Abstract
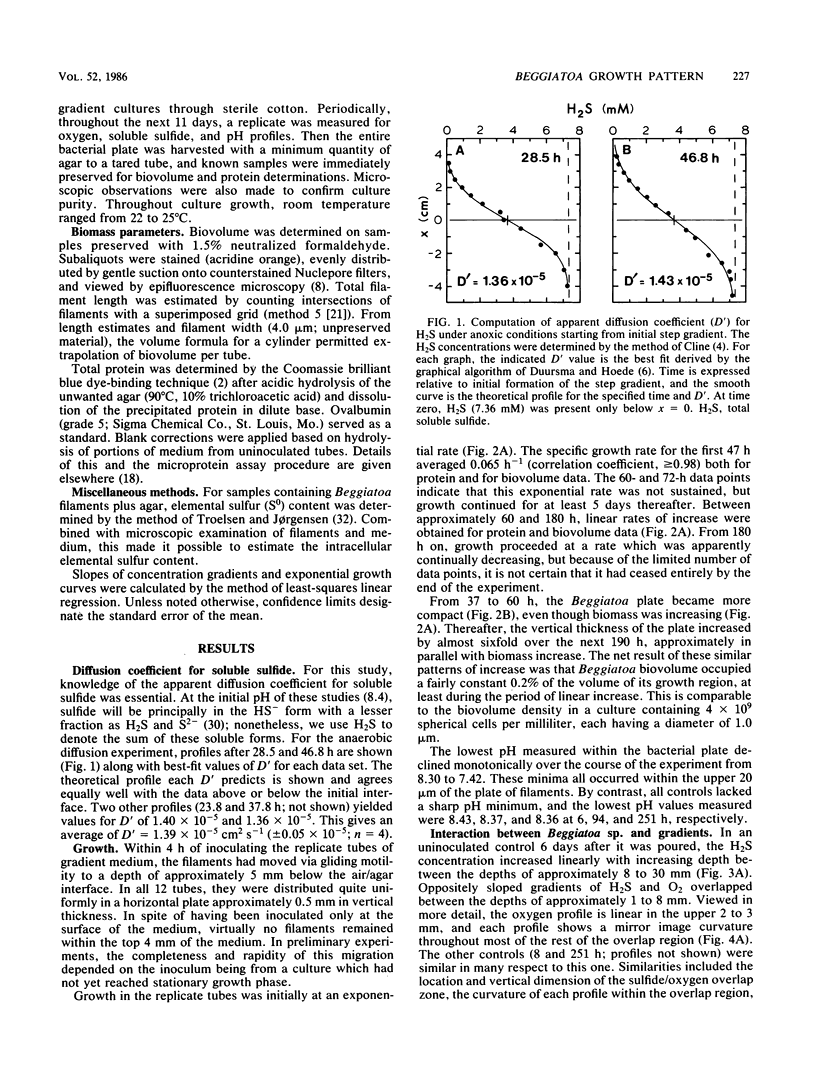
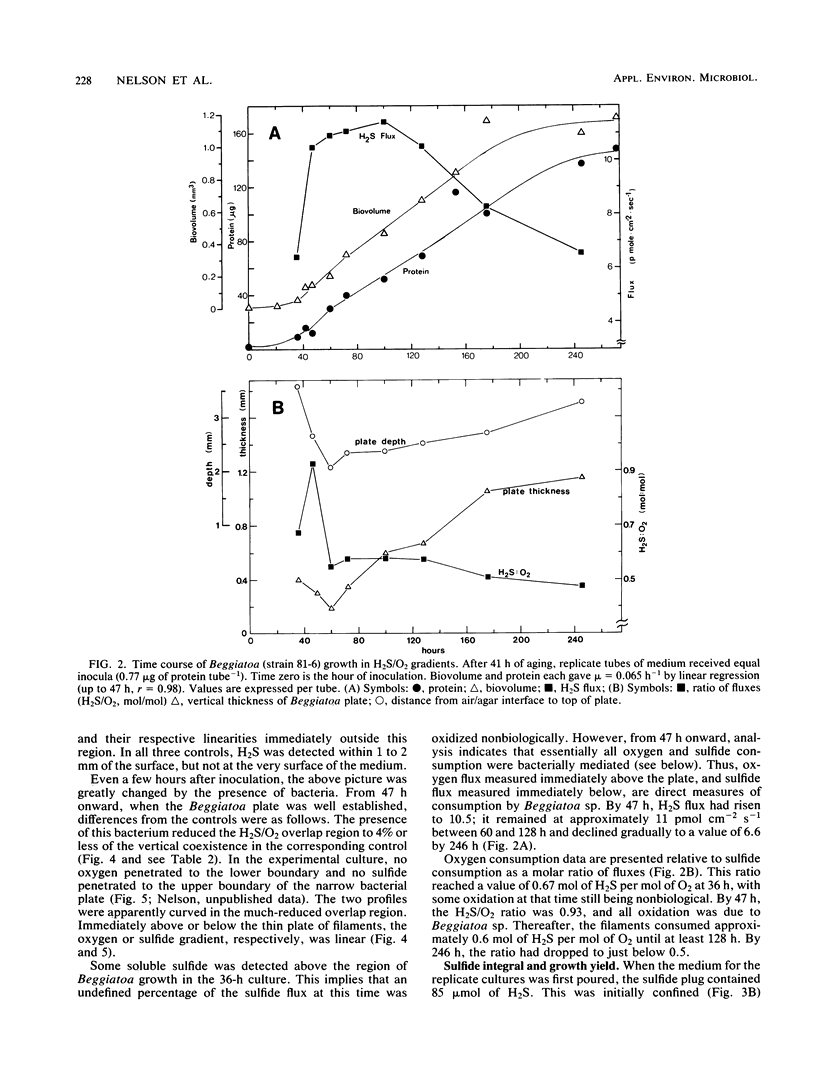
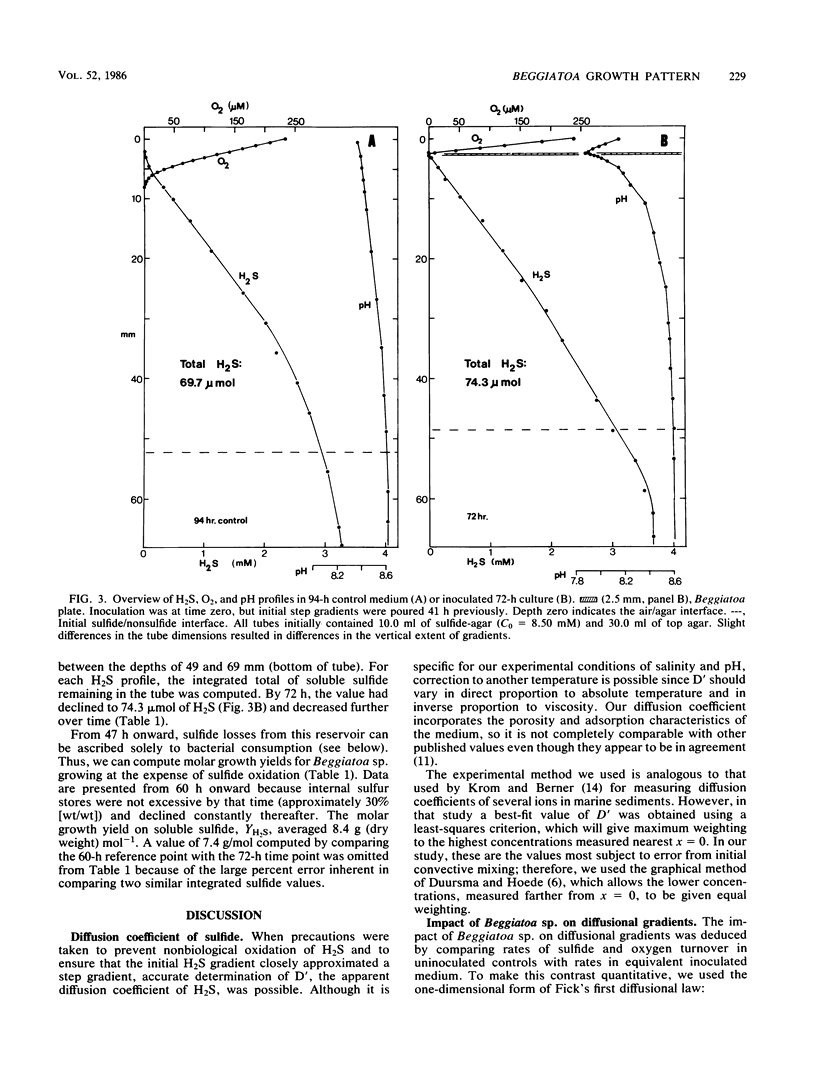
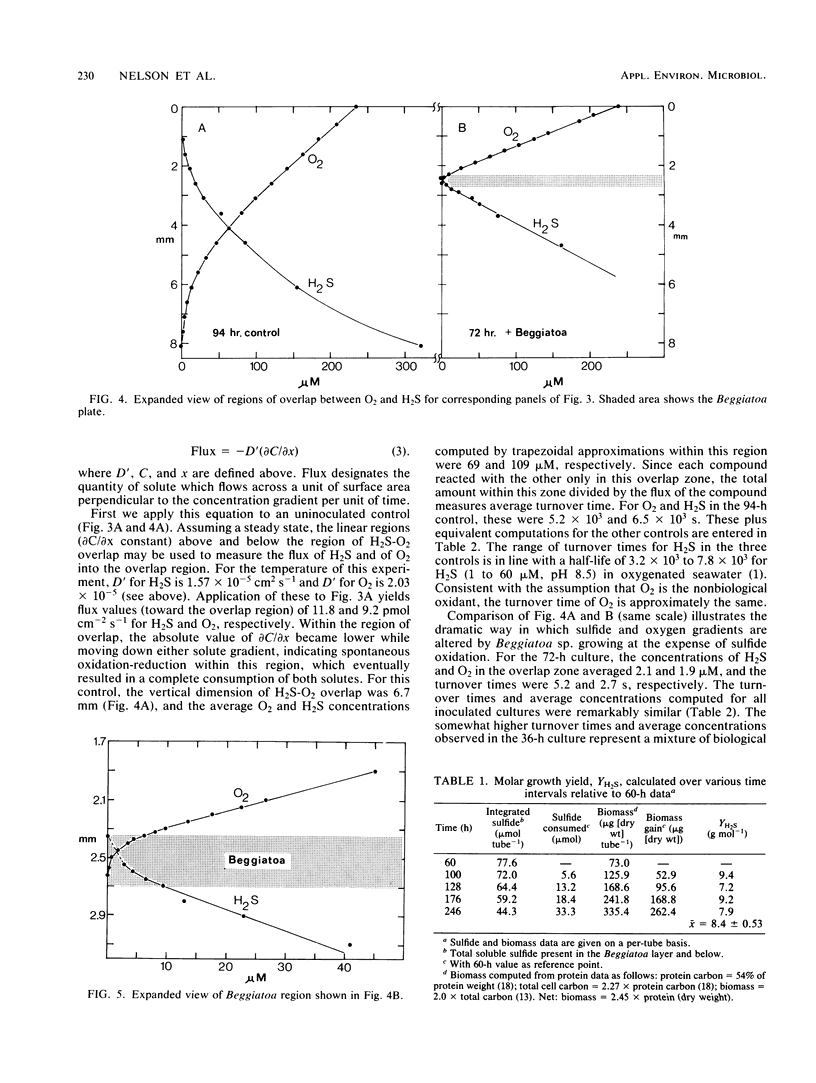
Recently developed techniques involving opposed, gel-stabilized gradients of O2 and H2S permit cultivation of a marine Beggiatoa strain as a chemolithoautotroph which uses gliding motility to precisely track the interface between H2S and O2. In the current study with microelectrodes, vertical profiles of H2, O2, and pH were measured in replicate cultures grown for various intervals. After an initial period of exponential biomass increase (doubling time, 11 h), linear growth prevailed throughout much of the time course. This H2S-limited growth was followed by a transition to stationary phase when the declining H2S flux was sufficient only to supply maintenance energy. During late-exponential and linear growth phases, the Beggiatoa sp. consumed a constant 0.6 mol of H2S for each 1.0 mol of O2, the ratio anticipated for balanced lithoautotrophic growth at the expense of complete oxidation of H2S to SO42−. Over the entire range of conditions studied, this consumption ratio varied by approximately twofold. By measuring the extent to which the presence of the bacterial plate diminished the overlap of O2 and H2S, we demonstrated that oxidation of H2S by Beggiatoa sp. is approximately 3 orders of magnitude faster than spontaneous chemical oxidation. By integrating sulfide profiles and comparing sulfide consumed with biomass produced, a growth yield of 8.4 g (dry weight) mol−1 of H2S was computed. This is higher than that found for sulfide-grown thiobacilli, indicating very efficient growth of Beggiatoa sp. as a chemoautotroph. The methods used here offer a unique opportunity to determine the yield of H2S-oxidizing chemolithoautotrophs while avoiding several problems inherent in the use of homogeneous liquid culture. Finally, by monitoring time-dependent formation of H2S profiles under anoxic conditions, we demonstrate a method for calculating the molecular diffusion coefficient of soluble substrates in gel-stabilized media.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON S. D., MORITA R. Y. EFFECT OF CATALASE AND CULTURAL CONDITIONS ON GROWTH OF BEGGIATOA. J Bacteriol. 1964 Dec;88:1755–1761. doi: 10.1128/jb.88.6.1755-1761.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- FAUST L., WOLFE R. S. Enrichment and cultivation of Beggiatoa alba. J Bacteriol. 1961 Jan;81:99–106. doi: 10.1128/jb.81.1.99-106.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen B. B., Revsbech N. P. Colorless Sulfur Bacteria, Beggiatoa spp. and Thiovulum spp., in O(2) and H(2)S Microgradients. Appl Environ Microbiol. 1983 Apr;45(4):1261–1270. doi: 10.1128/aem.45.4.1261-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P. Biochemistry of the chemolithotrophic oxidation of inorganic sulphur. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):499–528. doi: 10.1098/rstb.1982.0094. [DOI] [PubMed] [Google Scholar]
- Møller M. M., Nielsen L. P., Jørgensen B. B. Oxygen Responses and Mat Formation by Beggiatoa spp. Appl Environ Microbiol. 1985 Aug;50(2):373–382. doi: 10.1128/aem.50.2.373-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Castenholz R. W. Use of reduced sulfur compounds by Beggiatoa sp. J Bacteriol. 1981 Jul;147(1):140–154. doi: 10.1128/jb.147.1.140-154.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim E. G. Contributions toward the development of general microbiology (Ernst G. Pringsheim). Annu Rev Microbiol. 1970;24:1–16. doi: 10.1146/annurev.mi.24.100170.000245. [DOI] [PubMed] [Google Scholar]
- Pringsheim E. G. Die Mixotrophie von Beggiatoa. Arch Mikrobiol. 1967;59(1):247–254. [PubMed] [Google Scholar]
- Revsbech N. P., Ward D. M. Oxygen Microelectrode That Is Insensitive to Medium Chemical Composition: Use in an Acid Microbial Mat Dominated by Cyanidium caldarium. Appl Environ Microbiol. 1983 Mar;45(3):755–759. doi: 10.1128/aem.45.3.755-759.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTEN H. L., STOKES J. L. Isolation and properties of Beggiatoa. Arch Mikrobiol. 1962;42:353–368. doi: 10.1007/BF00409071. [DOI] [PubMed] [Google Scholar]
- Strohl W. R., Cannon G. C., Shively J. M., Güde H., Hook L. A., Lane C. M., Larkin J. M. Heterotrophic carbon metabolism by Beggiatoa alba. J Bacteriol. 1981 Nov;148(2):572–583. doi: 10.1128/jb.148.2.572-583.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. R., Larkin J. M. Enumeration, isolation, and characterization of beggiatoa from freshwater sediments. Appl Environ Microbiol. 1978 Nov;36(5):755–770. doi: 10.1128/aem.36.5.755-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol. 1984;38:459–486. doi: 10.1146/annurev.mi.38.100184.002331. [DOI] [PubMed] [Google Scholar]


