Abstract
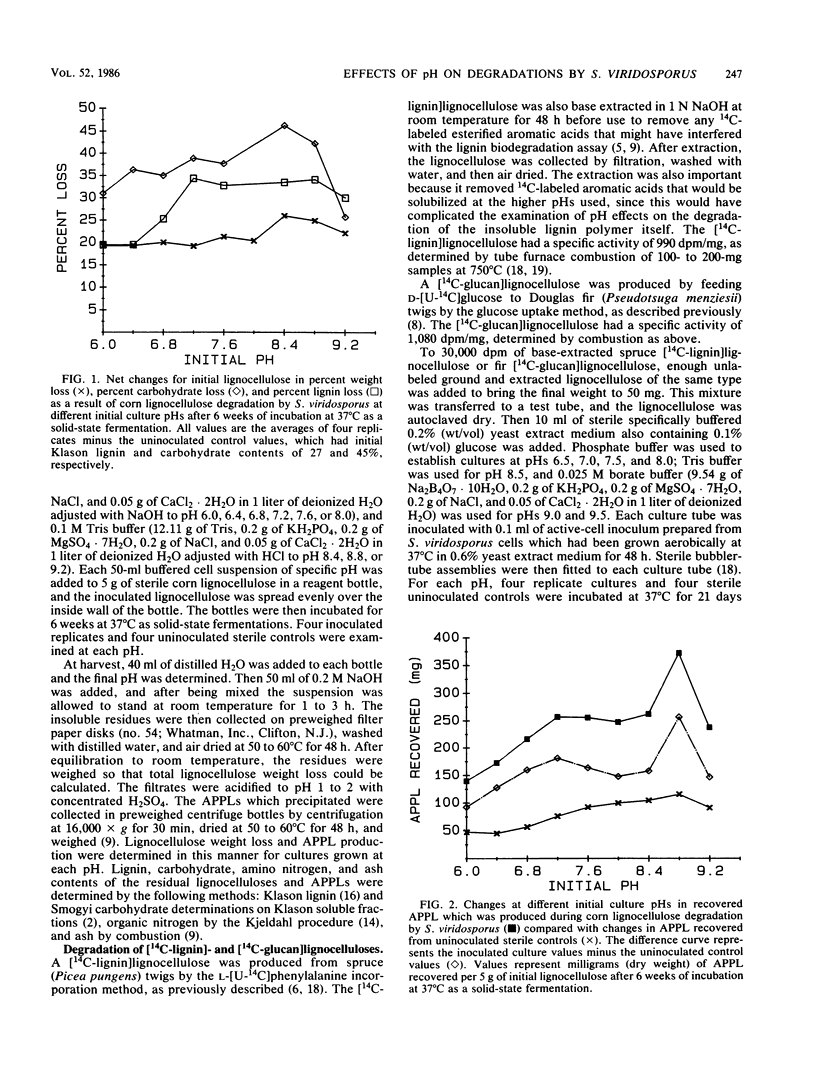
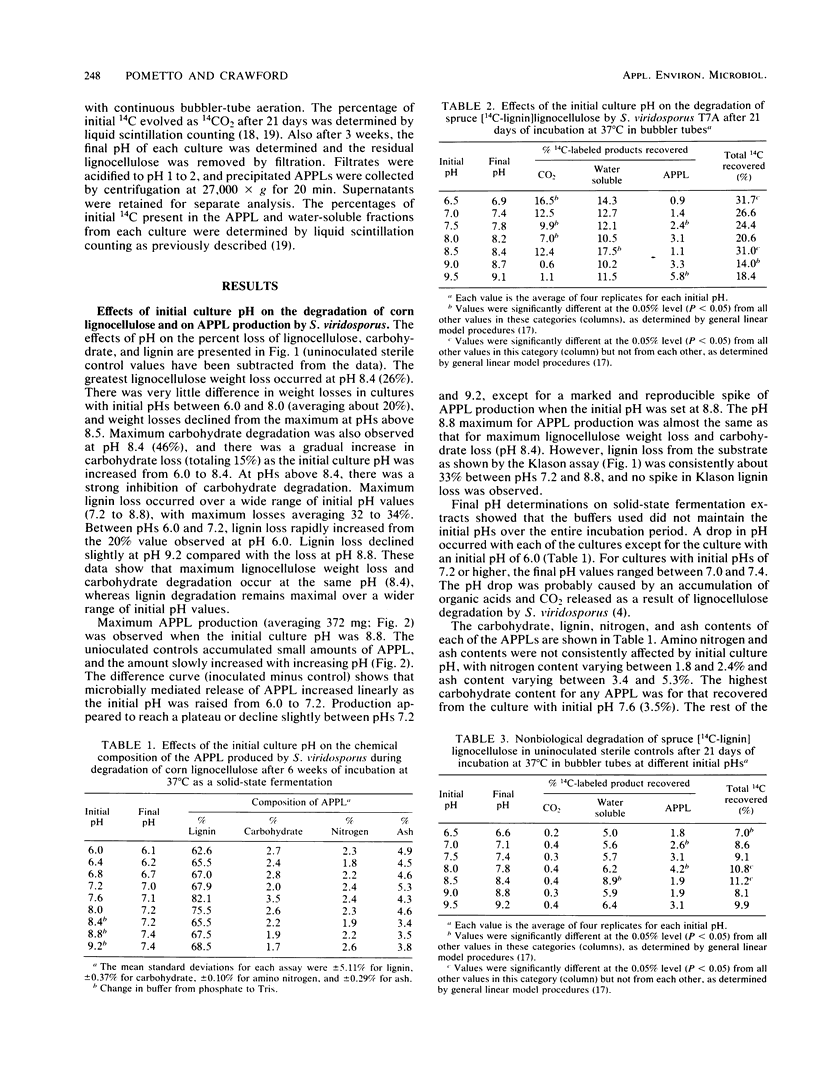
Lignocellulose degradation by Streptomyces viridosporus results in the oxidative depolymerization of lignin and the production of a water-soluble lignin polymer, acid-precipitable polymeric lignin (APPL). The effects of the culture pH on lignin and cellulose metabolism and APPL production by S. viridosporus are reported. Dry, ground, hot-water-extracted corn (Zea mays) lignocellulose was autoclaved in 1-liter reagent bottles (5 g per bottle) and inoculated with 50-ml volumes of S. viridosporus cells suspended in buffers of specific pH (pH 6.0 to 9.2 at 0.4 pH unit intervals). Four replicates of inoculated cultures and of uninoculated controls at each pH were incubated as solid-state fermentations at 37°C. After 6 weeks of incubation the percent loss of lignocellulose, lignin, and carbohydrate and the amount of APPL produced were determined for each replicate. Optimal lignocellulose degradation, as shown by substrate weight loss, was observed in the pH range of 8.4 to 8.8. Only minor differences were seen in the Klason lignin, carbohydrate, protein, and ash contents of the APPLS produced by cultures at each pH. The effects of pH on the degradation of a spruce (Picea pungens) [14C-lignin]lignocellulose and a Douglas fir (Pseudotsuga menziesii) [14C-glucan]-lignocellulose were also determined at pH values between 6.5 and 9.5 (0.5 pH unit intervals). The incubations were carried out for 3 weeks at 37°C with bubbler-tube cultures. The percentage of initial 14C recovered as 14CO2, 14C-labeled water-soluble products, and [14C]APPL was then determined. The mineralization of lignin and cellulose to CO2 was optimal at pHs 6.5 and 7.0, respectively. However, the optimum for lignin and cellulose solubilization was pH 8.5, which correlated with the pH 8.5 optimum for APPL production. Overall, the data show that, whereas lignin mineralization is optimal at neutral to slightly acidic pHs, lignocellulose degradation with lignin solubilization and APPL production is promoted by alkaline pHs. These findings indicate that lignin-solubilizing actinomycetes may play an important role in the metabolism of lignin in neutral to alkaline soils in which ligninolytic fungi are not highly competitive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antai S. P., Crawford D. L. Degradation of softwood, hardwood, and grass lignocelluloses by two streptomyces strains. Appl Environ Microbiol. 1981 Aug;42(2):378–380. doi: 10.1128/aem.42.2.378-380.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmeyer J. R., Crawford D. L. Production and Characterization of Polymeric Lignin Degradation Intermediates from Two Different Streptomyces spp. Appl Environ Microbiol. 1985 Feb;49(2):273–278. doi: 10.1128/aem.49.2.273-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976 May;31(5):714–717. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L., Pometto A. L. Preparation of specifically labeled C-(lignin)- and C-(cellulose)-lignocelluloses and their decomposition by the microflora of soil. Appl Environ Microbiol. 1977 Jun;33(6):1247–1251. doi: 10.1128/aem.33.6.1247-1251.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Pometto A. L., Crawford R. L. Lignin Degradation by Streptomyces viridosporus: Isolation and Characterization of a New Polymeric Lignin Degradation Intermediate. Appl Environ Microbiol. 1983 Mar;45(3):898–904. doi: 10.1128/aem.45.3.898-904.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan M. B., Crawford D. L., Pometto A. L., 3rd Isolation of lignocellulose-decomposing actinomycetes and degradation of specifically 14C-labeled lignocelluloses by six selected Streptomyces strains. Can J Microbiol. 1979 Nov;25(11):1270–1276. doi: 10.1139/m79-200. [DOI] [PubMed] [Google Scholar]
- Pometto A. L., Crawford D. L. Catabolic Fate of Streptomyces viridosporus T7A-Produced, Acid-Precipitable Polymeric Lignin upon Incubation with Ligninolytic Streptomyces Species and Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Jan;51(1):171–179. doi: 10.1128/aem.51.1.171-179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridham T. G., Gottlieb D. The Utilization of Carbon Compounds by Some Actinomycetales as an Aid for Species Determination. J Bacteriol. 1948 Jul;56(1):107–114. doi: 10.1128/jb.56.1.107-114.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. B., Crawford D. L., Pometto A. L. Catabolism of substituted benzoic acids by streptomyces species. Appl Environ Microbiol. 1981 Feb;41(2):442–448. doi: 10.1128/aem.41.2.442-448.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


