Abstract
Both insulin and insulin-like growth factor 1 (IGF-1) are known to reduce glucose-dependent insulin secretion from the β cells of pancreatic islets. In this paper we show that the mechanism by which IGF-1 mediates this effect is in large part through activation of a specific cyclic nucleotide phosphodiesterase, phosphodiesterase 3B (PDE3B). More specifically, in both isolated pancreatic islets and insulin-secreting HIT-T15 cells, IGF-1 inhibits insulin secretion that has been increased by glucose and glucagonlike peptide 1 (GLP-1). Moreover, IGF-1 decreases cAMP levels in parallel to the reduction of insulin secretion. Insulin secretion stimulated by cAMP analogs that activate protein kinase A and also are substrates for PDE3B is also inhibited by IGF-1. However, IGF-1 does not inhibit insulin secretion stimulated by nonhydrolyzable cAMP analogs. In addition, selective inhibitors of PDE3B completely block the ability of IGF-1 to inhibit insulin secretion. Finally, PDE3B activity measured in vitro after immunoprecipitation from cells treated with IGF-1 is higher than the activity from control cells. Taken together with the fact that pancreatic β cells express little or no insulin receptor but large amounts of IGF-1 receptor, these data strongly suggest a new regulatory feedback loop model for the control of insulin secretion. In this model, increased insulin secretion in vivo will stimulate IGF-1 synthesis by the liver, and the secreted IGF-1 in turn feedback inhibits insulin secretion from the β cells through an IGF-1 receptor-mediated pathway. This pathway is likely to be particularly important when levels of both glucose and secretagogues such as GLP-1 are elevated.
Insulin secretion from pancreatic islets is modulated by various nutrient levels and also is under tight control of a variety of circulating hormones. For example, glucagonlike peptide 1 (GLP-1), released in response to food passage through the internal lining of the gut, stimulates insulin secretion by elevating 3′,5′-cyclic adenosine monophosphate (cAMP) within pancreatic β cells (1). On the other hand, other hormones can attenuate insulin secretion and therefore help to bring insulin release under control. Insulin-like growth factor 1 (IGF-1) is one such example. When infused into normal healthy human subjects at a dose not causing hypoglycemia, IGF-1 can decrease the circulating insulin level (2) and IGF-1 can directly attenuate insulin secretion from isolated primary rat pancreatic β cells (3). Insulin itself has been postulated to exert a negative-feedback control of its own secretion (4, 5). However, as this effect of insulin requires high concentrations [ranging from 200 to 1,000 microunits (μU)/ml] it is also possible that this effect of insulin may be mediated through β cell IGF-1 receptors that have low affinity for insulin (4–7).
Many insulin secretagogues that utilize cAMP as a second messenger will potentiate glucose-stimulated insulin secretion (1, 8, 9), and agents that elevate cAMP, such as nonspecific phosphodiesterase (PDE) inhibitors (e.g., isobutylmethylxanthine and theophylline), are potent insulin secretagogues (10–12). Several recent studies have suggested the presence of both PDE3 and PDE4 activities within pancreatic islets (for a review about various types of PDEs, see ref. 13). Interestingly though, only the specific pharmacological inhibitors for PDE3 will actually potentiate insulin secretion (12, 14). These data suggest a possible functional importance for a PDE3 isozyme in the regulation of insulin secretion from pancreatic islets. The mammalian PDE3 family consists of two members, PDE3A and PDE3B, that have very similar pharmacological and kinetic properties but distinct expression profiles (13, 15). PDE3A is mainly expressed in the cardiovascular system and platelets (16). PDE3B has been recognized for its importance in mediating the antilipolytic and antiglycogenolytic action of insulin in adipose and liver tissues (16–18). Upon insulin binding to its receptor in adipose tissue, a Ser/Thr kinase is activated through a wortmannin-sensitive phosphorylation cascade (18). This insulin-sensitive kinase in turn activates PDE3B (16, 18). The activated PDE3B decreases cAMP and protein kinase A activity, thereby inactivating a hormone-sensitive lipase and thus inhibiting lipolysis.
As insulin and IGF-1 share a similar signal transduction pathway in other systems (19), we wondered if there might be a similar mechanism for activation of a PDE3B enzyme in the pancreatic β cells. We were also interested in determining if such an IGF-1-dependent mechanism could be an important regulator of insulin secretion. That is, could IGF-1 activate a β cell PDE and decrease cAMP, and if so, which specific PDE is activated? Some of these results have been presented in abstract form (20).
MATERIALS AND METHODS
Isolation of Mouse PDE3B cDNA.
A DNA fragment encoding a portion of the mouse PDE3B catalytic domain§ (amino acids 802–944) was used to screen a mouse brain cDNA library (5′-stretch λgt10; CLONTECH). This screening yielded four independent positive clones, among which the one containing the longest cDNA insert, named AD2–3, encoded a C-terminal portion of mouse PDE3B (amino acids 887-1108).
Generation of Anti-PDE3B Polyclonal Antibody.
The entire coding sequence of AD2–3 was fused in-frame (at the EcoRI site) with a glutathione S-transferase (GST) bacterial expression vector, pGEX-4T-1 (Pharmacia). The expression and purification of the GST-PDE3B protein were carried out according to a protocol from Pharmacia. We immunized two rabbits with this pure GST-PDE3B antigen. The antisera from the third bleed and afterwards were used for the Western blot and immunocytochemistry assays.
Antibodies and Chemical Reagents.
The anti-insulin monoclonal antibody was from Sigma. The anti-IGF-1 receptor and anti-insulin receptor antibodies were from Santa Cruz Biotechnology; both antibodies were raised against the N-terminal unique sequences (amino acids 1–20) of the corresponding receptor. IGF-1 was from Becton Dickinson or GIBCO/BRL. 8-Br-cAMP and N6-benzoyl-cAMP were from BioLog Chemical (San Diego).
Western Blotting.
The proteins of tissue or cell extracts were separated on SDS/8% polyacrylamide gels and the resolved protein bands were electrotransferred to nitrocellulose membranes. The membranes were preblocked with 20 mM Tris-buffered saline (pH = 7.2) containing 5% nonfat dry milk before incubation with diluted antiserum. Detection was carried out with appropriate peroxidase-conjugated secondary antibody (Bio-Rad) and chemiluminescent reagents (Pierce). The blots were exposed to X-Omat film (Kodak) for visualization.
Cultures of Neonatal Rat Pancreatic Monolayer Cells and HIT-T15 Cells.
The preparation and culture of neonatal rat pancreatic monolayer cells have been described (21). Briefly, pancreata dissected from neonatal rats at 3–5 days of age were digested with trypsin/collagenase and plated to allow attachment of fibroblasts. The cells in suspension were enriched in endocrine cells and transferred into a new set of 35-mm dishes for further growth of up to 4 days. Culture medium consisted of the following (vol/vol): 45% NCTC135, 45% medium 199, and 10% heat-inactivated fetal bovine serum. This medium is supplemented with glucose (16.5 mM) and gentamicin (50 μg/ml). The experiments normally were carried out on day 4 of culturing. For studies of insulin release, cultures were preincubated for 2 hr in Krebs–Ringer bicarbonate buffer supplemented with 0.1% bovine serum albumin and 1.6 mM glucose. This was followed by incubation for 1 hr in the same buffer under various conditions. Each condition was done in triplicate or quadruplicate and at least three independent experiments were carried out. HIT-T15 cells were cultured in Ham’s F-12 medium supplemented with 15% (vol/vol) heat-inactivated fetal bovine serum. HIT cells were perifused on columns of preswollen Bio-Gel P-2 as described previously (22, 23). After trypsin treatment to release the cells they were added to each syringe column at a density of 500,000 cells per column. Perifusion proceeded at 1 ml/min. Each condition was done in duplicate and at least three independent experiments were carried out. Static incubation of HIT cells was performed in a manner similar to that used for the neonatal rat pancreatic monolayer cells. Preincubation glucose was 0 mM, however, for HIT cells.
Insulin and cAMP Radioimmunoassays.
The insulin radioimmunoassay was carried out according to a previously described standard protocol (22). For measurement of cAMP, immediately after the culture medium was removed for insulin assay, the attached HIT-T15 cells were dissolved in ice-cold acidic ethanol (ethanol/concentrated HCl, 4:1, vol/vol). An aliquot (20 μl) of this extract was diluted into 80 μl of 1 M basic Tris buffer (pH = 11) so that the final pH was ≈7.0, and this mixture was analyzed for cAMP content by using a kit and protocol from DuPont/NEN. Insulin and cAMP are expressed as mean ± SEM.
Immunocytochemistry and Confocal Fluorescent Microscopy.
Rat pancreas was frozen in an OCT block on dry ice and 15-μm serial sections were prepared. The sections were immediately fixed in 4% paraformaldehyde in PBS for 20 min at room temperature, and subsequently washed once with 3× PBS and three times in 1× PBS. After being air-dried, the sections were preincubated in 5% normal goat serum and 5% normal donkey serum buffered by TPBS (PBS/0.1% Tween 20, pH 7.2) for 1 hr before being incubated with diluted antibody. The dilution of primary antibodies is as follows: anti-PDE3B, 1:1,000; anti-insulin, 1:300; anti-IGF-1 receptor, 1:100; and anti-insulin receptor, 1:100. We used either fluorescein-labeled goat anti-rabbit or rhodamine-labeled monkey anti-mouse secondary antibody to detect the specific binding. The stained sections were analyzed on a confocal fluorescent microscope (Bio-Rad MRC600) and the resultant digital images were recorded and analyzed in Photoshop 3.0.
Immunoprecipitations.
The HIT-T15 cells were extracted in an immunoprecipitation assay (IPA) buffer (50 mM NaF/150 mM NaCl/10 mM sodium phosphate, pH 7.2/2 mM EDTA/0.1% Triton X-100/0.5% Lubrol/3 mM benzamidine containing 5 μg/ml leupeptin and 20 μg/ml pepstatin A). One milliliter of IPA buffer was used to extract 1 × 106 cells. This extract was spun at 5,000 × g for 10 min at 4°C. A mixture of protein A-conjugated and protein G-conjugated agarose beads (GIBCO/BRL, 20 μl of a 5% suspension for each ml of extract) was added for preclearing. This preclearing did not significantly reduce the total PDE activity. After 20 min of incubation with rocking at 4°C, the beads were briefly spun down in a microcentrifuge. The supernatant (1 ml) was transferred to another tube and incubated with 2 μl of undiluted PDE3B antibody for 2 hr at 4°C. An additional aliquot of protein A- and protein G-conjugated agarose beads was added to the immunoprecipitation solution (40 μl of the 5% suspension for each ml of extract), and incubation was continued for another 3 hr. The beads were spun for 10 min at 4°C, and briefly washed twice in IPA buffer (1 ml per wash). Finally, the beads were resuspended in IPA buffer and analyzed for PDE3B activity. As a control, the PDE activity in the supernatant was also analyzed.
Measurement of PDE Activity.
The PDE assay was performed as described previously (24) with 1 μM cAMP as substrate. PDE3 activity is defined as the amount of PDE activity suppressed by 10 μM milrinone, whereas PDE4 activity is defined as the amount of PDE activity suppressed by 10 μM rolipram. The PDE activity is expressed as mean ± SEM.
RESULTS
PDE3B Is Expressed in the β Cells of Islets of Langerhans.
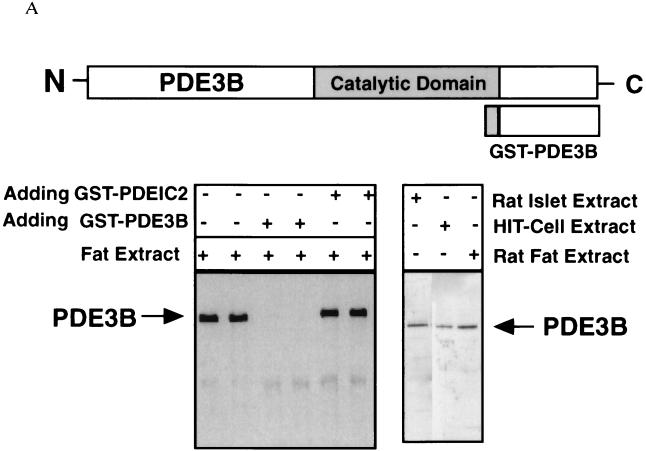
The mammalian PDE3 family consists of two gene members, PDE3A and PDE3B. To identify which specific PDE3 enzyme was expressed in pancreatic β cells, we raised a specific polyclonal antibody against a GST-PDE3B fusion protein (see Fig. 1A). This antibody recognizes a single protein band from mouse epididymal fat-pad tissue homogenates (Fig. 1A) that can be competed away by preincubation with the GST-PDE3B antigen, but not with a nonrelated GST-PDE1C fusion protein (Fig. 1A). In addition, the corresponding preimmune serum does not recognize this protein band (data not shown). We also tested this antiserum against a rat pancreatic islet extract and HIT-T15 cell extract by Western blot analysis. In both cases, it specifically detected a single band that corresponds in size to the PDE3B protein in the rat epididymal fat tissue (Fig. 1A).
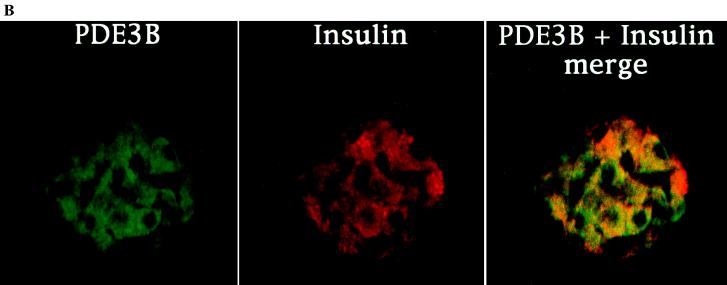
Figure 1.
PDE3B expression in the β cells of rat pancreatic islets. (A) The antibody against the C-terminal portion of a GST-PDE3B fusion protein recognizes a single band from a mouse epididymal fat pad extract. This band was competed away by preincubation with 200 ng of GST-PDE3B antigen but not with 200 ng of a GST-PDE1C2 polypeptide of similar length. This serum also specifically detects the PDE3B protein in the extracts of rat epididymal fat pad, rat pancreatic islets, and a hamster pancreatic β cell line, HIT-T15. (B) Confocal images of a rat pancreatic islet double-stained by anti-PDE3B and anti-insulin antibodies. The PDE3B signal was visualized by a fluorescein-conjugated goat anti-rabbit secondary antibody, and the insulin signal by a rhodamine-conjugated donkey anti-mouse secondary antibody. The side-by-side comparison of the two images clearly indicates that PDE3B is expressed in insulin-containing β cells, which is confirmed by the prevailing orange color in the merged image. (×40.) Note: the punctuate staining of insulin reflects insulin-containing secretory vesicles.
As shown in Fig. 1B, PDE3B protein is expressed in the β cells of islets of Langerhans, as evident by its costaining of insulin-containing cells (Fig. 1B). We have examined about 50 pancreatic serial sections, and 200 islets of various sizes. In all the islets examined, we consistently found that PDE3B is localized in the β cell. This localization pattern suggested that PDE3B might have a functional role to regulate the cAMP level in, and insulin secretion from, pancreatic β cells.
Expression of IGF-1 Receptors on the β Cell Surface of Islets of Langerhans.
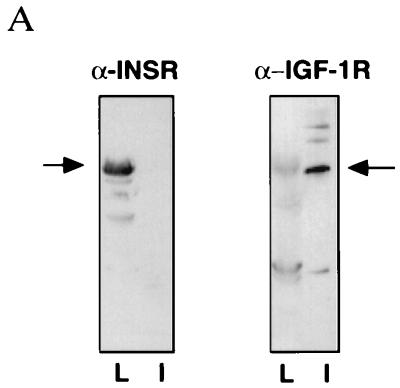
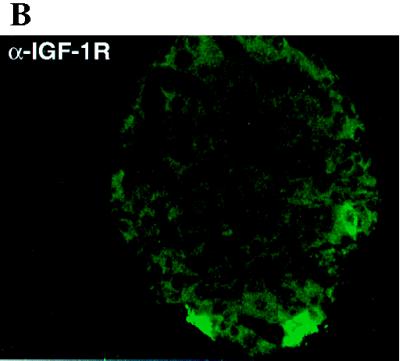
Both insulin and IGF-1 have been proposed as inhibitors of insulin secretion, and insulin has been shown to activate PDE3 in adipose and liver tissues (17, 25). As one step toward determining if either of these hormones regulates PDE3B in β cells, we performed Western blot analysis of the expression of IGF-1 receptor and insulin receptor in pancreatic islets (Fig. 2). Consistent with previous reports (26), our results confirm that IGF-1 receptor, but not insulin receptor, is expressed in the rat islets (Fig. 2A). In control experiments, insulin receptor was highly expressed in the rat liver tissue (Fig. 2A). We also immunostained for IGF-1 receptor (Fig. 2B). As expected, IGF-1 receptors were found on the β cells (Fig. 2B). These results, taken together with the data on PDE3B expression (Fig. 1), suggested that IGF-1 binding to its receptors on β cells might stimulate PDE3B and thereby attenuate insulin secretion.
Figure 2.
IGF-1 receptor, but not insulin receptor, is expressed in rat pancreatic islets. (A) Western blot detection of IGF-1 receptor α-subunit expression in rat pancreatic islets (lane I). Although insulin receptor is strongly expressed in rat liver (lane L), no detectable level of expression was seen in pancreatic islets. The solid arrows indicate either the IGF-1 or insulin receptor α subunits. Both antibodies are directed against the N-terminal 20 amino acids of the corresponding receptor α subunits. (B) Confocal image of a rat pancreatic islet. (×20.) The primary antibody is anti-IGF-1 receptor α subunit. The staining indicates that IGF-1 receptor is expressed in the pancreatic β cells.
Attenuation of Insulin Secretion by IGF-1 Is Mediated by a PDE, Specifically PDE3B.
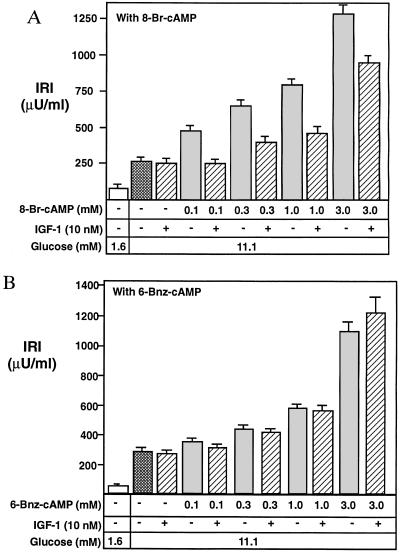
As a functional test for the importance of PDE3B in mediating the effect of IGF-1, we used a neonatal rat pancreatic monolayer cell culture that is substantially enriched in islet cells (27). This system shows phasic insulin secretion in response to glucose and also allows easy access of secretagogues and hormones to monolayers of islet cell clusters (27). As one test for whether or not the IGF-1 inhibition of insulin secretion is mediated through stimulation of PDE3B, we used two different cAMP analogs, 8-Br-cAMP and N6-benzoyl-cAMP, to examine the effect of IGF-1 on cAMP-potentiated secretion. It is known from previous studies that both of these analogs are capable of activating protein kinase A, but that only 8-Br-cAMP can be readily hydrolyzed by PDE3B (17, 25). Therefore, if IGF-1 did function mainly through activating PDE3B and decreasing intracellular cAMP, it should be able to suppress the 8-Br-cAMP-potentiated insulin secretion but have little or no effect on the N6-benzoyl-cAMP-potentiated insulin secretion.
As expected, both 8-Br-cAMP and N6-benzoyl-cAMP stimulated insulin secretion from the β cells of the islets (Fig. 3 A and B) in a concentration-dependent manner. Moreover, IGF-1 inhibited 8-Br-cAMP-potentiated insulin secretion (Fig. 3A), and especially at the low concentration of 8-Br-cAMP (0.1 mM), IGF-1 completely suppressed insulin secretion to the level induced by glucose alone (Fig. 3A). However, IGF-1 had little or no effect on N6-benzoyl-cAMP-potentiated insulin release (Fig. 3B). These data clearly support the model that the molecular signaling mechanism for IGF-1 attenuation of insulin secretion is through activation of a PDE. Moreover, these results make it unlikely that the IGF-1 effect on cAMP-potentiated insulin secretion is achieved through decreasing an adenylyl cyclase activity (see Discussion).
Figure 3.
Effect of IGF-1 on insulin secretion is potentiated by 8-Br-cAMP but not N6-benzoyl-cAMP (6-Bnz-cAMP). Newborn rat pancreatic islets in monolayer cell culture were preincubated in low-glucose medium (1.6 mM) for 2 hr before being switched to high glucose (11.1 mM). The cAMP analogs and/or 10 nM IGF-1 were added together with the high-glucose medium. After incubation for 30 min the medium was analyzed for immunoreactive insulin (IRI). Experiments under each set of conditions were carried out in triplicate. IGF-1 suppressed insulin secretion potentiated by 8-Br-cAMP (a relatively good PDE3B substrate) but had little or no effect on the insulin secretion potentiated by N6-benzoyl-cAMP, an analog highly resistant to hydrolysis by PDE3B. Twenty-five units of IRI = 1 mg of insulin.
PDE Selective Inhibitors Block the IGF-1 Effect.
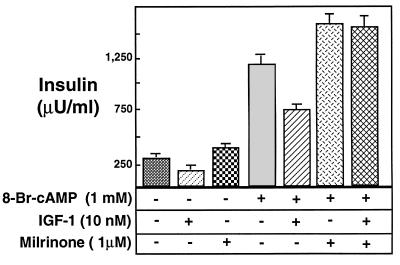
To further confirm that this IGF-1 function in β cells is mediated through PDE3, we also used a selective pharmacological antagonist. Milrinone, which has an IC50 value of ≈0.3 μM against PDE3 enzymes, shows at least 50-fold selectivity for PDE3 compared with all other known PDE enzymes (28). As expected for a PDE inhibitor, milrinone further potentiated the glucose- and cAMP-stimulated insulin secretion. More importantly, milrinone completely reversed the ability of IGF-1 to inhibit insulin secretion (Fig. 4). Since the major form of PDE expressed in β cells is PDE3B, these data strongly suggest that the molecular signaling mechanism for IGF-1 attenuation of insulin secretion is to activate specifically PDE3B in these cells.
Figure 4.
Milrinone prevents the inhibitory effect of IGF-1 on insulin secretion. The newborn rat pancreatic monolayer cells were cultured in low-glucose medium before transfer to high-glucose medium (see the legend of Fig. 2.). Different pharmacological conditions are represented by patterns or stippling. Milrinone, a highly selective inhibitor of PDE3B (IC50 = 0.3 μM), potentiates insulin secretion at low concentration (1 μM), suggesting the presence of PDE3 activity. Although IGF-1 significantly suppressed insulin secretion in the absence of milrinone, it could not inhibit insulin secretion in its presence.
IGF-1 Activates PDE3B and Reduces cAMP Levels.
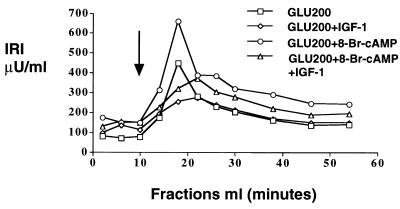
The model predicts that the binding of IGF-1 to its own receptors on the β cells should cause an increase in PDE3B activity and a concomitant reduction of cAMP. To directly measure effects of IGF-1 on cAMP and to rule out any indirect influence from non-β cells in the pancreatic cell mixtures, we needed to use a preparation that did not contain other cell types. We selected the β cell-derived HIT-T15 cell line to test this hypotheses because, as shown below, insulin secretion from these cells is regulated by IGF-1 (Fig. 5). Either with or without potentiation by 8-Br-cAMP, insulin secretion from the HIT-T15 cells was substantially inhibited by IGF-1 (Fig. 5). This result makes it unlikely that the IGF-1 effect on insulin secretion is indirectly modulated by non-β cells in the neonatal rat pancreatic monolayer culture. Similar results were found with a rat insulinoma cell line, RIN-5AH (data not shown).
Figure 5.
IGF-1 suppresses insulin secretion from HIT-T15 cells. HIT-T15 cells were perifused by Krebs–Ringer buffer containing 0.1% BSA and no glucose until the basal insulin level became stable. The perifused cells were then switched to the same buffer containing high glucose with or without the other agents. The starting point of exposure to high-glucose medium is indicated by the arrow. The results shown here are the average of fractions from duplicate columns. IGF-1 consistently suppressed insulin secretion both with and without 8-Br-cAMP potentiation.
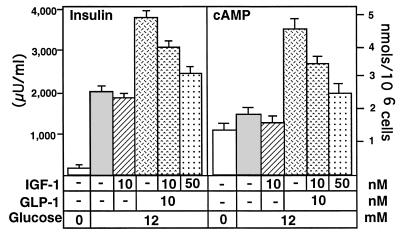
As expected, GLP-1 stimulated insulin secretion from the HIT-T15 cells (by ≈90%; see Fig. 6), and IGF-1 counteracted this effect in a dosage-dependent manner (Fig. 6). The cAMP content in these HIT-T15 cells also declined in parallel to the reduction of insulin release (Fig. 6), further supporting the idea that IGF-1 inhibits insulin secretion through reduction of intracellular cAMP.
Figure 6.
Inhibition of insulin secretion by IGF-1 is accompanied by a decrease in cAMP. HIT-T15 cells were preincubated with Krebs–Ringer buffer containing no glucose for 1 hr before treatment with GLP-1 and/or IGF-1 in the presence of glucose at a high concentration. Different conditions are represented by different stippling patterns. GLP-1, as expected, increased cAMP and potentiated insulin secretion from the HIT-T15. IGF-1 counteracted the GLP-1 effect on insulin secretion and caused a drop in cAMP.
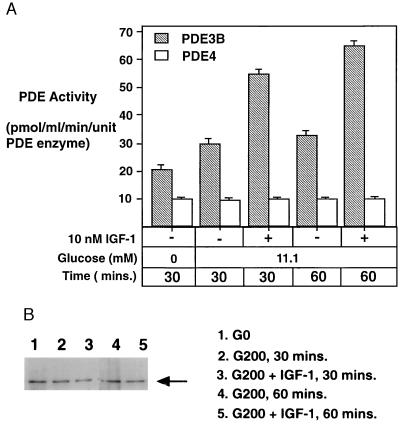
If the IGF-1 inhibitory effects on insulin secretion as well as intracellular cAMP level are both mediated by activation of PDE3B, then one would expect an increase of PDE3B activity upon IGF-1 binding to the pancreatic β cells. To test this idea, we immunoprecipitated PDE3B from the HIT-T15 cell extract. Under the low-glucose condition, the PDE3B activity did not change significantly (data not shown). A high concentration of glucose (200 mg/dl) by itself stimulated the PDE3B activity by about 50–55% relative to the low-glucose condition (30 mg/dl). The mechanism for this effect is not known. IGF-1 (10 nM) further stimulated the PDE3B activity about 90–95% (Fig. 7A). Since the amount of PDE3B enzyme used in these assays remained relatively constant (Fig. 7B), the activity changes of the PDE3B could not be attributed to changes in the total amount of the PDE3B enzyme. As a control, we also monitored PDE4 activity in the supernatant after the immunoprecipitation (Fig. 7A). Under all the conditions tested, there was very little change in PDE4 activity, suggesting that the PDE enzyme mediating IGF-1 action in pancreatic β cells is primarily PDE3B.
Figure 7.
Activation of PDE3B by IGF-1 in HIT-T15 cells. (A) When PDE3B activity was immunoprecipitated from the HIT-T15 extract, the pellet contained only PDE3B, as all of the PDE activity was completely suppressed by milrinone. A significant increase of PDE3B activity was observed when the HIT-T15 cells were treated with IGF-1. As a control, the PDE4 activity remaining in the supernatant did not change under any of the conditions tested. (B) Western blot assay indicating the integrity and relative amount of PDE3B protein present in each assay.
DISCUSSION
An inhibitory effect of IGF-1 on insulin secretion has been well demonstrated in previous studies (2, 3) as well as in this paper. We have further shown here that the molecular mechanism by which this effect occurs is through activation of a PDE, specifically PDE3B, which causes a reduction of cAMP concentration. IGF-1 attenuates GLP-1-stimulated insulin secretion by suppressing the GLP-1 elevated cAMP level in pancreatic β cells (Fig. 6). Similarly, IGF-1 inhibited insulin secretion potentiated by a cAMP analog that is a substrate of PDE3B but not by one that is not a good substrate (Fig. 3). This result essentially rules out the possibility that IGF-1 inhibits insulin secretion by directly influencing the signaling components downstream of cAMP. It also indicates that the reduction of intracellular cAMP level is most likely not due to an inhibition of an adenylyl cyclase in the β cells, as the stimulus for secretion is the continuous influx of the cAMP analog. Our data, when combined with several previous studies on the cross-regulation between insulin and IGF-1 (1, 2, 3, 27, 28), points to a regulatory feedback loop involving both endocrine insulin and endocrine IGF-1. In this scenario (Fig. 8), an increased circulating insulin level (in response to an elevated nutrient level and hormonal action) will act on the liver and perhaps other tissues to stimulate the synthesis of IGF-1 (29, 30). IGF-1 then will in turn bind to its own receptors on the β cell surface, thereby activating PDE3B and decreasing cAMP. In completion of this loop, insulin secretion from pancreatic islets will be reduced. The data further suggest that the inhibitory effect of IGF-1 and the stimulatory effect of GLP-1 share a common molecular switch—i.e., modulation of intracellular cAMP levels through balanced activation of adenylyl cyclase and PDE3B.
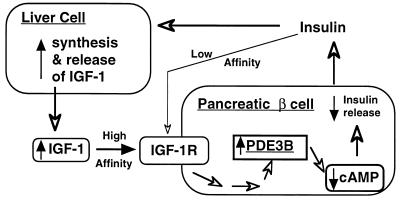
Figure 8.
Model for the role of PDE3B in IGF-1 regulation of insulin secretion from pancreatic β cells. In this model increased circulating insulin stimulates the synthesis and release of IGF-1 (thick arrows) from the liver (27, 28). The elevated IGF-1 in the circulation then binds to IGF-1 receptors (IGF-1R) on the pancreatic β cell surface, activating a series of still unidentified kinases that eventually cause activation of PDE3B. As a result, the cAMP levels within the β cells declines, leading to attenuation of insulin release. If the local insulin concentration is too high near the β cells, this regulatory feedback loop may also allow insulin to exert an immediate inhibitory effect on its own secretion through the IGF-1 receptors (as indicated by the thin open arrows). This model does not exclude participation of potential paracrine IGF-1 synthesized locally within the pancreatic islets.
Several studies have suggested that one major hormonal activator of PDE3B is insulin, which stimulates this enzyme in fat and liver tissues and thereby decreases the intracellular cAMP levels (16, 17). Although there have been published reports about the role of insulin as a direct feedback inhibitor of its own secretion from pancreatic β cells, this idea is controversial, with conflicting results from different groups (4–7). Even in those cases where the inhibitory effect of insulin was observed, a very high level of insulin was required (4, 5, 31). On the other hand, IGF-1 can attenuate insulin secretion at a physiological concentration range both in vivo and in vitro (1–3). For example, in an isolated primary β cell system, van Schravendijk et al. (3) showed that while 5 nM IGF-1 could inhibit insulin secretion by 30%, it would take 1 μM insulin to achieve the same effect. Since IGF-1 receptors, but not insulin receptors, are highly expressed on the β cells (Fig. 2), it seems most likely that the IGF-1 acts through its own receptors on the β cell surface to attenuate insulin secretion. If there is a direct inhibitory effect of insulin on its own secretion, it could easily be due to the low-affinity binding of insulin to the IGF-1 receptors. This may be functionally very important, however, if conditions exist whereby high local concentrations of insulin surrounding the β cells develop in vivo. Such a mechanism could help to prevent oversecretion of insulin acutely.
The regulation of insulin secretion is complex. Although metabolic fuels (e.g., glucose and amino acids) are the primary secretagogues, a number of other factors are known to modulate insulin secretion. Thus, GLP-1, an “incretin,” is stimulated by nutrient ingestion and is a positive modulator of insulin secretion (1). Somatostatin-28, a “decretin,” is also stimulated by nutrient ingestion but is a negative modulator of insulin secretion (32, 33). The data presented here suggest another longer-term modulation of islet β cell function by the liver. While GLP-1 and somatostatin-28 are produced rapidly by the intestine, where the nutrient insulin secretagogues are absorbed, IGF-1 is produced mainly by the liver, an organ that is a target for insulin action. Thus, it seems likely that IGF-1 inhibition of insulin secretion through activation of PDE3B may constitute part of a major homeostatic control mechanism for regulation of insulin secretion.
Finally, the data suggest that two of the important aspects of insulin physiology that are often impaired in diabetes, the regulation of insulin secretion and insulin sensitivity of peripheral tissues to insulin, are controlled at least in part by a common underlying molecular theme (i.e., regulation of cAMP by PDE3B). Activation of PDE3B in adipose and liver tissues is anabolic in nature in that it promotes lipogenic and glycogenic actions of insulin by reducing intracellular cAMP levels (16, 17). In pancreatic islets, however, the activation of PDE3B attenuates insulin secretion. Therefore, one could envision that any genetic mutation leading to partial or complete loss of PDE3B function would cause dyslipidemia and hyperglycemia due to effects on fat and liver tissues, but that such mutations should be compensated for by an abnormal accumulation of cAMP in and an increased insulin secretion from the pancreatic β cells. Similarly, the high expression of PDE3B in pancreatic β cells also may have pharmacological implications for the therapeutic safety of current PDE3-specific inhibitors. Several such agents, including milrinone, enoximone, and cilostazol, are currently being explored as therapeutic drugs for treatment of certain cardiovascular diseases (28). The fact that these agents would be expected not only to inhibit insulin function but also to stimulate insulin secretion may explain why they have not been found to cause diabeteslike symptoms during their clinical trials (28).
Acknowledgments
We thank Ms. Paulette Brunner for her technical assistance on confocal microscopy carried out in the W. M. Keck Center for Advanced Studies in Neural Signaling. We also thank the Cell and Tissue Core of the Diabetes Endocrinology Research Center (National Institutes of Health Grant DK17047) for providing rat islets and facilitating the experiments using cultured islet cells. A.Z. was a recipient of a Dick and Julia McAbee Diabetes Research Fellowship. This work was also supported by National Institutes of Health grants to J.A.B. (DK21723 and HL44948).
ABBREVIATIONS
- IGF-1
insulin-like growth factor 1
- PDE
phosphodiesterase
- GLP-1
glucagonlike peptide 1
- μU
microunits
- GST
glutathione S-transferase
- IRI
immunoreactive insulin
Footnotes
References
- 1.Holz G G, Habener J F. Trends Biochem Sci. 1992;17:388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guler H P, Schmid C, Zapf J, Froesch E R. Proc Natl Acad Sci USA. 1989;86:2868–2872. doi: 10.1073/pnas.86.8.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Schravendijk C F, Heylen L, Van den Brande J L, Pipeleers D G. Diabetologia. 1990;33:649–653. doi: 10.1007/BF00400565. [DOI] [PubMed] [Google Scholar]
- 4.Koranyi L, James D E, Kraegen E W, Permutt M A. J Clin Invest. 1992;89:432–436. doi: 10.1172/JCI115602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchetti P, Scharp D W, McLear M, Finke E H, Olack B, Swanson C, Giannarelli R, Navalesi R, Lacy P E. Acta Diabetol. 1995;32:75–77. doi: 10.1007/BF00569560. [DOI] [PubMed] [Google Scholar]
- 6.Grodsky G M, Fanska R, Schmid F G. Diabetes. 1973;22:256–263. doi: 10.2337/diab.22.4.256. [DOI] [PubMed] [Google Scholar]
- 7.Marincola F, Frank W, Clark W, Douglas M, Merrell R. Diabetes. 1983;32:1162–1167. doi: 10.2337/diab.32.12.1162. [DOI] [PubMed] [Google Scholar]
- 8.Pipeleers D G, Schuit F C, in’t Veld P A, Maes E, Hooghe P E L, Van d W M, Gepts W. Endocrinology. 1985;117:824–833. doi: 10.1210/endo-117-3-824. [DOI] [PubMed] [Google Scholar]
- 9.Holz G G, Leech C A, Habener J F. J Biol Chem. 1995;270:17749–17757. [PMC free article] [PubMed] [Google Scholar]
- 10.Fehmann H C, Goke R, Goke B, Bachle R, Wagner B, Arnold R. Biochim Biophys Acta. 1991;1091:356–363. doi: 10.1016/0167-4889(91)90200-h. [DOI] [PubMed] [Google Scholar]
- 11.D’Ambra R, Surana M, Efrat S, Starr R G, Fleischer N. Endocrinology. 1990;126:2815–2822. doi: 10.1210/endo-126-6-2815. [DOI] [PubMed] [Google Scholar]
- 12.Shafiee N R, Pyne N J, Furman B L. Br J Pharmacol. 1995;115:1486–1492. doi: 10.1111/j.1476-5381.1995.tb16641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beavo J A. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 14.Parker J C, VanVolkenburg M A, Ketchum R J, Brayman K L, Andrews K M. Biochem Biophys Res Commun. 1995;217:916–923. doi: 10.1006/bbrc.1995.2858. [DOI] [PubMed] [Google Scholar]
- 15.Beavo J A. In: Multiple Phosphodiesterase Isoenzymes: Background, Nomenclature, and Implications. Beavo J A, Houslay M D, editors. Vol. 2. Chichester, U.K.: Wiley; 1990. pp. 3–19. [Google Scholar]
- 16.Manganiello V C, Taira M, Degerman E, Belfrage P. Cell Signalling. 1995;7:445–455. doi: 10.1016/0898-6568(95)00017-j. [DOI] [PubMed] [Google Scholar]
- 17.Beebe S J, Redmon J B, Blackmore P F, Corbin J D. J Biol Chem. 1985;260:15781–15788. [PubMed] [Google Scholar]
- 18.Rahn T, Ridderstrale M, Tornqvist H, Manganiello V, Fredrikson G, Belfrage P, Degerman E. FEBS Lett. 1994;350:314–318. doi: 10.1016/0014-5793(94)00797-7. [DOI] [PubMed] [Google Scholar]
- 19.Wang L M, Keegan A, Frankel M, Paul W E, Pierce J H. Stem Cells (Dayton) 1995;13:360–368. doi: 10.1002/stem.5530130407. [DOI] [PubMed] [Google Scholar]
- 20.Zhao, A. Z. & Beavo, J. A. (1996) Biochem. Soc. Trans. 24, Part 4, 582S.
- 21.Metz S A, Robertson R P, Fujimoto W Y. Diabetes. 1981;30:551–557. doi: 10.2337/diab.30.7.551. [DOI] [PubMed] [Google Scholar]
- 22.Kanatsuna T, Lernmark A, Rubenstein A H, Steiner D F. Diabetes. 1981;30:231–234. doi: 10.2337/diab.30.3.231. [DOI] [PubMed] [Google Scholar]
- 23.Hagopian W A, Karlsen A E, Petersen J S, Teague J, Gervassi A, Jiang J, Fujimoto W, Lernmark A. Endocrinology. 1993;132:2674–2681. doi: 10.1210/endo.132.6.8504767. [DOI] [PubMed] [Google Scholar]
- 24.Yan C, Zhao A Z, Bentley J K, Loughney K, Ferguson K, Beavo J A. Proc Natl Acad Sci USA. 1995;92:9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beebe S J, Beasley L A, Corbin J D. Methods Enzymol. 1988;159:531–540. doi: 10.1016/0076-6879(88)59052-3. [DOI] [PubMed] [Google Scholar]
- 26.Van Schravendijk C F, Foriers A, Van den Brande J L, Pipeleers D G. Endocrinology. 1987;121:1784–1788. doi: 10.1210/endo-121-5-1784. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto W Y, Metz S A. Diabetes. 1984;33:872–878. doi: 10.2337/diab.33.9.872. [DOI] [PubMed] [Google Scholar]
- 28.Beavo J A, Reifsnyder D H. Trends Pharmacol Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- 29.Phillips L S, Harp J B, Goldstein S, Klein J, Pao C I. Proc Nutr Soc. 1990;49:451–458. doi: 10.1079/pns19900053. [DOI] [PubMed] [Google Scholar]
- 30.Scheiwiller E, Guler H P, Merryweather J, Scandella C, Maerki W, Zapf J, Froesch E R. Nature (London) 1986;323:169–171. doi: 10.1038/323169a0. [DOI] [PubMed] [Google Scholar]
- 31.Rappaport A M, Ohira S, Coddling J A, Empey G, Kalnins A, Lin B J, Haist R E. Endocrinology. 1972;91:168–176. doi: 10.1210/endo-91-1-168. [DOI] [PubMed] [Google Scholar]
- 32.Bell G I, Yasuda K, Kong H, Law S F, Raynor K, Reisine T. Ciba Found Symp. 1995;190:65–79. doi: 10.1002/9780470514733.ch5. [DOI] [PubMed] [Google Scholar]
- 33.Harris, A. G. (1994) Gut 35, Suppl. 3, S1–S4. [DOI] [PMC free article] [PubMed]