Abstract
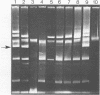
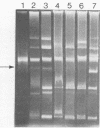
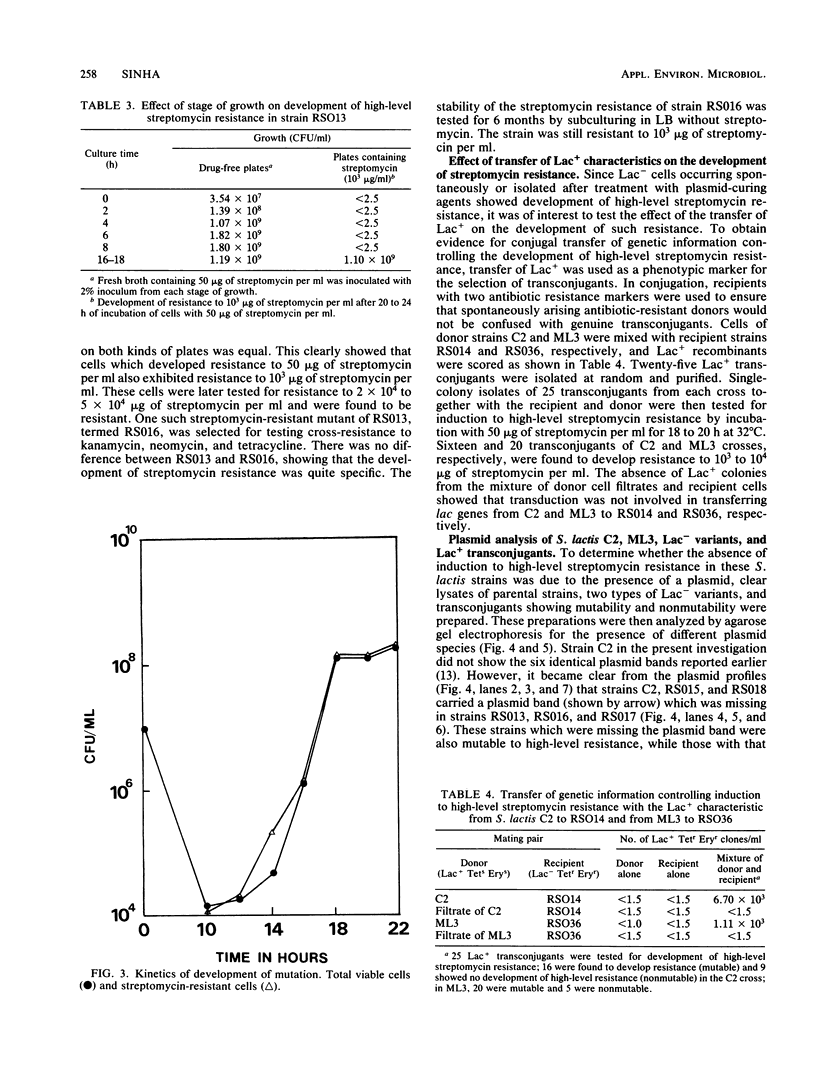
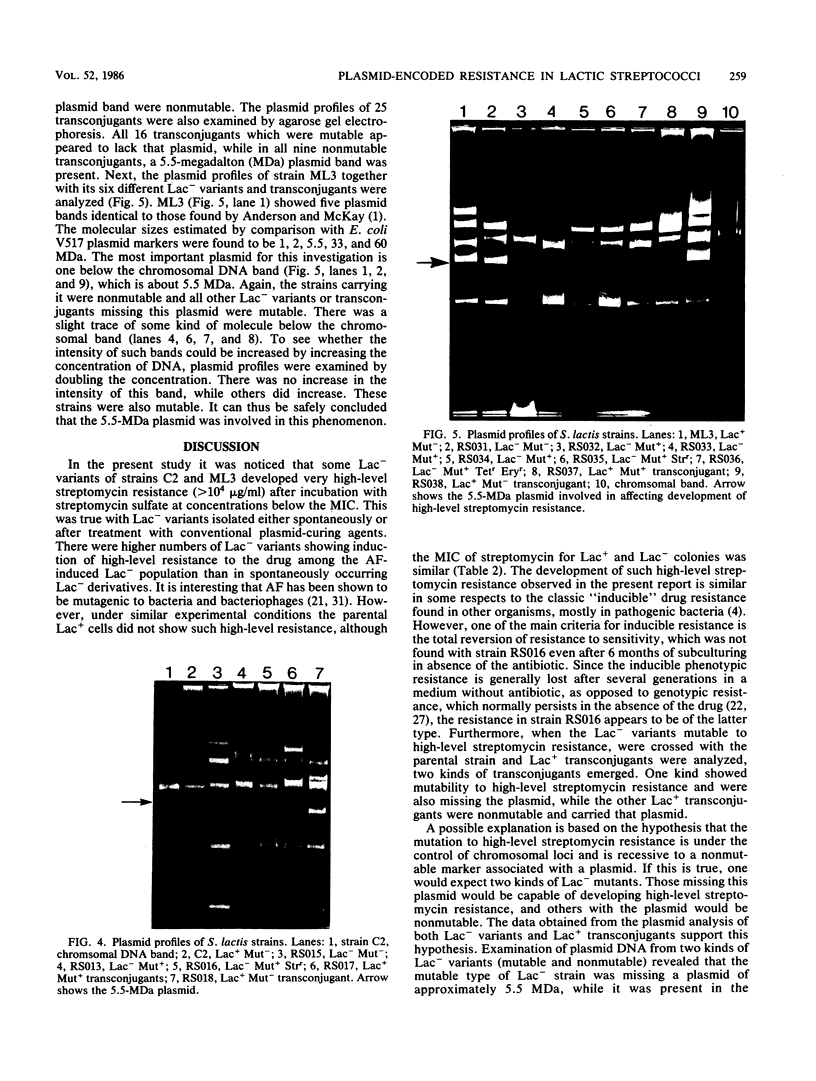
Some lactose-negative (Lac-) mutants of Streptococcus lactis C2 and ML3 exhibited development of very high level streptomycin resistance after incubation with subinhibitory concentrations of the drug for 18 to 22 h. These drug-resistant mutants showed no loss of resistance even after 6 months of subculturing in broth without any drug. The parental Lac+ strains did not show mutation to high-level streptomycin resistance. The Lac+ characteristic of the parental strain was conjugally transferred to Lac- derivatives of C2 and ML3, showing the ability to mutate to high-level resistance. When transconjugants were analyzed for this characteristic, they showed both mutable and nonmutable Lac+ types. The results suggested that genetic information for mutation to high-level streptomycin resistance in lactic streptococci resides on the chromosome, and its expression is affected by a plasmid. The plasmid profiles of strains C2, ML3, C2 Lac-, ML3 Lac-, and two kinds of transconjugants confirmed the presence of a plasmid of approximately 5.5 megadaltons in strains showing no mutation to high-level streptomycin resistance, while strains missing such a plasmid exhibited high-level streptomycin resistance after incubation with subinhibitory concentrations of the drug.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan T. M. Susceptibility of cheese and yoghurt starter bacteria to antibiotics. Appl Microbiol. 1972 May;23(5):960–965. doi: 10.1128/am.23.5.960-965.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connamacher R. H. Inducible bacterial resistance. Antibiot Chemother (1971) 1975;20:8–66. doi: 10.1159/000398461. [DOI] [PubMed] [Google Scholar]
- Danbara H., Yoshikawa M. Plasmid-determined epistatic susceptibility to kasugamycin. Antimicrob Agents Chemother. 1975 Sep;8(3):243–250. doi: 10.1128/aac.8.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. G., Zajdel J., Dobrzański W. T. Possible plasmid nature of the determinant for production of the antibiotic nisin in some strains of Streptococcus lactis. J Gen Microbiol. 1975 May;88(1):189–192. doi: 10.1099/00221287-88-1-189. [DOI] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Inducible and constitutive resistance to macrolide antibiotics and lincomycin in clinically isolated strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 1973 Sep;4(3):327–331. doi: 10.1128/aac.4.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Genetic Evidence for Plasmid-Linked Lactose Metabolism in Streptococcus lactis subsp. diacetylactis. Appl Environ Microbiol. 1979 May;37(5):1041–1043. doi: 10.1128/aem.37.5.1041-1043.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Conjugative 40-megadalton plasmid in Streptococcus lactis subsp. diacetylactis DRC3 is associated with resistance to nisin and bacteriophage. Appl Environ Microbiol. 1984 Jan;47(1):68–74. doi: 10.1128/aem.47.1.68-74.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Efstathiou J. D. Transductional evidence for plasmid linkage of lactose metabolism in streptococcus lactis C2. Appl Environ Microbiol. 1976 Jul;32(1):45–52. doi: 10.1128/aem.32.1.45-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Walsh P. M. Conjugal transfer of genetic information in group N streptococci. Appl Environ Microbiol. 1980 Jul;40(1):84–89. doi: 10.1128/aem.40.1.84-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORGEL A., BRENNER S. Mutagenesis of bacteriophage T4 by acridines. J Mol Biol. 1961 Dec;3:762–768. doi: 10.1016/s0022-2836(61)80081-8. [DOI] [PubMed] [Google Scholar]
- Pitton J. S. Mechanisms of bacterial resistance to antibiotics. Ergeb Physiol. 1972;65:15–93. doi: 10.1007/3-540-05814-1_2. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Evidence for Plasmid Linkage of Restriction and Modification in Streptococcus cremoris KH. Appl Environ Microbiol. 1981 Dec;42(6):944–950. doi: 10.1128/aem.42.6.944-950.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherwitz K. M., Baldwin K. A., McKay L. L. Plasmid linkage of a bacteriocin-like substance in Streptococcus lactis subsp. diacetylactis strain WM4: transferability to Streptococcus lactis. Appl Environ Microbiol. 1983 May;45(5):1506–1512. doi: 10.1128/aem.45.5.1506-1512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. P. Effect of buffering media with phosphates on antibiotic resistance of lactic streptococci. Appl Environ Microbiol. 1984 May;47(5):1175–1177. doi: 10.1128/aem.47.5.1175-1177.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M. Chromosomal mutation to drug resistance in bacteria. Antibiot Chemother (1971) 1975;20:67–86. doi: 10.1159/000398463. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Sevag M. G. Sensitivity of Escherichia coli to atabrine conferred by R factor and its potential clinical significance. J Bacteriol. 1967 Jan;93(1):245–253. doi: 10.1128/jb.93.1.245-253.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]