha R, Patterson B H, Mangels A R, Levander O A, Gibson T, Taylor P R, Block G. Cancer Epidemiol Biomarkers Prev. 1993;2:473–479. [PubMed] [Google Scholar]
- 51.Kamal-Eldin A, Pettersson D, Appelqvist L Å. Lipids. 1995;30:499–505. doi: 10.1007/BF02537023. [DOI] [PubMed] [Google Scholar]